Abstract
Purpose
ω-3 and ω-6 polyunsaturated fatty acids modulate inflammatory processes throughout the body through distinct classes of lipid mediators that possess both proinflammatory and proresolving properties. The purpose of this cross-sectional study was to explore the relationship between lipid profiles in human tears and dry eye (DE) symptoms and signs.
Methods
Forty-one patients with normal eyelid and corneal anatomy were prospectively recruited from a Veterans Administration Hospital over 18 months. Symptoms and signs of DE were assessed, and tear samples was analyzed by mass spectrometry–based lipidomics. Statistical analyses comparing the relationship between tear film lipids and DE included Pearson/Spearman correlations and t-tests.
Results
Arachidonic acid (AA), docosahexaenoic acid (DHA), and eicosapentaenoic acid (EPA) were present in more than 90% of tear film samples. The ratio of ω-6 (AA) to ω-3 (DHA+EPA) fatty acids was correlated with multiple measures of tear film dysfunction (tear breakup time, Schirmer 2 scores, and corneal staining; all P < 0.05). Arachidonic acid–derived prostaglandin E2 was detected in the majority of samples and correlated with low tear osmolarity, meibomian gland plugging, and corneal staining.
Conclusions
Both ω-3 and ω-6 lipid circuits are activated in the human tear film. The ratio of ω-6:ω-3 tear lipids is elevated in DE patients in proportion to the degree of tear film dysfunction and corneal staining. Metabolic deficiency of ω-3 tear film lipids may be a driver of chronic ocular surface inflammation in DE.
Keywords: dry eyes, tears, fatty acids, prostaglandins, nutrition
Dry eye (DE) is a multifactorial disorder characterized by symptoms of ocular pain and visual disturbance and a myriad of signs including decreased tear production, increased evaporation, hyperosmolarity, and damage to the ocular surface.1 Dry eye symptoms range in quality, severity, and chronicity and affect tens of millions of Americans.2,3 Inflammation is a well-described component of DE,4,5 and various proinflammatory mediators, including innate and adaptive immune cells (infiltrating macrophages and monocytes, γδ, and regulatory T cells, intraepithelial lymphocytes, and natural killer cells5), cytokines (IL-1β and −6, IFN-γ, TNF-α6), chemokines (CCL3-5, CXCL9-11, CXCR37), and prostaglandins (PGE2 and PGD28) have been found at elevated levels in the tears of DE patients compared with controls. Prostaglandins belong to a diverse class of inflammatory lipid mediators called eicosanoids, which are derived from the oxygenation of arachidonic acid (AA), an ω-6 polyunsaturated fatty acid (PUFA), that is enzymatically released from cell membranes of activated cells in response to environmental stress. The release of AA and subsequent generation of eicosanoid lipid mediators is responsible for triggering the acute inflammatory response to corneal injury.9
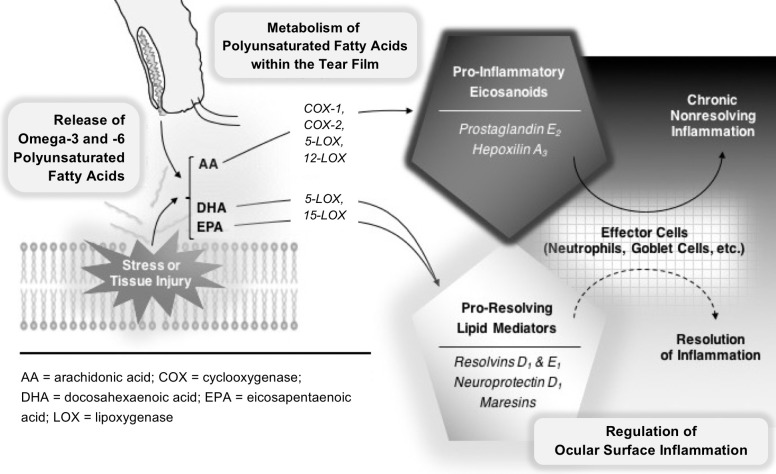
A less well-described aspect of the inflammatory process is its active resolution, mediated by proresolving lipids, such as resolvins, protectins, and maresins. In experimental models of acute, self-resolving inflammation, the early metabolism of AA into proinflammatory eicosanoids is superseded by a resolving phase, in which proresolving lipid mediators predominate.10 Resolution of inflammation is thus an active process, and chronic nonresolving inflammation may result from underactivation of the resolving phase mediators.11 The interplay between proinflammatory and proresolving lipid mediators is an emerging framework for understanding the pathogenesis of chronic inflammatory diseases,12,13 including DE.14–20 Most proresolving lipid mediators are derived from ω-3 PUFA precursors, such as docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA). These proresolving lipid mediators have essential roles in controlling epithelial wound healing, inflammatory cell migration, and nerve regeneration.21–23 Thus, the bioavailability of ω-3 and ω-6 PUFAs may influence the initiation, duration, and resolution of the inflammatory response to injury on the ocular surface (Fig. 1).
Figure 1.
Hypothesized model of chronic ocular surface inflammation in dry eye. ω-3 and ω-6 polyunsaturated fatty acids are released into the tear film as part of meibum and/or from the ocular surface epithelium in response to injury from dessicating stress. Cyclooxygenases and lipoxygenases are expressed on the ocular surface by corneal epithelial cells and resident polymorphonuclear leukocytes. The ω-6 species, AA, is a substrate for cyclooxygenases and lipoxygenases and is converted to several classes of proinflammatory eicosanoids. In contrast, the ω-3 species, DHA and EPA, are also substrates for 15-lipoxygenase and 5-lipoxygenase, but are converted to several classes of proresolving, anti-inflammatory, and neuroprotective mediators. The formation of proinflammatory and proresolving lipid mediators regulates activation of effector cells on the ocular surface. In healthy eyes, proresolving lipid mediators counteract the eicosanoids and promote speedy resolution of inflammation. In dry eye, metabolic deficiency of ω-3 species leads to underproduction of proresolving lipid mediators and a state of chronic nonresolving inflammation on the ocular surface.
