Abstract
Background
Many onychomycosis treatments have not been directly compared in head-to-head clinical trials. Objective: To determine the relative efficacy of onychomycosis treatments using network meta-analysis (NMA).
Methods
We conducted a systematic review and NMA of mycological cure rates.
Results
Nineteen trials were included in the network. Terbinafine 250 mg was significantly superior to all treatments except itraconazole 400 mg pulse therapy. The itraconazole 400 mg pulse regimen was significantly superior to all topicals except efinaconazole 10% nail solution. Itraconazole 200 mg was significantly superior to all topical treatments, while fluconazole 150-450 mg, efinaconazole 10% nail solution, tavaborole 5% nail solution, ciclopirox nail lacquer 8%, terbinafine nail solution, and amorolfine 5% nail lacquer were significantly superior to placebo.
Conclusions
Newly developed topicals have improved the odds ratios (ORs) of mycological cure, yet these ORs were not significantly greater than preexisting topical treatments. Further experience with these agents will reveal their clinical significance, and head-to-head trials are warranted.
© 2015 S. Karger AG, Basel
Key Words: Onychomycosis, Systematic review, Network meta-analysis, Antifungals
Introduction
Onychomycosis is a progressive fungal infection of the nail unit and surrounding tissue. It is characterized by marked thickening and discoloration of the nail and separation of the nail unit from the nail bed [1]. The infection is frequently caused by dermatophyte fungi but may also be attributed to yeasts and nondermatophyte molds [2]. Onychomycosis is the most common nail disease, with an average prevalence of approximately 4% in Europe and North America [3]. However, the prevalence of onychomycosis is >10% in certain patient populations, such as the elderly, diabetic, and immunocompromised, who are at a greater risk of infection due to the presence of preexisting nail deformity, hyperkeratinization, reduced peripheral circulation, and impaired immune function [4].
Oral antifungals are often the first line of treatment for onychomycosis, yet these agents are not recommended in patients at high risk of experiencing drug interactions [5]. Therefore, topical therapies are indispensable in the treatment of onychomycosis, particularly in patients most susceptible to the disease and where polypharmacy is common [6]. For years, ciclopirox 8% nail lacquer was the only topical treatment approved for this indication in North America [6]. Newer agents have been developed with the aim of improving nail penetrance and efficacy. In 2014, efinaconazole topical solution 10% and tavaborole topical solution 5% received FDA approval as treatments for toenail onychomycosis caused by dermatophytes [7,8]. However, neither of these agents has been evaluated against active comparators. Indeed, onychomycosis treatments have seldom been directly compared in clinical trials as pivotal trials often evaluate new agents against placebo.
Network meta-analysis (NMA) enables treatments, which have not been directly compared in head-to-head trials to be evaluated against each other via their relationship to a common comparator. For example, if drug A has not been compared to drug B, yet both drugs A and B have been compared to drug C (or in many instances, placebo), then the relative efficacy of drugs A and B can be evaluated by comparing their efficacy rates when compared to drug C [9]. In NMA, this type of evidence is known as indirect evidence (as opposed to direct evidence from a head-to-head trial). However, the precision of indirect comparisons tends to decrease when the treatments involved are farther apart in the network. When available, both direct and indirect evidence is considered when evaluating treatments in a NMA. Similarity of the study design, participant characteristics, and outcome criteria (both definition and measurement) between trials are essential for NMA to yield valid results [9]. The objective of this study was to use NMA to compare the relative efficacy of onychomycosis treatments for the outcome mycological cure.
Methods
Search Strategy and Selection Criteria
Our previous systematic review of the Scopus, PubMed, Medline, OLDMedline, Healthstar, Embase, Embase Classic, and International Pharmaceutical Abstracts databases via OVID was conducted up until March 25, 2013 [10]. This search was updated to include results as of October 31, 2014, using the terms ‘onychomycosis and treatment’ and further refining the results to clinical trials. The clinicaltrails.gov website was also searched for relevant trials.
Only randomized controlled trials with a parallel-group design and a minimum of 48 weeks study duration were included. Trials must have pertained to oral or topical monotherapy for toenail onychomycosis caused by dermatophytes and must have reported mycological cure rates defined as negative potassium hydroxide mount (KOH) and culture. A previous study by Gupta et al. [11] found that fluconazole dosages of 150, 300, and 450 mg administered for 6 or 9 months were equivalent in efficacy; therefore, these dosages were combined. All other treatments and dosages were analyzed separately. For multi-arm trials, only the arms reporting standard dosages indicated for onychomycosis and the treatment durations specified above were included in the analyses. Consequently, arms that included terbinafine treatment durations >12 weeks were omitted. Phase II studies were also excluded because of their narrower inclusion and exclusion criteria.
Two reviewers (D.D. and K.A.F.) performed the title, abstract, and content review and rated the methodological quality of each trial. The methodological quality of the studies was determined with study quality assessment tools based on the Consolidated Standards of Reporting Trials (CONSORT) statement, which includes items on the reporting of randomization, blinding, and statistical analysis in clinical trials [12,13]. A cutoff score of 11 on this assessment tool denotes a study of high quality [12]. Data extraction was performed by one reviewer (D.D.) and entered into the Aggregate Data Drug Information System (ADDIS) software and was verified by the second reviewer (K.A.F.). The data recorded included the study design, number of participants randomized into each treatment group, baseline characteristics, drug name, treatment duration, length of follow-up, and mycological cure rates.
