Abstract
Acneiform rash is the most common side effect of epidermal growth factor receptor (EGFR) inhibitors (EGFRis), and it occurs in 50-100% of patients. This condition can affect the quality of life of these patients and can sometimes lead to a discontinuation of the antineoplastic therapy. Several recent prospective studies have addressed and evaluated different interventions to mitigate or reduce the severity of EGFRis-associated skin rash. With this aim, we have established a dermocosmetological outpatient clinic for cancer patients at the Department of Clinical Medicine and Surgery, University of Naples Federico II in collaboration with the Department of Dermatology and Cutaneous Surgery, Miller School of Medicine, University of Miami. An interdisciplinary network of physicians can improve the quality of life of the cancer patients, focusing on such important aspects as dermocosmetological skin care, but also on the evaluation of new therapeutic and diagnostic algorithms in order to make further progress in the field of prevention. In this review, we summarize the state of the art of the epidemiology, pathogenesis, and treatment of EGFRis acneiform rash, and we describe our outpatient clinical experience.
Key Words: EGFR inhibitors, Acneiform rash, Papulopustular rash
Introduction
Acneiform rash is the most common side effect of epidermal growth factor receptor (EGFR) inhibitors (EGFRis). EGFR belongs to a family (ErbB) of tyrosine kinase receptors which regulate tumor cell differentiation, survival, and proliferation. EGFR can be inhibited by the monoclonal antibodies cetuximab (Erbitux®) and panitumumab (Vectibix®), and by the small molecule tyrosine kinase inhibitors erlotinib (Tarceva®) and gefitinib (Iressa®). EGFR is expressed in many different cell types in normal tissues, such as epithelial tissue, skin, hair follicles, and the gastrointestinal tract, and during treatment with EGFRis cutaneous adverse events occur in about 65-90% of patients. This condition can affect the quality of life of these patients and can sometimes lead to a discontinuation of the antineoplastic therapy [1]. The most common cutaneous side effect is a dose-dependent follicular papulopustular (acneiform) eruption on the face, scalp, chest, and upper back (fig. 1). Acneiform rash is a dose-dependent skin drug reaction, which usually develops in the first 1-2 weeks, peaks at 3-4 weeks on therapy, and its intensity decreases after 2 weeks but can often persist over some months. It is not well known why monoclonal antibodies are the most frequent trigger of acneiform rash [2,3]. This skin reaction is defined ‘acneiform’ rash because the lesions, such as papules, nodules, and pustules, look like acne, but in these patients, comedones, which are a distinguishing factor of acne, are never present. Acneiform rash can be distinguished from acne vulgaris by the monotonous lesion morphology. Several recent prospective studies have addressed and evaluated different therapies to mitigate or reduce the severity of EGFRis-associated skin rash [4,5,6]. With this aim, we have established a dermocosmetological outpatient clinic for cancer patients at the Department of Clinical Medicine and Surgery, University of Naples Federico II [4] in collaboration with the Department of Dermatology and Cutaneous Surgery, Miller School of Medicine, University of Miami.
Fig. 1.
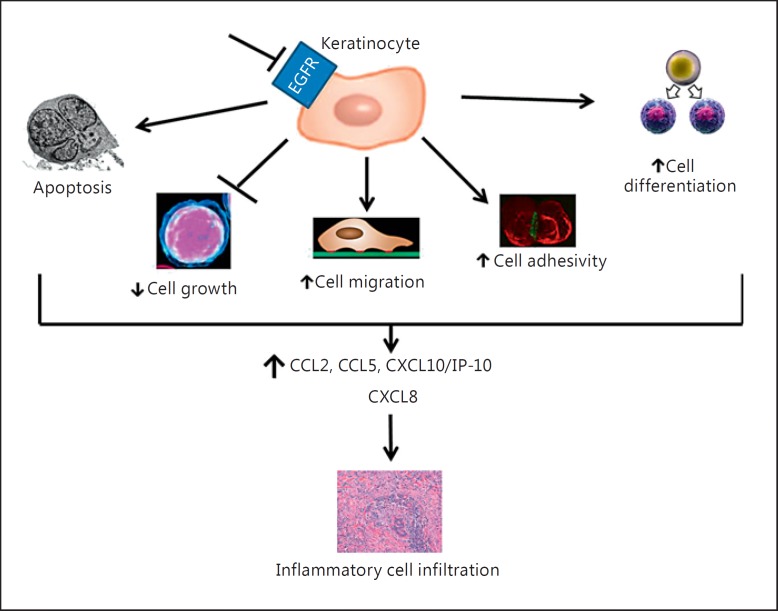
Pathogenesis of inflammatory cell infiltration occurring in the EGFRis-induced papulopustular rash.