We hypothesize that DE is a metabolic disorder characterized by an imbalance of ω-3 and ω-6 PUFAs, leading to underproduction of proresolving lipid mediators, which promotes nonresolving inflammation on the ocular surface. Our hypothesis is informed by several lines of epidemiologic, clinical, and experimental evidence. Epidemiologic studies suggest that individuals with higher dietary intake of DHA and EPA are protected against DE.24 Several randomized clinical trials have also shown that dietary ω-3 supplementation (DHA+EPA) has favorable effects on DE signs and symptoms.25,26 The ocular surface highly expresses lipoxygenase (LOX) and cyclooxygenase (COX) enzymes that metabolize PUFAs into lipid mediators, which regulate inflammatory, immune, and wound-healing responses.17,23,27–31 Experimental evidence from cell culture and animal models of DE suggests that DHA-derived neuroprotectin D1 (NPD1), resolvin D1 (RvD1), and EPA-derived resolvin E1 (RvE1) are proresolving lipid mediators that are particularly relevant in maintaining ocular surface health and tear film function.14,15,17,18,32 Here, for the first time, we evaluate whether ω-3 and ω-6 lipid profiles can be detected in human tears and whether these measures correlate with DE disease severity in human subjects.
Methods
Study Population
The Miami Veterans Administration eye clinic serves veterans in South Florida and evaluates patients with a variety of ophthalmic conditions including refractive issues, cataracts, glaucoma, and retinal pathologies in addition to performing screening for eye pathology in patients with systemic conditions (diabetes, hypertension). Patients were prospectively recruited from the eye clinic between November 2013 and April 2015. Informed consent was obtained from each patient, and protected health information was accessed by the study team in a Health Insurance Portability and Accountability Act (HIPPA)-compliant manner. Patients underwent a complete ocular surface examination, and those without notable abnormalities of their eyelids or ocular surface were included. As we wished to study “idiopathic” DE, that is DE symptoms not associated with well-established ocular or systemic conditions, patients were excluded from the study if they had concomitant ocular or systemic processes that could confound their clinical presentation, such as anatomic abnormalities of their eyelids (e.g., ectropion), conjunctiva (e.g., pterygium), and/or cornea (e.g., edema); history of glaucoma, refractive, or retinal surgery; an active external ocular process; cataract surgery within the last 6 months; use of contact lenses or ocular medications with the exception of artificial tears; HIV; sarcoidosis; graft-versus host disease; multiple sclerosis; stroke; or collagen vascular disease. Patients were asked not to use any artificial tears within 2 hours of testing. This study was conducted with adherence to the tenets of the Declaration of Helsinki and with approval from the Miami Veterans Administration Institutional Review Board.
Data Collection
Demographic information was collected for each patient, including age, sex, race, ethnicity, smoking status, medications, and medical history.
Dry Eye Symptoms and Ocular Pain
Dry eye symptoms were assessed via the ocular surface disease index (OSDI), which assesses the impact of DE on visual function, and the Dry Eye Questionnaire Score 5 (DEQ5), which assesses specific discomforts (tearing, dryness, etc.) independent of visual function.33,34 A numerical rating scale (NRS; score 0–10) was used to assess the “average intensity of eye pain during the past week.”
Dry Eye Signs
Further ocular surface examination for DE signs included, in the order performed, measurement of tear osmolarity, corneal sensitivity, tear breakup time (TBUT), corneal staining, Schirmer score with anesthesia, and eyelid assessment. Tear osmolarity was measured once in each eye (TearLAB, San Diego, CA, USA). Fluorescein dye was instilled, and TBUT was measured three times in each eye and averaged. Corneal staining was assessed in five areas of the cornea and scored 0–3 in each area (National Eye Institute [NEI] scale). A Schirmer 2 score was recorded as millimeters of wetting at 5 minutes. Eyelid vascularity and meibomian gland plugging were graded on a scale of 0–3 (0 = none; 1 = mild; 2 = moderate; 3 = severe).35 Meibomian gland drop out was measured via noncontact meibography (a technique that uses transillumation to evaluate degree of area loss of glands according to the Meiboscale). Finally, meibum quality was graded on a scale of 0–4 (0 = clear consistency; 1 = cloudy consistency; 2 = granular consistency; 3 = toothpaste; 4 = no meibum expressed16 using digital pressure).
Determination of Corneal Sensitivity
Mechanical detection and pain thresholds of the central cornea were assessed with a modified Belmonte noncontact aesthesiometer, which was developed based on the original Belmonte instrument.36 The tip of the aesthesiometer (0.5 mm in diameter) was placed perpendicular to and 4 mm from the surface of the cornea of the right eye. Stimulation consisted of pulses of air at room temperature (approximately 23°C–26°C)37 applied to the corneal surface. The method of limits, using ascending series only, was used to measure threshold.
For corneal detection threshold measurements, subjects were presented with a stimulus immediately following a blink and asked to indicate whether they felt the stimulus by pressing a button. The initial flow rate was set at a level below threshold (50 mL/min for most individuals) and increased by 10 mL/min (with 15-second intervals between stimuli) until the subject stated that they felt the stimulus or the maximum allowable flow rate (400 mL/min) was reached. Two ascending series were conducted, and detection threshold was defined as the arithmetic mean of the value at which the subject pressed the button across the two series. To estimate ocular pain threshold, the flow rate was further increased beyond the detection threshold in 10 mL/min increments until the subject reported the stimulus as painful or the maximum allowable flow rate (400 mL/min) was reached. Two ascending series were conducted in this way, and pain threshold was defined as a mean of the two series. All threshold measures were performed during the morning hours by the same operator (ALM). Corneal sensitivity was evaluated after tear osmolarity followed by a 2-hour break, after which time the remaining dry eye testing was performed.