Statistical Analysis
The degree of inter-rater agreement for the assessment of study quality was quantified with the kappa statistic in SPSS 20 [14]. Between-trial heterogeneity was assessed by calculating the I2 from Mantel-Haenszel random effects pairwise meta-analyses in Review Manager 5.3 [15]. Between-trial heterogeneity was also qualitatively assessed based on the study design and participant baseline characteristics. The node-splitting analyses, which are conducted to assess the inconsistency between the direct and indirect evidence in the network, were conducted in ADDIS version 1.16.3 software with 0.05 significance levels [16]. The odds ratios (ORs) of mycological cure between treatments were compared with a consistency model based on a Bayesian random effects model. Minimally-informative prior distributions were selected for the treatment effect parameters and arbitrary starting values for the Markov Chain were assigned by the ADDIS program and updated with each iteration [17]. Model convergence was assessed by the Brooks-Gelman diagnostic [18]. Convergence was reached (a) when the potential scale reduction factor values approximated 1 and (b) when the iterative potential scale reduction factor graphically converged [19]. The results of the NMA are presented as ORs with 95% credible intervals (CrI). A treatment ranking was also produced based on the probability of mycological cure.
Results
Literature Search and Study Quality
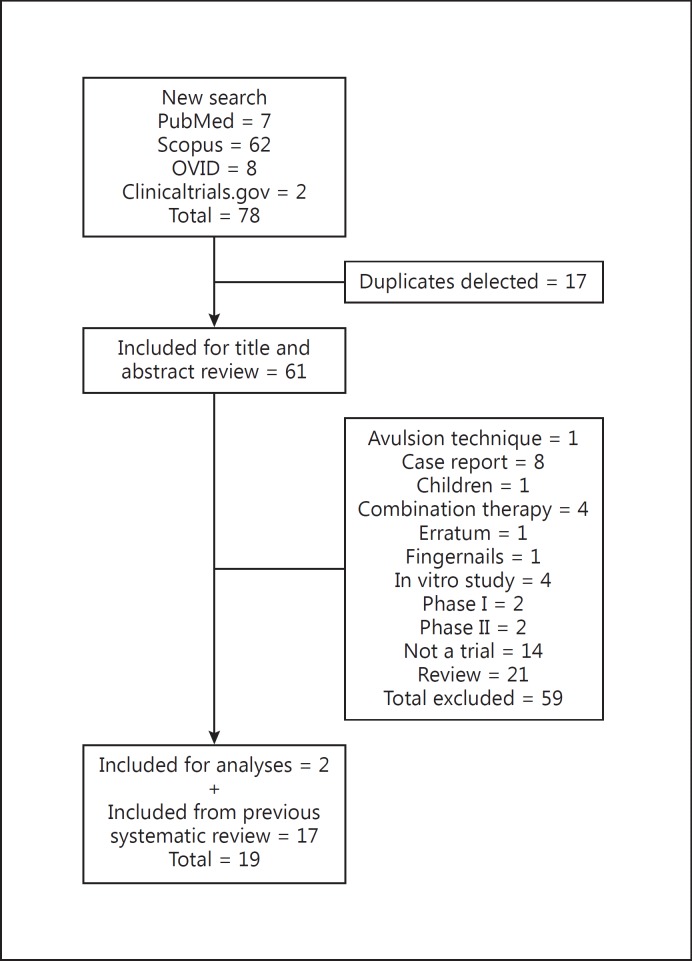
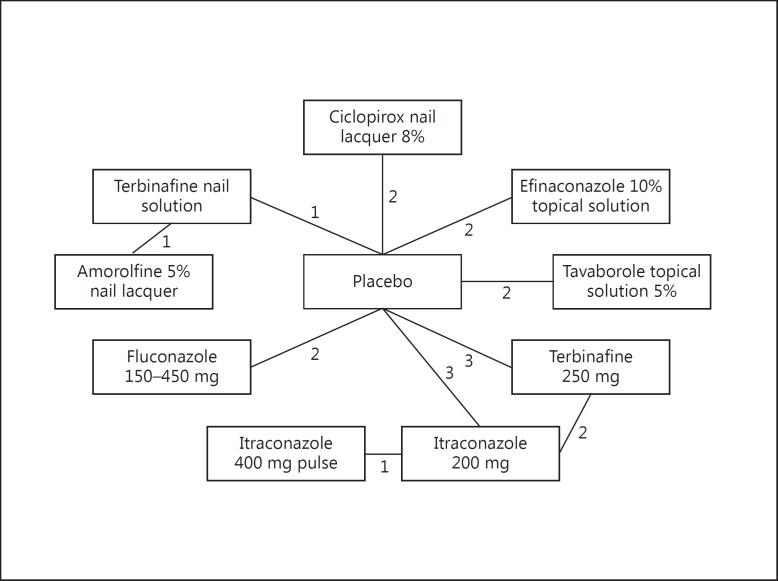
Search results are presented in figure 1. Seventeen trials were included from the previous search [10]. An updated search of the PubMed, Scopus and OVID, and clinical trials.gov databases yielded 7, 72, and 6 articles, respectively, and two clinicaltrials.gov records (n = 85). Of these, only the two clinicaltrials.gov records met the inclusion criteria; thus, a total of 19 trials (n = 5,551 patients) were included for the NMA (table 1) [20,21,22,23,24,25,26,27,28,29,30,31,32,33]. The evidence network consisted of five topical treatments [amorolfine 5% nail lacquer, ciclopirox nail lacquer 8%, efinaconazole 10% topical solution, terbinafine nail solution (TNS), and tavaborole topical solution 5%] and four oral treatment regimens (fluconazole 150-450 mg, itraconazole 200 mg continuous dosing, itraconazole 400 mg pulse, and terbinafine 250 mg continuous dosing; fig. 2).