An interdisciplinary network of physicians can improve the quality of life of the cancer patients, focusing on such important aspects as dermocosmetological skin care, but also on the evaluation of new therapeutic and diagnostic algorithms in order to make further progress in the field of prevention. In this review, we summarize the state of the art of the epidemiology, pathogenesis, and treatment of EGFRis acneiform rash, and we describe our outpatient clinical experience.
Epidemiology
Papulopustular rash is the most common cutaneous adverse event during EGFRis therapy; it has been reported to occur in 50-100% of patients [7].
When its incidence is analyzed in relation to a single drug, it is observed in 24-62% of patients on gefitinib, in 49-67% of patients on erlotinib, and in 75-91% of patients on cetuximab [8].
Acneiform rash can affect the quality of life of these patients, but it also seems to be associated with a good antitumor activity (although not all literature reports agree [9]), and it can be used as a biomarker for prognosis in patients receiving EGFRis [7,8,9,10,11,12,13,14,15].
Pathogenesis
The pathogenesis of EGFRis-induced skin rash is not well clarified. Nevertheless, some modifications have been identified; in particular the inhibition of EGFR tyrosine kinase activity seems to be determinant [15].
Lichtenberger et al. [16] showed that EGFR is expressed in the basal layer of the epidermis. In papulopustular rash, the main pathogenetic target is the keratinocyte and not the cutaneous adnexa. Indeed, the EGFR signal in the epidermal keratinocytes plays a crucial role in several cell activities, such as inflammation, barrier function, and innate host defense.
Obviously, EGFRis inhibit both EGFR overexpressed in tumor cells and the one expressed in normal cells of the epidermis [16]. In this way, the inhibition of EGFR in keratinocytes induces apoptosis, arrests cell growth, reduces cell migration, and increases cell adhesivity and cell differentiation. All these processes induce keratinocytes to release inflammatory chemokines [chemokine (C-C motif) ligand 2 (CCL2), chemokine (C-C motif) ligand 5 (CCL5), and C-X-C motif chemokine 10 (CXCL10)/interferon γ-inducible protein 10 (IP-10)]. EGFR inhibition is also associated with a reduction of C-X-C motif chemokine 8 (CXCL8) expression. Consequently, an inflammatory cell infiltration occurs (fig. 1). These modifications in the skin lead to the typical cutaneous manifestation described above [15]. In particular, the arrest of growth, the migration of keratinocytes, and inflammation cause xerosis and papulopustular acneiform rash [16].
Histology
Histologically, two major patterns of reaction have been described: (1) hyperkeratotic or ectatic follicular infundibula surrounded by a superficial dermal inflammatory cell infiltrate, particularly in the upper portion of the hair follicle, and (2) neutrophilic suppurative folliculitis with rupture of the epithelial lining [12]. No changes in dermal capillaries, eccrine glands or sebaceous glands are reported. Microbiological stains and cultures have demonstrated the absence of infection, confirming that this is a sterile process.
These histopathological findings underline the different characteristics of papulopustular rash and acne vulgaris, which is characterized by sebaceous gland hypertrophy, comedo development, and inflammatory infiltrate associated with colonization by Propionibacterium acnes[14].
Clinical Aspects
EGFRis-induced rash is an early event: it can occur between 2 days and 6 weeks after the first drug administration, but it usually develops within the first 2 weeks [10].
Clinically, the rash is characterized by tender erythematous papules, which after a few days evolve into pustules and then into crusts. It affects skin areas with a high density of sebaceous glands (scalp, face, upper chest, and back); however, less commonly, it can involve the extremities, lower back, and the abdomen (fig. 2, 3).
Fig. 2.
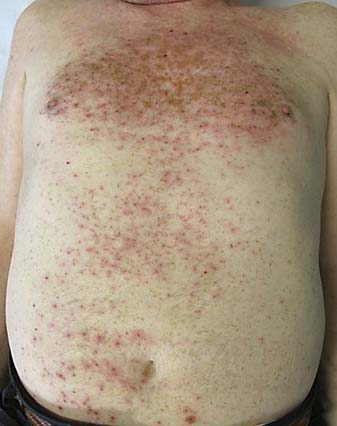
Papulopustular rash on the trunk of a patient affected by lung cancer and treated with erlotinib.