Tear Collection
Fifty microliters of sterile saline was instilled by a pipette to the inferior cul-de-sac of each eye prior to assessment of meibum quality. Tears were immediately collected by capillary action using a 1-μL microcaps pipette (Drummond Scientific Company, Broomall, PA, USA) applied gently to the temporal margin of the lower eyelid. Each study eye was sampled once. A minimum of 50 μL tears (approximately six disposable micropipettes) was collected and released by bulb dispenser into 1.5-mL Nalgene polypropylene cryogenic vials (Sigma-Aldrich Corp., St Louis, MO, USA). These vials were labeled with de-identified subject codes and immediately placed in a −80°C freezer.
Laboratory Methodology
Inflammatory and proresolving lipid mediators and specific ω-6 PUFA and ω-3 PUFA pathway markers were identified and quantified by liquid chromatography (LC)-tandem mass spectrometry (MS/MS). In brief, 400 pg class-specific deuterated (-d) internal standards (AA-d8, DHA-d5, PGE2-d4, lipoxin A4-d5, leukotriene B4-d4, 15-hydroxyeicosatetraenoic acid-d8) were added to each tear sample prior to processing to calculate the recovery of specific classes of PUFA, LOX, and COX metabolites. Collected tears (30–50 μL) containing internal standards were combined with 2 mL methanol, dried under a gentle stream of nitrogen, immediately resuspended in high-performance LC mobile, and placed in a refrigerated autosampler for lipidomic analysis. Eicosanoids and docosanoids were identified and quantified by LC-MS/MS–based lipidomics based on published methods.30,38 Processed tear samples were analyzed by a triple-quadrupole linear ion trap LC-MS/MS system (MDS SCIEX 3200 QTRAP; Applied Biosystems, Foster City, CA, USA) equipped with a Kinetex C18 mini-bore column (Phenomenex, Torrance, CA, USA). The mobile phase was a gradient of water/acetonitrile/acetic acid (72:28:0.01, vol:vol:vol) and isopropanol/acetonitrile (60:40, vol:vol) with a 450-μL/min flow rate. Tandem MS/MS analyses were performed in negative ion mode, and prominent fatty acid metabolites were quantified in multiple reaction monitoring mode using established and specific transitions as previously described.30,31,38–41 Calibration curves (1–1000 pg) and specific LC retention times for each compound were established with synthetic standards (Cayman Chemical, Ann Arbor, MI, USA). Structures were confirmed for selected autacoids by MS/MS analyses using enhanced product ion mode with appropriate selection of the parent ion in quadrupole 1.
Tear samples were analyzed by LC-MS/MS in two batches. In the first batch (N = 21 patients), tear samples from both eyes were analyzed separately, and the results were averaged. In the second batch (N = 20 distinct patients), tear samples from both eyes were pooled and analyzed together. ω-6:ω-3 ratios and PGE2 levels were statistically comparable between the two batches.
Statistical Analysis