Fig. 1.
Study selection flow chart.
Table 1.
Trials included in the evidence network
| Treatment arms | Age, years | Clinical type | % Nail involvement | Mycological cure rate | Assessment week | |
|---|---|---|---|---|---|---|
| Topical treatment trials | ||||||
| Elewski et al. [20], 2013 trial b | terbinafine 10% nail solution placebo | 54.0 ± 11.78 53.9 ± 12.09 | DLSO, PSO, TDO | 49.3 ± 14.48 48.4 ± 14.84 | 51/271 14/256 | 52 |
| Elewski et al. [20], 2013 trial c | terbinafine 10% nail solution amorolfine 5% nail lacquer | 53.0 ± 12.15 53.0 ± 12.97 | DLSO | 51.9 ± 17.13 50.4 ± 16.32 | 82/507 82/522 | 52 |
| Elewski et al. [21], 2013 trial 1 | efinaconazole 10% topical solution placebo | 52 (20 – 71) 52 (18 – 70) | DLSO | 36.7 (20 – 50) 36.8 (20 – 50) | 362/656 36/214 | 52 |
| Elewski et al. [21], 2013 trial 2 | efinaconazole 10% topical solution placebo | 51 (18 – 71) 51 (18 – 70) | DLSO | 36.2 (20 – 60) 36.7 (20 – 50) | 311/583 34/202 | 52 |
| Gupta et al. [22], 2000 trial 1 | ciclopirox 8% nail lacquer placebo | 50 (20 – 70) 49 (18 – 70) | DLSO | 36.6 ± 10.0 40.3 ± 9.6 | 30/112 12/111 | 48 |
| Gupta et al. [22], 2000 trial 2 | ciclopirox 8% nail lacquer placebo | 50 (19 – 70) 50 (23 – 70) | DLSO | 37.7 ± 10.8 38.3 ± 8.6 | 41/119 10/118 | 48 |
| NCT01302119 [23] | tavaborole 5% topical solution placebo | 55.5 ± 11.5 55.4 ± 11.0 | DLSO | − | 142/396 25/205 | 52 |
| NCT01270971 [24] | tavaborole 5% topical solution placebo | 53.6 ± 12.5 53.4 ± 12.3 | DLSO | – | 124/399 14/194 | 52 |
| Oral treatment trials | ||||||
| Billstein et al. [25], 1999 | terbinafine 250 mg placebo | 42 – 47 | – | – | 11/15 0/16 | 48 |
| Drake et al. [26], 1997 | terbinafine 250 mg placebo | 45 ± 1 45 ± 2 | DLSO | 58 ± 2 57 ± 3 | 99/142 6/71 | 48 |
| Goodfield et al. [27], 1992 | terbinafine 250 mg placebo | 44 (19 – 78) | - | – | 38/70 1/29 | 48 |
| Bräutigam et al. [28], 1996 | terbinafine 250 mg itraconazole 200 mg | 49 (21 – 70) | DLSO, PSO | 65% > 60% NI 60% > 60% NI | 70/86 53/84 | 52 |
| De Backer et al. [29], 1998 | terbinafine 250 mg itraconazole 200 mg | – | – | – | 136/186 85/186 | 48 |
| Elewski et al.[30], 1997 trial 1 | itraconazole 200 mg placebo | 46 (18 – 70) 50 (30 – 68) | DLSO, PSO, TD | – | 24/35 2/33 | 48 |
| Elewski et al. [30], 1997 trial 2 | itraconazole 200 mg placebo | 47 (24 – 69) 48 (23 – 69) | DLSO, PSO, TD | – | 18/38 3/35 | 48 |
| Elewski et al. [30], 1997 trial 3 | itraconazole 200 mg placebo | 49 (23 – 67) 49 (22 – 69) | DLSO, PSO, TD | – | 17/37 1/39 | 48 |
| Havu et al. [31], 1997 | itraconazole 200mg itraconazole 400 mg (pulse) | 45 41 | DLSO | – | 44/65 43/64 | 48 |
| Ling et al. [32], 1998 | fluconazole 450 mg (weekly) placebo | 18 – 70 | DLSO | 80% ≥ 50% NI 80% ≥ 50% NI | 86/192 6/96 | 48 – 60 |
| Scher et al. [33], 1998 | fluconazole 150 – 450 mg (weekly) placebo | 48 49 | DLSO | 69% ≥ 50% NI 66% ≥ 50% NI | 139/269 12/92 | 48 |
Participant age and % nail involvement are presented as mean ± standard deviation or (range) when available. NI = Nail involvement; DLSO = distolateral subungual onychomycosis, PSO = proximal subungual onychomycosis, TD = total dystrophic nail bed disease.