Fig. 3.
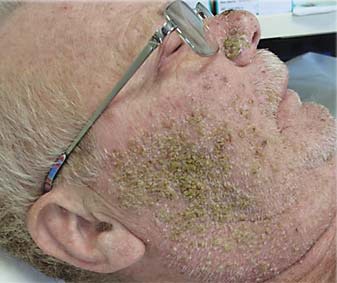
Papulopustular rash on the face of a patient affected by lung cancer and treated with erlotinib.
Lesions can be painful and itching. In some cases, folliculitis may extend to the whole body surface, always sparing the palms, soles, and mucosa [11]. Even if the mucosa is not involved by the rash, they could be interested by xerosis (xerophthalmia, xerostomia) with an incidence varying from 12 to 35%, and this is considered one of the most important aspects that influence a patient's quality of life [16].
Although this rash has been referred to as ‘acneiform’ and ‘acne-like’, it has clinically, etiologically, and histopathologically different characteristics compared to acne [12]. In fact, in contrast to acne, EGFRis-associated rash is predominantly pustular and not associated with comedones (blackheads or whiteheads).
There are several proposed score systems to grade the severity of EGFRis-associated rash. The most commonly used is the US Common Terminology Criteria for Adverse Events (CTCAE) version 4.0, which recognizes 5 grades (table 1) [7].
Table 1.
NCI-CTCAE version 4.0 grading scale of skin and subcutaneous tissue disorders
| EGFRis-associated rash | |
|---|---|
| Grade 1 | Papules and/or pustules covering <10% of the BSA associated or not associated with symptoms of pruritis or tenderness |
| Grade 2 | Papules and/or pustules covering 10–30% of the BSA associated or not associated with symptoms of pruritis or tenderness; psychosocial impact; limiting instrumental ADL |
| Grade 3 | Papules and/or pustules covering >30% of the BSA associated or not associated with pruritis or tenderness; limiting self-care ADL, associated with local superinfection (oral antibiotics indicated) |
| Grade 4 | Covering any percentage of the BSA associated or not with pruritis or tenderness; associated with severe superinfection (intravenous antibiotics indicated); life-threatening consequences |
| Grade 5 | Death |
BSA = Body surface area; ADL = activity of daily living.
Complications
Among the complications of acneiform rash, impetiginization is very frequent, and it can be clinically confused with a severe grade of papulopustular rash. A bacterial overlapping should be suspected when a sudden worsening of the rash (more polymorphous lesions and characteristic honey-colored crust) and different symptoms occur. The major bacterium responsible for the impetiginization is Staphylococcus aureus, especially in patients with nasal carriage and mid-face involvement [11].
Therapy
In the recent literature, few studies on the management of cutaneous side effects of EGFRis can be found. However, different therapeutic strategies have been proposed for the effective management of dermatological adverse events associated with EGFRis therapy. With the increasing rate of EGFRis therapy, a higher proportion of patients develop papulopustular exanthemas and other cutaneous side effects. This led a panel of German dermatologists to draw up recommendations on the management of these cutaneous side effects. These recommendations provide a 3-step guideline that ranges from general measures/preventive measures (step 1) to measures that can be performed by the primary treating physician (step 2) and to advanced therapy by an experienced dermatologist (step 3). Step 1 includes general preventive measures: before starting treatment, patients should be informed of the various cutaneous side effects and the typical manifestation. At the beginning of the EGFRis therapy, patients should be advised to moisturize dry areas of their body twice a day. For this purpose, a thick alcohol-free emollient is recommended. Patients should also be advised to minimize their exposure to sunlight because rash may be more severe in sun-exposed areas of the skin [17,18]. In this phase, preexisting skin diseases such as rosacea or seborrheic dermatitis should be diagnosed and an adequate therapy prescribed if needed. Other preventive measures are summarized in table 2.
Table 2.