All statistical analyses were performed using SPSS Version 22 (SPSS, Inc., Chicago, IL, USA) statistical package. Analyses included comparison of means (t-test), medians (Mann-Whitney U), and correlations (Pearson and Spearman); P < 0.05 was considered statistically significant.
Results
Study Population
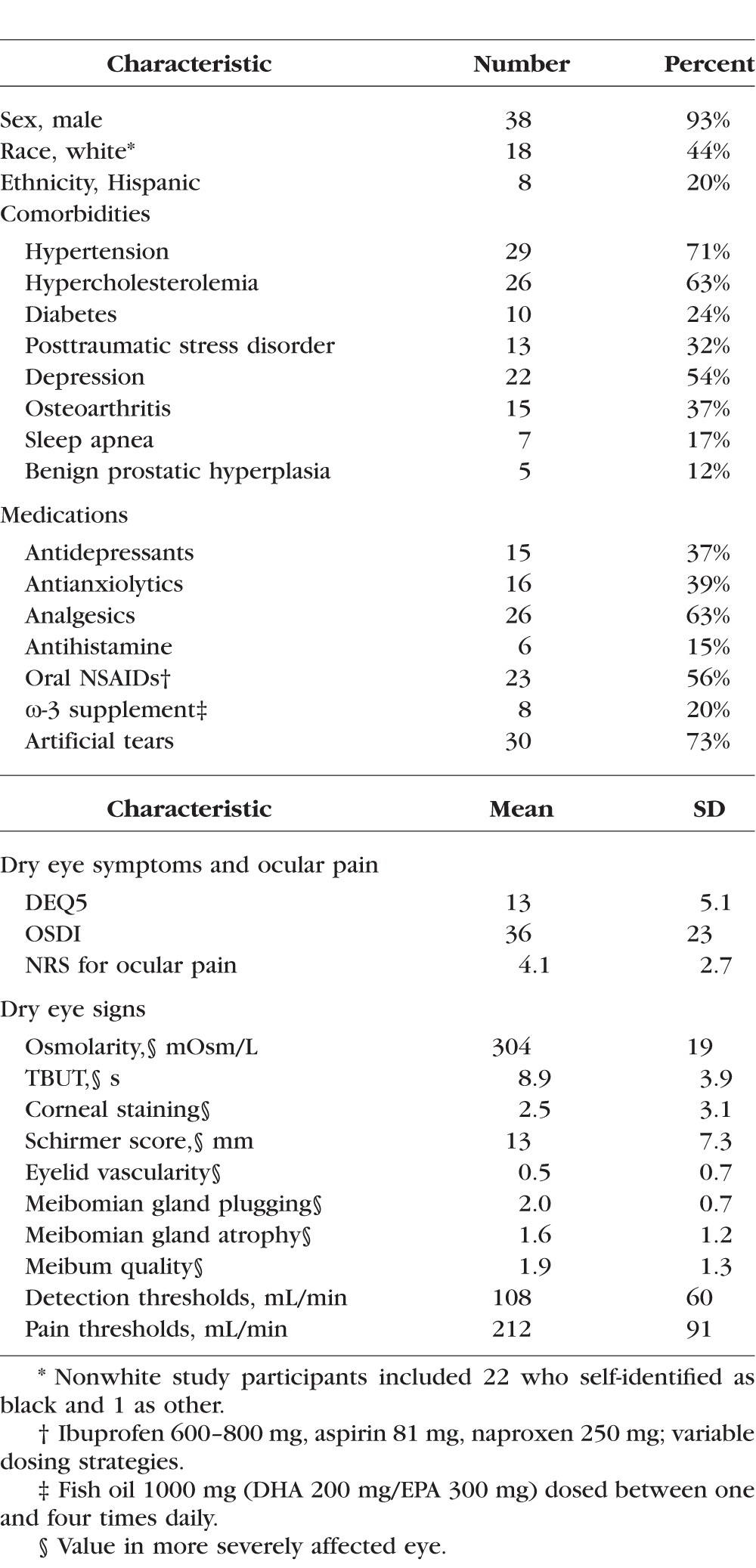
The study group (N = 41) comprised a racially and ethnically diverse population of late middle-aged and elderly, predominately male, subjects (mean age, 62 years; SD, 11; range, 27–83 years), as described in Table 1. Seventy-one percent of subjects were actively using artificial tears, on average 3.0 ± 1.4 times daily, for an average duration of 36 ± 38 months. Fifty-six percent of patients were taking nonsteroidal anti-inflammatory drugs (NSAIDs), and 20% were taking an ω-3 supplement. Subjects displayed a wide range of symptoms and signs of DE, ranging from none to severe (93% with DEQ5 ≥ 6, 81% with OSDI ≥ 20; 56% with NRS ≥ 4). The majority of subjects had one or more eyelid abnormalities including meibomian gland plugging (78%), a score of 2 or greater on meibum quality (57%), meibomian gland atrophy (44%), and increased eyelid vascularity (10%). A majority of subjects also had evidence of tear dysfunction with TBUT ≤ 8 seconds in 56%, Schirmer < 8 mm in 29%, and osmolarity ≥ 308 mOsm in 28%.
Table 1.
Demographic and Clinical Information of Study Population
Mass Spectroscopy
Five principal species, AA, DHA, EPA, PGE2, and hepoxilin A3 (HxA3), were detected in the majority of samples. Arachidonic acid, DHA, EPA, and PGE2 were detected in >90% of samples and HxA3 in 81%.
Correlations Between Lipid Species
The five principal lipid species were positively correlated with one another (Supplementary Table S1). Associations were strongest between DHA and AA and less strong for the other pairwise comparisons.
Clinico-Pathologic Correlation
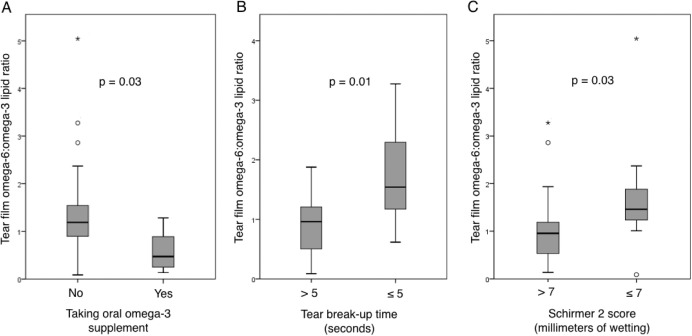
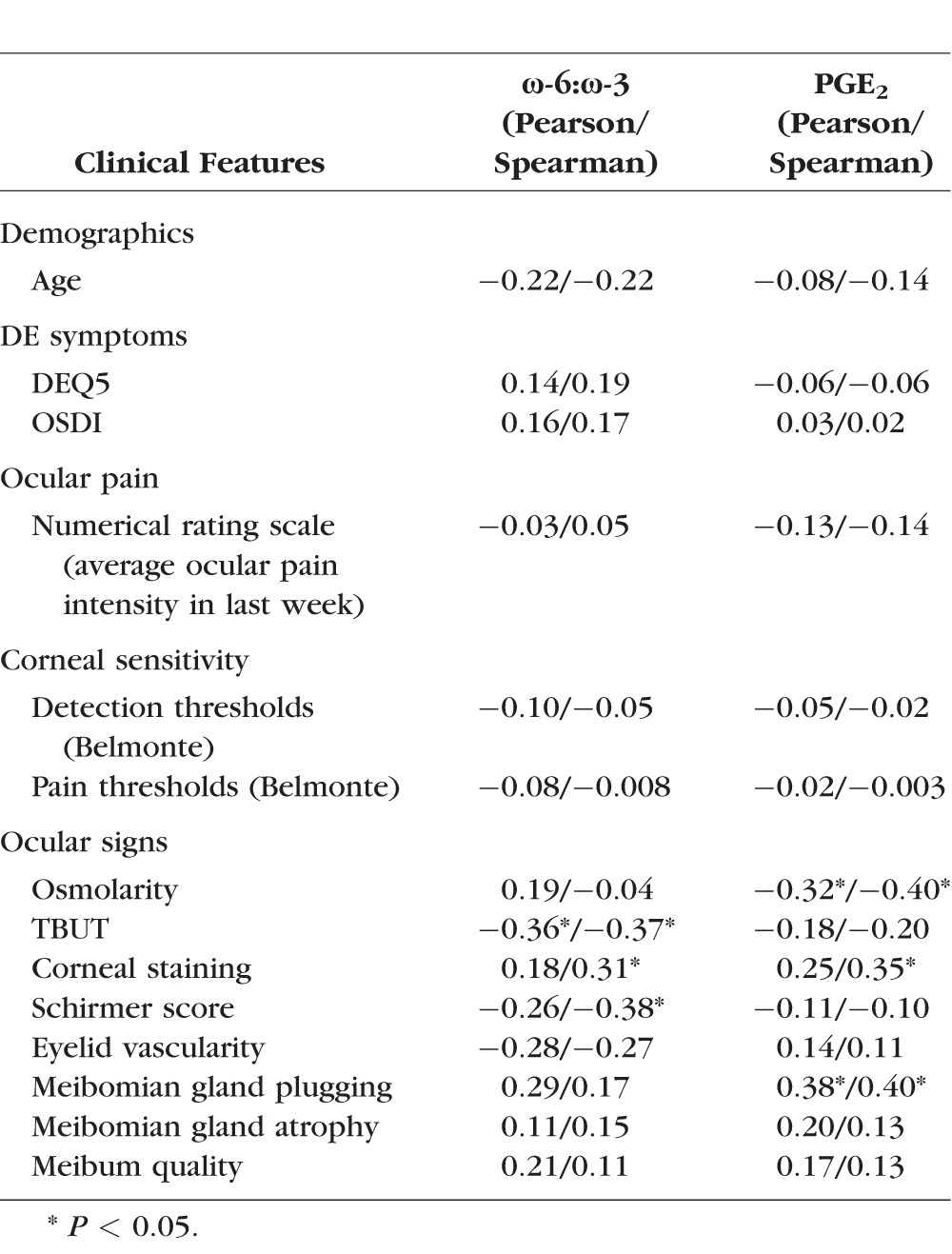
The ω-6:ω-3 ratio (proinflammatory/anti-inflammatory) and PGE2 levels were evaluated for correlations with demographic characteristics and symptoms and signs of DE. There was no significant correlation of either measure with age or sex in our study population. White patients demonstrated a lower ω-6:ω-3 ratio compared with blacks (0.82 ± 0.61 vs. 1.6 ± 1.1; P = 0.01) and lower PGE2 levels (11.3 ± 2.7 vs 13.2 ± 3.2; P = 0.05), suggesting a less inflammatory tear lipid profile. Patients with osteoarthritis had a higher ω-6:ω-3 ratio compared with those without arthritis (1.7 ± 1.2 vs. 0.95 ± 0.64; P = 0.02) but similar PGE2 levels, suggesting a more proinflammatory tear lipid profile. Differences were also seen between groups with respect to medication use. Those taking NSAIDs had lower levels of all lipid mediators compared with those not taking NSAIDS, with a significant difference in PGE2 levels between the groups (11.3 ± 2.6 vs. 13.7 ± 3.1; P = 0.01; Supplementary Fig. S1). Those taking ω-3 supplements had modestly higher tear film levels of all lipid mediators compared with those not taking ω-3 and a significantly lower ω-6:ω-3 ratio (0.58 ± 0.43 vs. 1.37 ± 0.96; P = 0.03; Fig. 2A), suggesting a less inflammatory lipid profile. Means were not significantly different by the other demographics, comorbidities, and medications listed in Table 1.