Fig. 2.
Evidence network showing the number of trials for each comparison.
The inter-rater reliability coefficient for the assessment of study quality was 0.81, indicating a substantial agreement between raters [34]. Trials included in the analyses were rated to be of high quality (quality scores >11) [12]. All trials were randomized and double blind, except one open trial by Elewski et al. [20], which compared TNS to amorolfine 5% nail lacquer.
Between-Trial Heterogeneity
Baseline characteristics of trial participants are presented in table 1. The mean age of the participants was similar across trials, with most participants being middle-aged. Efficacy assessments for all trials were performed between weeks 48 and 60, and the majority of onychomycosis infections presented as distolateral subungual onychomycosis caused by dermatophytes. Severity of disease was somewhat more difficult to compare across studies as measures of nail involvement differed between trials. Overall, trials of topical treatments involved more patients with milder disease than trials for oral treatments. The Elewski et al. trial [20], comparing TNS with amorolfine 5% nail lacquer, and those comparing TNS to placebo, had higher percent nail involvement (∼50%) at baseline than trials of efinaconazole 10% topical solution and ciclopirox nail lacquer 8% (both with participants who had ∼30% baseline nail involvement) [21,22]. The I2 values were 0% for all pairwise meta-analyses (efinaconazole 10% nail solution vs. vehicle, tavaborole topical solution 5% vs. vehicle, fluconazole vs. placebo, itraconazole 200 mg vs. placebo, itraconazole 200 mg vs. terbinafine 250 mg, and terbinafine 250 mg vs. placebo), which indicated an absence of quantifiable between-trial heterogeneity. The I2 for the pairwise comparison of ciclopirox nail lacquer 8% versus placebo was 29%; however, this amount of heterogeneity was negligible [35].
Node-Splitting Analyses
Results from the node-splitting analysis are presented in table 2. The evidence network (fig. 2) contains one loop, and each of the three edges in that loop represents a direct comparison between the treatments that it connects (e.g. terbinafine 250 mg vs. placebo). If the loop is broken by removing a particular direct comparison, the two treatments are still connected by the remaining edges of the loop, and that connection yields an indirect comparison between those two treatments. Leaving the network intact also yields a comparison corresponding to each edge, and the ‘Overall’ column of table 2 reports these estimates. The absence of significant inconsistency between the direct and indirect evidence justified the use of a consistency model for the NMA.
Table 2.
Results from the node-splitting analyses
| Comparison | Direct comparison OR (95% CrI) | Indirect comparison OR (95% CrI) | Overall OR (95% CrI) | p value |
|---|---|---|---|---|
| Itraconazole 200 mg and placebo | −3.09 (−4.05, −2.18) | −2.38 (−3.55, – 1.49) | −2.79 (−3.54, – 2.19) | 0.32 |
| Itraconazole 200 mg and terbinafine 250 mg | 3.55 (2.71, 4.55) | 4.25 (3.12, 5.30) | 3.84 (3.19, 4.62) | 0.33 |
| Terbinafine 250 mg and placebo | 1.12 (0.54, 1.64) | 0.55 (−0.77, 1.89) | 1.05 (0.51, 1.53) | 0.40 |
p < 0.05 indicates a statistically significant inconsistency between direct and indirect comparisons.
NMA of Mycological Cure
Convergence of the consistency model was achieved after 20,000 tuning iterations and 100,000 simulation iterations. The ORs and 95% CrIs of mycological cure for pairs of interventions are presented in table 3. The ORs of mycological cure were significantly higher with terbinafine 250 mg than all other treatments except itraconazole 400 mg pulse therapy. The ORs of mycological cure with the itraconazole 400 mg pulse regimen were significantly greater than those of tavaborole 5% nail solution, ciclopirox nail lacquer 8%, amorolfine 5% nail lacquer, TNS, and placebo. The ORs of mycological cure with fluconazole 150-450 mg, efinaconazole 10% topical solution, tavaborole 5% nail solution, ciclopirox nail lacquer 8%, amorolfine 5% nail lacquer, and TNS were significantly higher than placebo only.
Table 3.
ORs of mycological cure between onychomycosis treatments
| Treatment | Terbinafine 250 mg | Itraconazole 400 mg pulse | Itraconazole 200 mg | Fluconazole 150–450 mg | Efinaconazole 10% topical solution | Tavaborole 5% nail solution | Ciclopirox nail lacquer 8% | Amorolfine 5% nail lacquer | Terbinafine nail solution | Placebo |
|---|---|---|---|---|---|---|---|---|---|---|
| Terbinafine 250 mg | – | 2.57 (0.86, 6.82) | 2.85 (1.67, 4.61) | 5.12 (2.01, 13.66) | 7.83 (3.52, 19.79) | 9.78 (4.15, 24.37) | 11.08 (4.34, 28.98) | 12.13 (3.42, 42.57) | 11.73 (3.93, 34.74) | 46.72 (24.38, 101.34) |
| Itraconazole 400 mg pulse | 0.39 (0.15, 1.16) | – | 1.10 (0.44, 2.84) | 2.01 (0.53, 8.06) | 3.10 (0.94, 11.79) | 3.83 (1.14, 14.44) | 4.27 (1.22, 18.15) | 4.69 (1.04, 22.94) | 4.49 (1.15, 19.77) | 18.36 (6.14, 63.33) |
| Itraconazole 200 mg | 0.35 (0.22, 0.60) | 0.91 (0.35,2.27) | – | 1.81 (0.75,4.85) | 2.74 (1.31, 6.93) | 3.42 (1.54, 8.54) | 3.88 (1.62, 10.66) | 4.28 (1.16, 15.58) | 4.02 (1.35, 12.97) | 16.33 (8.95, 34.53) |
| Fluconazole 150–450 mg | 0.20 (0.07, 0.50) | 0.50 (0.12, 1.89) | 0.55 (0.21, 1.34) | – | 1.55 (0.70, 3.47) | 1.94 (0.83, 4.41) | 2.15 (0.88, 5.40) | 2.29 (0.70, 7.94) | 2.24 (0.80, 6.60) | 9.15 (4.95, 17.79) |
| Efinaconazole 10% topical solution | 0.13 (0.05, 0.28) | 0.32 (0.08, 1.06) | 0.36 (0.14, 0.76) | 0.65 (0.29, 1.43) | – | 1.26 (0.58, 2.48) | 1.41 (0.63, 3.08) | 1.52 (0.45, 4.74) | 1.48 (0.53, 3.76) | 5.95 (3.67, 9.67) |
| Tavaborole 5% nail solution | 0.10 (0.04, 0.24) | 0.26 (0.07, 0.88) | 0.29 (0.12, 0.65) | 0.52 (0.23, 1.20) | 0.80 (0.40, 1.72) | – | 1.12 (0.50, 2.69) | 1.22 (0.37, 3.95) | 1.19 (0.43, 3.11) | 4.75 (2.86, 8.49) |
| Ciclopirox nail lacquer 8% | 0.09 (0.03, 0.23) | 0.23 (0.06, 0.82) | 0.26 (0.09, 0.62) | 0.47 (0.19, 1.13) | 0.71 (0.32, 1.59) | 0.89 (0.37, 1.99) | – | 1.09 (0.31, 3.61) | 1.04 (0.36, 2.92) | 4.25 (2.25, 8.12) |
| Amorolfine 5% nail lacquer | 0.08 (0.02, 0.29) | 0.21 (0.04, 0.96) | 0.23 (0.06, 0.86) | 0.44 (0.13, 1.43) | 0.66 (0.21, 2.22) | 0.82 (0.25, 2.70) | 0.92 (0.28, 3.23) | – | 0.96 (0.50, 1.85) | 3.94 (1.41, 11.31) |
| Terbinafine nail solution | 0.09 (0.03, 0.25) | 0.22 (0.05, 0.87) | 0.25 (0.08, 0.74) | 0.45 (0.15, 1.25) | 0.68 (0.27, 1.88) | 0.84 (0.32,2.31) | 0.96 (0.34, 2.77) | 1.04 (0.54, 1.99) | – | 4.03 (1.82, 9.57) |
| Placebo | 0.02 (0.01, 0.04) | 0.05 (0.02, 0.16) | 0.06 (0.03, 0.11) | 0.11 (0.06, 0.20) | 0.17 (0.10, 0.27) | 0.21 (0.12, 0.35) | 0.24 (0.12, 0.44) | 0.25 (0.09, 0.71) | 0.25 (0.10, 0.55) | – |
Significant ORs (i.e. do not include the null value 1) are shown in italics. The OR (95% CrI) in a particular cell is the ratio of the ORs of mycological cure for the treatment in that row to the treatment in that column. Median of the posterior distribution of the random effects standard deviation in the log-odds scale = 0.17 (0.02, 0.66).
The Markov Chain Monte Carlo process produces a treatment ranking for each inference sample based on the probability of mycological cure for each of the treatments. The treatment ranking presented in table 4 reflects the proportion of inference samples in which that drug had that rank; thus, treatments were ranked according to which treatment had the highest probability for each rank. The treatment ranking was as follows: (1) terbinafine 250 mg, (2) itraconazole 400 mg pulse regimen, (3) itraconazole 200 mg, (4) fluconazole 150-450 mg, (5) efinaconazole 10% nail solution, (6) tavaborole 5% nail solution, (7) ciclopirox nail lacquer 8%, (8) TNS, (9) amorolfine 5% nail lacquer, and (10) placebo.
Table 4.