Figure 2.
Box-and-whisker plots comparing tear film ω-6:ω-3 lipid ratios in patients with and without (A) use of ω-3 supplements (0.58 ± 0.43 vs. 1.37 ± 0.96; P = 0.03), (B) evaporative deficiency defined by TBUT ≤ 5 seconds (1.89 ± 1.24 vs. 0.87 ± 0.47; P = 0.01), and (C) aqueous tear deficiency defined by Schirmer 2 score ≤7 mm (1.70 ± 1.20 vs. 1.02 ± 0.74; P = 0.03). Gray boxes represent the interquartile range between the 25th to 75th percentile, middle line represents the median, and vertical line extends from the minimum to the maximum value, excluding outliers (open circles represent values larger than the upper quartile plus 1.5 times the interquartile range; asterisks represent values larger than the upper quartile plus 3 times the interquartile range).
Dry eye symptoms and ocular pain were not significantly correlated with ω-6:ω-3 ratio or PGE2, nor were metrics of corneal sensitivity (stimulus detection and pain thresholds). However, several clinically important signs of ocular surface disease were correlated with inflammatory tear lipids (Table 2). Higher levels of PGE2 were correlated with lower tear osmolarity, more meibomian gland plugging, and more corneal staining. Less healthy tear parameters including shorter TBUT, lower Schirmer scores, and more corneal staining correlated with higher ω-6:ω-3 ratios (Figs. 2B, 2C).
Table 2.
Correlations Between Lipid Species, Demographics, Comorbidities, and Dry Eye Metrics
Discussion
This is the first study to demonstrate that the major biologically relevant ω-3 PUFAs (DHA and EPA) are detectable in the human tear film, suggesting activation of these proresolving lipid circuits in DE. The anti-inflammatory potential of ω-3 series proresolving lipid mediators has been established by multiple animal and cell culture models of DE.14,16–19,32,42 Changes in tear volume have been shown to correlate with dietary ω-3 intake in mice,42 and here we demonstrated a significant correlation between the tear film ω-6:ω-3 ratio and tear volume (Schirmer score), as well as tear stability (TBUT) and corneal staining. Additionally, human studies have shown that ω-3 (DHA+EPA) supplementation has beneficial effects on the signs and symptoms of DE.25,26 Although our study was noninterventional, we found that patients taking ω-3 supplements had higher tear film PUFA levels and a lower, less inflammatory ω-6:ω-3 ratio. This suggests that oral intake of ω-3 supplements has sufficient bioavailability to directly impact inflammatory lipid expression in the human tear film.
Previous investigations have measured AA43 and related ω-6 series eicosanoids including 12-HETE44 and PGE28 in the tear film of human DE subjects. Here we detected AA, PGE2, and, for the first time in human tears, the nonclassical eicosanoid HxA3, in the majority of subjects. Although previous investigations reported differences in DE tears compared with healthy controls, we have gone further to show that inflammatory lipid profiles correlate with phenotypic variations in ocular surface disease severity among patients.
The role of eicosanoids in the regulation of corneal inflammation was first recognized by Srinivasan and Kulkarni, who showed in various models of corneal injury that polymorphonuclear leukocytes (PMNs) were recruited from conjunctival vessels through the tear film, ultimately attaching to injured corneal epithelium in a AA- and PGE2-dependent manner.9 Prostaglandin E2 is synthesized from AA by COX and PGE synthase, and both these enzymes are up-regulated on the ocular surface of mice placed in a DE environmental chamber.8 Chronic corneal injury results in recruitment 5-LOX expressing PMNs and expression of COX in corneal epithelial cells, inducing formation and release of proinflammatory and proangiogenic mediators (PGE2 and leukotriene B4) that drive and amplify ocular surface inflammation.31
The role of ω-3 fatty acids in down-regulating inflammation is an emerging paradigm for understanding the pathogenesis of chronic inflammatory diseases,12,13 including DE.14–20 DHA and EPA are conditionally essential fatty acids, which can be produced from α-linolenic acid, but the rate of conversion is generally insufficient to replace metabolic consumption of DHA and EPA.45 In a survey of dietary fat intake across 28 countries, 20 failed to meet minimum World Health Organization recommended levels of DHA and EPA intake, including the United States and the majority of European nations.46 DHA and EPA are metabolic precursors to proresolving lipid mediators such as NPD1, RvD1, and RvE1, which counteract the proinflammatory actions of PGE2, HxA3, and other eicosanoids.10,12,13,18,47 Corneal epithelial cells and resident regulatory PMNs in the corneal limbus and lacrimal gland highly express 15-LOX, a key enzyme for generating and releasing specialized proresolving mediators (lipoxins, resolvins, and neuroprotectins) that are critical for controlling ocular surface immune and wound healing responses).17,23,27–30 Lipoxin A4 promotes corneal epithelial wound healing,30 inhibits pathologic angiogenesis and proinflammatory cytokine expression,28,29 and controls effector T-cell activation.27 Neuroprotectin D1 is implicated in epithelial cell survival,48 recovery from oxidative stress,48 and wound healing.14 Neuroprotectin D1 has also been shown to promote corneal nerve regeneration and return of corneal sensitivity.14,17 Resolvin E1 exerts proresolving effects directly through G-protein–coupled receptors10 and indirectly through negative feedback on COX-2 expression.20 In murine models of DE, RvD1 and RvE1 play a role in tear film homeostasis by enhancing tear production, as well as goblet cell survival and secretion in response to desiccating stress.16,18,20 These data underscore the biologic significance of ω-3 proresolving lipid mediators in the tear film and lend support to the hypothesis that DE has a metabolic basis. Nutritional or metabolic deficiency of DHA and EPA may lead to underproduction of proresolving lipid mediators in tears, ultimately resulting in nonresolving inflammation on the ocular surface.
As with all studies, our findings need to be considered with our study limitations, which include: a cross-sectional study design, a predominantly male DE population, a small sample size, and specific metrics used to capture DE features. Because we studied tears, we cannot discern whether the lipid mediators originated from meibum, the ocular surface epithelium, or both. In addition, more work needs to be done to optimize tear collection and lipid identification in human tears, as many of the naturally occurring ω-3 metabolites are unstable and short-lived species.49 One strength of our study was the ability to calculate a standardized ω-6:ω-3 ratio, which helps to mitigate some of the variation in lipid concentrations between individual samples. However, the ω-6:ω-3 ratio may be an overly simplistic metric, as some ω-6–derived eicosanoids (e.g., PGE1, Lipoxin A4) can be anti-inflammatory. Future investigation will be needed to characterize the active metabolites of the proresolving lipid mediators DHA and EPA in the human tear film (e.g., DHA-derived NPD1 and RvD1 and EPA-derived RvE1).16–19,32
To summarize, DE has been previously described as a chronic inflammatory disorder of the ocular surface. In additional to known proinflammatory lipids (e.g., AA, PGE2), we have shown for the first time that proresolving lipid biomarkers (DHA, EPA) can be simultaneously detected in the tear film of human subjects with DE. The ratio of proinflammatory to proresolving lipid pathway markers in the tear film was clinically significant as a biomarker for tear film dysfunction. Our findings support the hypothesis that a higher ω-6 (proinflammatory) to ω-3 (proresolving) ratio of lipid mediators supports nonresolving inflammation on the ocular surface. This theoretical framework may help to devise new, more targeted therapies toward lipid pathways in DE.
Supplementary Material
Acknowledgments
The authors thank Mireya Hernandez for study coordination support.
Presented at the American Society for Cataract and Refractive Surgery Annual Symposium and Congress, San Diego, California, United States, April 2015.
Supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Sciences Research and Development's Career Development Award CDA-2-024-10S (AG), National Eye Institute Research Project Grant (RO1) EY022208 (KG), Contact Lens Association of Ophthalmologists and Sjögren's Syndrome Foundation Educational Research Fund Grants (SDW, KG), Heed Ophthalmic Foundation Fellowship (SDW), National Institutes of Health Center Core Grant P30EY014801, and Research to Prevent Blindness Unrestricted Grant, Department of Defense (DOD; Grant W81XWH-09-1-0675 and Grant W81XWH-13-1-0048 ONOVA) (institutional). The sponsor or funding organizations had no role in the design or conduct of this research.