Onychomycosis treatment ranking based on the ORs of mycological cure
| Drug | Rank 1 | Rank 2 | Rank 3 | Rank 4 | Rank 5 | Rank 6 | Rank 7 | Rank 8 | Rank 9 | Rank 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Terbinafine 250 mg | 0.96 | 0.04 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Itraconazole 400 mg pulse | 0.04 | 0.54 | 0.29 | 0.09 | 0.02 | 0.01 | 0.01 | 0.00 | 0.00 | 0.00 |
| Itraconazole 200 mg | 0.00 | 0.36 | 0.59 | 0.05 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Fluconazole 150 – 450 mg | 0.00 | 0.05 | 0.10 | 0.65 | 0.12 | 0.05 | 0.02 | 0.01 | 0.00 | 0.00 |
| Efinaconazole 10% topical solution | 0.00 | 0.00 | 0.01 | 0.09 | 0.45 | 0.21 | 0.15 | 0.06 | 0.02 | 0.00 |
| Tavaborole 5% nail solution | 0.00 | 0.00 | 0.00 | 0.03 | 0.13 | 0.30 | 0.23 | 0.18 | 0.14 | 0.00 |
| Ciclopirox nail lacquer 8% | 0.00 | 0.00 | 0.00 | 0.02 | 0.10 | 0.17 | 0.25 | 0.16 | 0.29 | 0.00 |
| Terbinafine nail solution | 0.00 | 0.00 | 0.00 | 0.02 | 0.09 | 0.14 | 0.19 | 0.36 | 0.19 | 0.00 |
| Amorolfine 5% nail lacquer | 0.00 | 0.01 | 0.01 | 0.04 | 0.09 | 0.12 | 0.15 | 0.23 | 0.34 | 0.01 |
| Placebo | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.99 |
Each entry denotes the proportion of inference samples in which the drug's probability of mycological cure had the particular rank. Values in italics indicate the highest probability for that rank.
Discussion
This is the first study to compare the relative efficacy of onychomycosis treatments including recently approved efinaconazole 10% nail solution and tavaborole 5% nail solution. All treatments showed significantly greater ORs of achieving mycological cure compared to placebo. Terbinafine 250 mg and the itraconazole 400 mg pulse regimen had the greatest relative ORs of mycological cure. Terbinafine 250 mg had significantly greater ORs of mycological cure compared to oral and topical treatments, with the exception of itraconazole 400 mg pulse, while itraconazole 400 mg pulse had significantly greater ORs only in comparison to topical treatments, with the exception of efinaconazole 10% nail solution. The ORs of mycological cure with fluconazole 150-450 mg were not significantly different from either itraconazole regimen or any topical treatments. This analysis confirms that oral treatment for toenail onychomycosis is superior to topical treatment, with 250 mg terbinafine or itraconazole 400 mg pulse being the oral treatments of choice for dermatophyte infections.
Although statistically significant superiority of one topical treatment over another was not observed, efinaconazole demonstrated the highest rank probability preceding oral treatments. Efinaconazole 10% nail solution was also the only topical treatment to demonstrate ORs of mycological cure not statistically superior to the itraconazole 400 mg pulse regimen. Tavaborole was the next highest rank for topical treatments. Again, the ORs of mycological cure with efinaconazole 10% nail solution or tavaborole 5% nail solution were not significantly different from each other or other topical treatments. Nevertheless, these rankings and similar ORs of mycological cure between efinaconazole 10% nail solution and itraconazole 200 mg are encouraging for the future of topical treatments. It appears that the evolution of topical treatments has achieved some success in improving nail penetrance and efficacy.
Mycological cure rates were used in this NMA. Patient-centered outcomes, such as clinical cure (improvement in the appearance of the nail), were not considered. We were unable to perform this analysis for the outcome clinical cure because it is defined differently across trials, and homogenous efficacy measures between trials are necessary for NMA to be valid. It is important to note that the treatment ranking could change depending on the choice of outcome.
Although randomization produces a similarity of participants between trial arms, participants are not necessarily similar across studies. In this network, oral and topical antifungals were compared despite the variation in disease severity between trials for these two types of treatment. As illustrated in table 1, participants in trials of topical treatments typically have less extensive nail involvement at baseline than participants in trials for oral treatments. We attempted to minimize between-trial heterogeneity by implementing strict inclusion criteria, yet this also produced a thin evidence network, which resulted in a lower precision, as reflected in relatively wide 95% CrIs for some comparisons.
Conclusions
Terbinafine 250 mg confers the greatest ORs of mycological cure and is the treatment of choice for toenail onychomycosis. Newly developed topicals have improved ORs of mycological cure, yet these ORs were not significantly greater than those of preexisting topical treatments. Further experience with topical agents will reveal their clinical significance, and head-to-head trials of onychomycosis treatments are warranted.
Statement of Ethics
The authors have no ethical conflicts to disclose.
Disclosure Statement
A.K.G. has been a clinical trials investigator for Valeant Canada, Bristol-Myers Squibb, Eli Lilly, Merck, Novartis, Janssen Pharmaceuticals, and Allergan. He has served as a speaker for Valeant Canada, Bayer, Janssen Pharmaceuticals, and Novartis. D.D. and K.A.F. are employees of Mediprobe Research Inc., which conducts clinical trials under the supervision of A.K.G.