Disclosure: S.D. Walter, None; K. Gronert, None; A.L. McClellan, None; R.C. Levitt, None; K.D. Sarantopoulos, None; A. Galor, None
References
- 1. Galor A,, Levitt RC,, Felix ER,, et al. Neuropathic ocular pain: an important yet underevaluated feature of dry eye. Eye (Lond). 2015; 29: 301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007; 5: 93–107. [DOI] [PubMed] [Google Scholar]
- 3. Pouyeh B,, Viteri E,, Feuer W,, et al. Impact of ocular surface symptoms on quality of life in a United States Veterans Affairs population. Am J Ophthalmol. 2012; 153: 1061–1066. [DOI] [PubMed] [Google Scholar]
- 4. Stern ME,, Gao J,, Schwalb TA,, et al. Conjunctival T-cell subpopulations in Sjogren's and non-Sjogren's patients with dry eye. Invest Ophthalmol Vis Sci. 2002; 43: 2609–2614. [PubMed] [Google Scholar]
- 5. Stern ME,, Schaumburg CS,, Pflugfelder SC. Dry eye as a mucosal autoimmune disease. Int Rev Immunol. 2013; 32: 19–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wei Y,, Gadaria-Rathod N,, Epstein S,, et al. Tear cytokine profile as a noninvasive biomarker of inflammation for ocular surface diseases: standard operating procedures. Invest Ophthalmol Vis Sci. 2013; 54: 8327–8336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yoon KC,, Park CS,, You IC,, et al. Expression of CXCL9, −10, −11, and CXCR3 in the tear film and ocular surface of patients with dry eye syndrome. Invest Ophthalmol Vis Sci. 2010; 51: 643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shim J,, Park C,, Lee HS,, et al. Change in prostaglandin expression levels and synthesizing activities in dry eye disease. Ophthalmology. 2012; 119: 2211–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Srinivasan BD,, Kulkarni PS. The role of arachidonic acid metabolites in the mediation of the polymorphonuclear leukocyte response following corneal injury. Invest Ophthalmol Vis Sci. 1980; 19: 1087–1093. [PubMed] [Google Scholar]
- 10. Serhan CN. Resolution phase of inflammation: Novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol. 2007; 25: 101–137. [DOI] [PubMed] [Google Scholar]
- 11. Nathan C,, Ding A. Nonresolving inflammation. Cell. 2010; 140: 871–882. [DOI] [PubMed] [Google Scholar]
- 12. Serhan CN. Novel chemical mediators in the resolution of inflammation: Resolvins and protectins. Anesthesiol Clin. 2006; 24: 341–364. [DOI] [PubMed] [Google Scholar]
- 13. Spite M,, Claria J,, Serhan CN. Resolvins specialized proresolving lipid mediators, and their potential roles in metabolic diseases. Cell Metab. 2014; 19: 21–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cortina MS,, He J,, Li N,, et al. Recovery of corneal sensitivity, calcitonin gene-related peptide-positive nerves, and increased wound healing induced by pigment epithelial-derived factor plus docosahexaenoic acid after experimental surgery. Arch Ophthalmol. 2012; 130: 76–83. [DOI] [PubMed] [Google Scholar]
- 15. Cortina MS,, Bazan HE. Docosahexaenoic acid, protectins and dry eye. Curr Opin Clin Nutr Metab Care. 2011; 14: 132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Paiva CS,, Schwartz CE,, Gjorstrup P,, et al. Resolvin E1 (RX-10001) reduces corneal epithelial barrier disruption and protects against goblet cell loss in a murine model of dry eye. Cornea. 2012; 31: 1299–1303. [DOI] [PubMed] [Google Scholar]
- 17. Cortina MS,, He J,, Li N,, et al. Neuroprotectin D1 synthesis and corneal nerve regeneration after experimental surgery and treatment with PEDF plus DHA. Invest Ophthalmol Vis Sci. 2010; 51: 804–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dartt DA,, Hodges RR,, Li D,, et al. Conjunctival goblet cell secretion stimulated by leukotrienes is reduced by resolvins D1 and E1 to promote resolution of inflammation. J Immunol. 2011; 186: 4455–4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Esquenazi S,, Bazan HE,, Bui V,, et al. Topical combination of NGF and DHA increases rabbit corneal nerve regeneration after photorefractive keratectomy. Invest Ophthalmol Vis Sci. 2005; 46: 3121–3127. [DOI] [PubMed] [Google Scholar]
- 20. Li N,, He J,, Schwartz CE,, et al. Resolvin E1 improves tear production and decreases inflammation in a dry eye mouse model. J Ocul Pharmacol Ther. 2010; 26: 431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gronert K. Resolution, the grail for healthy ocular inflammation. Exp Eye Res. 2010; 91: 478–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bazan NG,, Molina MF,, Gordon WC. Docosahexaenoic acid signalolipidomics in nutrition: significance in aging neuroinflammation, macular degeneration, Alzheimer's, and other neurodegenerative diseases. Annu Rev Nutr. 2011; 31: 321–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kenchegowda S,, Bazan HE. Significance of lipid mediators in corneal injury and repair. J Lipid Res. 2010; 51: 879–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Miljanovic B,, Trivedi KA,, Dana MR,, et al. Relation between dietary n-3 and n-6 fatty acids and clinically diagnosed dry eye syndrome in women. Am J Clin Nutr. 2005; 82: 887–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kangari H,, Eftekhari MH,, Sardari S,, et al. Short-term consumption of oral omega-3 and dry eye syndrome. Ophthalmology. 2013; 120: 2191–2196. [DOI] [PubMed] [Google Scholar]
- 26. Kawakita T,, Kawabata F,, Tsuji T,, et al. Effects of dietary supplementation with fish oil on dry eye syndrome subjects: randomized controlled trial. Biomed Res. 2013; 34: 215–220. [DOI] [PubMed] [Google Scholar]
- 27. Gao Y,, Min KJ,, Zhang YB,, et al. Female-specific downregulation of tissue polymorphonuclear neutrophils drives impaired regulatory T cell and amplified effector T cell responses in autoimmune dry eye disease. J Immunol. 2015; 195: 3086–3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Leedom AJ,, Sullivan AB,, Dong B,, et al. Endogenous LXA4 circuits are determinants of pathological angiogenesis in response to chronic injury. Am J Pathol. 2010; 176: 74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Biteman B,, Hassan IR,, Walker E,, et al. Interdependence of lipoxin A4 and heme-oxygenase in counter-regulating inflammation during corneal wound healing. FASEB J. 2007; 21: 2257–2266. [DOI] [PubMed] [Google Scholar]
- 30. Wang SB,, Hu KM,, Seamon KJ,, et al. Estrogen negatively regulates epithelial wound healing and protective lipid mediator circuits in the cornea. FASEB J. 2012; 26: 1506–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liclican EL,, Nguyen V,, Sullivan AB,, et al. Selective activation of the prostaglandin E2 circuit in chronic injury-induced pathologic angiogenesis. Invest Ophthalmol Vis Sci. 2010; 51: 6311–6320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cortina MS,, He J,, Russ T,, et al. Neuroprotectin D1 restores corneal nerve integrity and function after damage from experimental surgery. Invest Ophthalmol Vis Sci. 2013; 54: 4109–4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Begley CG,, Caffery B,, Chalmers RL,, et al. Use of the dry eye questionnaire to measure symptoms of ocular irritation in patients with aqueous tear deficient dry eye. Cornea. 2002; 21: 664–670. [DOI] [PubMed] [Google Scholar]
- 34. Schiffman RM,, Christianson MD,, Jacobsen G,, et al. Reliability and validity of the ocular surface disease index. Arch Ophthalmol. 2000; 118: 615–621. [DOI] [PubMed] [Google Scholar]
- 35. Foulks GN,, Bron AJ. Meibomian gland dysfunction: A clinical scheme for description diagnosis, classification, and grading. Ocul Surf. 2003; 1: 107–126. [DOI] [PubMed] [Google Scholar]
- 36. Belmonte C,, Acosta MC,, Schmelz M,, et al. Measurement of corneal sensitivity to mechanical and chemical stimulation with a CO2 esthesiometer. Invest Ophthalmol Vis Sci. 1999; 40: 513–519. [PubMed] [Google Scholar]
- 37. Situ P,, Simpson TL,, Fonn D. Eccentric variation of corneal sensitivity to pneumatic stimulation at different temperatures and with CO2. Exp Eye Res. 2007; 85: 400–405. [DOI] [PubMed] [Google Scholar]
- 38. von Moltke J,, Trinidad NJ,, Moayeri M,, et al. Rapid induction of inflammatory lipid mediators by the inflammasome in vivo. Nature. 2012; 490: 107–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Prüss H,, Rosche B,, Sullivan AB,, et al. Proresolution lipid mediators in multiple sclerosis—differential, disease severity-dependent synthesis—a clinical pilot trial. PLoS One. 2013; 8: e55859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kalish BT,, Le HD,, Fitzgerald JM,, et al. Intravenous fish oil lipid emulsion promotes a shift toward anti-inflammatory proresolving lipid mediators. Am J Physiol Gastrointest Liver Physiol. 2013; 305: G818–G828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sapieha P,, Stahl A,, Chen J,, et al. 5-Lipoxygenase metabolite 4-HDHA is a mediator of the antiangiogenic effect of omega-3 polyunsaturated fatty acids. Sci Transl Med. 2011; 3:69ra12. [DOI] [PMC free article] [PubMed]
- 42. Harauma A,, Saito J,, Watanabe Y,, et al. Potential for daily supplementation of n-3 fatty acids to reverse symptoms of dry eye in mice. Prostaglandins Leukot Essent Fatty Acids. 2014; 90: 207–213. [DOI] [PubMed] [Google Scholar]
- 43. Chen D,, Wei Y,, Li X,, et al. sPLA2-IIa is an inflammatory mediator when the ocular surface is compromised. Exp Eye Res. 2009; 88: 880–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mieyal PA,, Dunn MW,, Schwartzman ML. Detection of endogenous 12-hydroxyeicosatrienoic acid in human tear film. Invest Ophthalmol Vis Sci. 2001; 42: 328–332. [PubMed] [Google Scholar]
- 45. Calder PC,, Dangour AD,, Diekman C,, et al. Essential fats for future health. Proceedings of the 9th Unilever Nutrition Symposium, 26-27 May 2010. Eur J Clin Nutr. 2010; 64( suppl 4): S1–S13. [DOI] [PubMed] [Google Scholar]
- 46. Elmadfa I,, Kornsteiner M. Dietary fat intake—a global perspective. Ann Nutr Metab. 2009; 54(s uppl 1): 8–14. [DOI] [PubMed] [Google Scholar]
- 47. Gronert K,, Maheshwari N,, Khan N,, et al. A role for the mouse 12/15-lipoxygenase pathway in promoting epithelial wound healing and host defense. J Biol Chem. 2005; 280: 15267–15278. [DOI] [PubMed] [Google Scholar]
- 48. Mukherjee PK,, Marcheselli VL,, Serhan CN,, et al. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc Natl Acad Sci U S A. 2004; 101: 8491–8496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pace-Asciak CR. Formation of hepoxilin A4, B4 and the corresponding trioxilins from 12(S)-hydroperoxy-5,8,10,14,17-icosapentaenoic acid. Prostaglandins Leukot Med. 1986; 22: 1–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.