References
- 1.Scher RK, Tavakkol A, Sigurgeirsson B, Hay RJ, Joseph WS, Tosti A, et al. Onychomycosis: diagnosis and definition of cure. J Am Acad Dermatol. 2007;56:939–944. doi: 10.1016/j.jaad.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 2.Gupta AK, Ryder JE, Summerbell RC. Onychomycosis: classification and diagnosis. J Drugs Dermatol. 2004;3:51–56. [PubMed] [Google Scholar]
- 3.Sigurgeirsson B, Baran R. The prevalence of onychomycosis in the global population – a literature study. J Eur Acad Dermatol Venereol. 2014;28:1480–1491. doi: 10.1111/jdv.12323. [DOI] [PubMed] [Google Scholar]
- 4.Gupta AK, Daigle D, Foley KA. The prevalence of culture-confirmed toenail onychomycosis in at-risk patient populations. J Eur Acad Dermatol Venereol. 2015;29:1039–1044. doi: 10.1111/jdv.12873. [DOI] [PubMed] [Google Scholar]
- 5.Roberts DT, Taylor WD, Boyle J, British Association of Dermatologists Guidelines for treatment of onychomycosis. Br J Dermatol. 2003;148:402–410. doi: 10.1046/j.1365-2133.2003.05242.x. [DOI] [PubMed] [Google Scholar]
- 6.Del Rosso JQ. The role of topical antifungal therapy for onychomycosis and the emergence of newer agents. J Clin Aesthet Dermatol. 2014;7:10–18. [PMC free article] [PubMed] [Google Scholar]
- 7.Valeant Pharmaceuticals, Inc. : Valeant Pharmaceuticals announces FDA approval of Jublia® for the treatment of onychomycosis. 2014. http://ir.valeant.com/investor-relations/news-releases/news-release-details/2014/Valeant-Pharmaceuticals-Announces-FDA-Approval-Of-Jublia-for-the-Treatment-of-Onychomycosis/default.aspx (accessed June 27, 2014).
- 8.Markham A. Tavaborole: first global approval. Drugs. 2014;74:1555–1558. doi: 10.1007/s40265-014-0276-7. [DOI] [PubMed] [Google Scholar]
- 9.Jansen JP, Fleurence R, Devine B, Itzler R, Barrett A, Hawkins N, Lee K, Boersma C, Annemans L, Cappelleri JC. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 1. Value Health. 2011;14:417–428. doi: 10.1016/j.jval.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Gupta AK, Daigle D, Paquet M: Therapies for Onychomycosis: a systematic review and network meta-analysis of mycological cure. J Am Podiatr Med Assoc 2014, Epub ahead of print. [DOI] [PubMed]
- 11.Gupta AK, Drummond-Main C, Paquet M. Evidence-based optimal fluconazole dosing regimen for onychomycosis treatment. J Dermatol Treat. 2013;24:75–80. doi: 10.3109/09546634.2012.703308. [DOI] [PubMed] [Google Scholar]
- 12.Gupta AK, Ryder JE, Bluhm R, Johnson A, Summerbell RC. Onychomycosis: quality of studies. J Cutan Med Surg. 2003;7:312–316. doi: 10.1007/s10227-002-0137-y. [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Schulz KF, Altman D, CONSORT Group The CONSORT Statement: revised recommendations for improving the quality of reports of parallel-group randomized trials 2001. Explore (NY) 2005;1:40–45. doi: 10.1016/j.explore.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 14.IBM Corp. IBM SPSS Statistics for Windows. N.Y.: Armonk; 2011. [Google Scholar]
- 15.The Nordic Cochrane Centre TCC: Rev Man. 2011. http://ims.cochrane.org/revman/download.
- 16.Van Valkenhoef G, Tervonen T, Zwinkels T, de Brock B, Hillege H: ADDIS: a decision support system for evidence-based medicine. 2012. http://drugis.org/files/addis-dss.pdf (accessed August 29, 2012).
- 17.Van Valkenhoef G, Lu G, de Brock B, Hillege H, Ades AE, Welton NJ. Automating network meta-analysis. Res Synth Methods. 2012;3:285–299. doi: 10.1002/jrsm.1054. [DOI] [PubMed] [Google Scholar]
- 18.Gelman A, Rubin DB. Inference from iterative simulation using multiple sequences. Stat Sci. 1992;7:457–472. [Google Scholar]
- 19.Brooks SP, Gelman A. General methods for monitoring convergence of iterative simulations. J Comput Graph Stat. 1998;7:434. [Google Scholar]
- 20.Elewski BE, Ghannoum MA, Mayser P, Gupta AK, Korting H-C, Shouey RJ, Baker DR, Rich PA, Ling M, Hugot S, Damaj B, Nyirady J, Thangavelu K, Notter M, Parneix-Spake A, Sigugeirsson B. Efficacy, safety and tolerability of topical terbinafine nail solution in patients with mild-to-moderate toenail onychomycosis: results from three randomized studies using double-blind vehicle-controlled and open-label active-controlled designs. J Eur Acad Dermatol Venereol. 2013;27:287–294. doi: 10.1111/j.1468-3083.2011.04373.x. [DOI] [PubMed] [Google Scholar]
- 21.Elewski BE, Rich P, Pollak R, Pariser DM, Watanabe S, Senda H, Ieda C, Smith K, Pillai R, Ramarkrishna T, Olin JT. Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase III multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013;68:600–608. doi: 10.1016/j.jaad.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Gupta AK, Fleckman P, Baran R. Ciclopirox nail lacquer topical solution 8% in the treatment of toenail onychomycosis. J Am Acad Dermatol. 2000;43:S70–S80. doi: 10.1067/mjd.2000.109071. [DOI] [PubMed] [Google Scholar]
- 23.Anacor Pharmaceuticals Inc. : Efficacy and safety of AN2690 topical solution to treat onychomycosis of the toenail NCT01302119. 2014. https://clinicaltrials.gov/ct2/show/NCT01302119?term=tavaborole&phase=2&rank=1.
- 24.Anacor Pharmaceuticals Inc. : Efficacy and safety evaluation of AN2690 topical solution to treat onychomycosis of the toenail NCT01270971. 2014. https://clinicaltrials.gov/ct2/show/NCT01270971?term=tavaborole&phase=2&rank=2.
- 25.Billstein S, Kianifard F, Justice A. Terbinafine versus placebo for onychomycosis in black patients. Int J Dermatol. 1999;38:377–379. doi: 10.1046/j.1365-4362.1999.00713.x. [DOI] [PubMed] [Google Scholar]
- 26.Drake LA, Shear NH, Arlette JP, Cloutier R, Danby FW, Elewski BE, Garnis-Jones S, Giroux J-M, Gratton D, Gulliver W, Hull P, Jones HE, Journet M, Krol AL, Leyden JJ, Maddin SC, Ross JB, Savin RC, Scher RK, Sibbald GR, Tawfik NH, Zaias N, Tolpin M, Evans S, Marsolais C, Chin T, Lin T-H, Maher T, Birnbaum JE. Oral terbinafine in the treatment of toenail onychomycosis: North American multicenter trial. J Am Acad Dermatol. 1997;37:740–745. doi: 10.1016/s0190-9622(97)70111-7. [DOI] [PubMed] [Google Scholar]
- 27.Goodfield MJ, Andrew L, Evans EG. Short term treatment of dermatophyte onychomycosis with terbinafine. BMJ. 1992;304:1151–1154. doi: 10.1136/bmj.304.6835.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bräutigam M, Nolting S, Schopf RE, Weidinger G. German randomized double-blind multicentre comparison of terbinafine and itraconazole for the treatment of toenail tinea infection. Br J Dermatol. 1996;134(suppl 46):18–21. doi: 10.1111/j.1365-2133.1996.tb15654.x. discussion 38. [DOI] [PubMed] [Google Scholar]
- 29.De Backer M, De Keyser P, De Vroey C, Lesaffre E. A 12-week treatment for dermatophyte toe onychomycosis: terbinafine 250 mg/day versus itraconazole 200 mg/day – a double-blind comparative trial. Br J Dermatol. 1996;134(suppl 46):16–17. doi: 10.1111/j.1365-2133.1996.tb15653.x. discussion 38. [DOI] [PubMed] [Google Scholar]
- 30.Elewski BE, Scher RK, Aly R, Daniel R, Jones HE, Odom RB, Zaias N, Jacko ML. Double-blind, randomized comparison of itraconazole capsules versus placebo in the treatment of toenail onychomycosis. Cutis. 1997;59:217–220. [PubMed] [Google Scholar]
- 31.Havu V, Brandt H, Heikkilä H, Hollmen A, Oksman R, Rantanen T, Saari S, Stubb S, Turjanmaa K, Piepponen T. A double-blind, randomized study comparing itraconazole pulse therapy with continuous dosing for the treatment of toe-nail onychomycosis. Br J Dermatol. 1997;136:230–234. [PubMed] [Google Scholar]
- 32.Ling MR, Swinyer LJ, Jarratt MT, Falo L, Monroe EW, Tharp M, Kalivas J, Weinstein GD, Asarch RG, Drake L, Martin AG, Leyden JJ, Cook J, Pariser DM, Pariser R, Thiers BH, Lebwohl MG, Babel D, Stewart DM, Eaglstein WH, Falanga V, Katz HI, Bergfeld WF, Hanifin JM, Young MR. Once-weekly fluconazole (450 mg) for 4, 6, or 9 months of treatment for distal subungual onychomycosis of the toenail. J Am Acad Dermatol. 1998;38:S95–S102. doi: 10.1016/s0190-9622(98)70492-x. [DOI] [PubMed] [Google Scholar]
- 33.Scher RK, Breneman D, Rich P, Savin RC, Feingold DS, Konnikov N, Shupack JL, Pinnell S, Levine N, Lowe NJ, Aly R, Odom RB, Greer DL, Morman MR, Bucko AD, Tschen EH, Elewski BE, Smith EB. Once-weekly fluconazole (150, 300, or 450 mg) in the treatment of distal subungual onychomycosis of the toenail. J Am Acad Dermatol. 1998;38:S77–S86. doi: 10.1016/s0190-9622(98)70490-6. [DOI] [PubMed] [Google Scholar]
- 34.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 35.Higgins J, Green S: Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0. The Cochrane Collaboration. 2011. www.cochrane-handbook.org.