General preventive measures to adopt before EGFRis treatment to reduce follicular rash severity
| Avoidance of skin contact with skin irritants such as solvents, disinfectants, polishes |
| Use of mild bath or shower oils or syndets (no soap) |
| Use of moisturizers and/or urea-containing skin care products (ointment, cream) without fragrances or other skin irritants (no lotion or gel) |
| Avoidance of activities which mechanically stress the skin (e.g. garden work, carrying heavy objects, and hot hair drying) |
| Avoidance of frequent hand washing, daily, long showers or frequent, long baths |
Some authors also proposed the use of some topical agents in this phase with the aim of reducing follicular rash severity. Tomková et al. [19] assessed the possible effect of topical phytomenadione (vitamin K1) pretreatment on diminishing the extent and severity of acne-like follicular rash associated with EGFRis therapy. The rationale for this study was the observation that menadione stabilizes the phosphorylation of EGFR, and it can be useful for a targeted prophylaxis of EGFRis-induced cutaneous lesions. However, generally, topical (and eventually systemic) agents are prescribed when rash appears (step 2). In these cases, the following interventions are suggested based on the severity of the reaction:
(1) Mild toxicities: patients may not require any form of intervention; however, it may be appropriate to treat some mild toxicities with topical hydrocortisone (1 or 2.5% cream) or clindamycin (1% gel).
(2) Moderate toxicities: the treatment used includes hydrocortisone (2.5% cream), clindamycin (1% gel), or pimecrolimus (1% cream) with the addition of either doxycycline (100 mg, p.o. bid) or minocycline (100 mg, p.o. bid) [20,21].
With these measures, usually 80% of the rash manifestations can be controlled well. Both in mild and in moderate toxicities, the EGFRis dosage should not be modified. In case of an inadequate response, the patient needs a step 3 therapy for the management of severe toxicities: the concomitant intervention is the same as for moderate toxicities – i.e., hydrocortisone (2.5% cream), clindamycin (1% gel), or pimecrolimus (1% cream), with the addition of either doxycycline (100 mg, p.o. bid) or minocycline (100 mg, p.o. bid) – but with the addition of methylprednisolone dose pack. Microbial diagnostics in order to recognize and early treat a possible bacterial superinfection is necessary. If toxicities are not reduced after 2-4 weeks, despite treatment, then the interruption of EGFRis therapy is recommended in accordance with prescribing information. Sometimes, only a reduction in EGFRis dose might be necessary. In table 3, we report some additional recommendations to increase therapy response and its maintenance.
Table 3.
Additional recommendations to increase therapy response and its maintenance
| Recommendations | Aim |
|---|---|
| Maintain topical or systemic therapy even if EGFRis therapy is decreased or is interrupted | To avoid skin toxicity recurrence |
| Do not use topical corticosteroids for >14 days without interruption and give preference to pulse therapy application of topical corticosteroids (e.g. 14 days on treatment, then 7 days off treatment) | To avoid dermal toxicity and reduce the risk for bacterial or viral superinfection |
| Prescribe systemic therapy when papulopustular exanthema develops on the scalp | To offer an effective protection from the development of a severe papulopustular exanthema |
| Perform an antibiogram in all cases of resistant and severe papulopustular rash | To individuate and treat early bacterial superinfection |
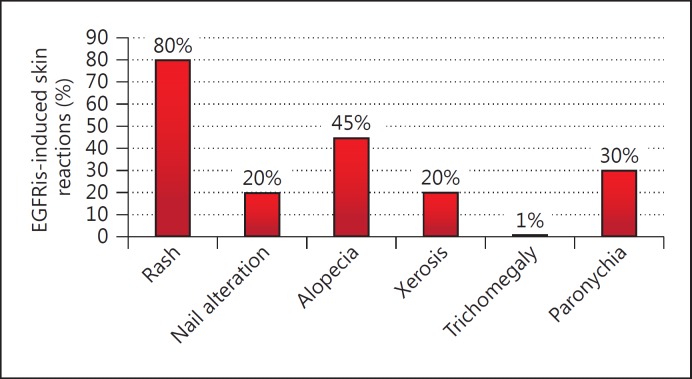
From October 2010 through July 2014, 350 patients were referred to our dermatological outpatient clinic for cancer patients at the Section of Dermatology, Department of Clinical Medicine and Surgery, University of Naples Federico II (fig. 4). The patients’ data were registered using a software set up specifically to record general information, tumor grading, type of chemotherapy, and skin manifestations.
Fig. 4.
EGFRis-induced skin reactions.
Patients were between 19 and 81 years old (mean age, 56 years); 124 were men and 226 women. Among them, 120 patients were treated with EGFRis (cetuximab, erlotinib, lapatinib, gefitinib, and panitumumab). This cluster consisted of 38 men and 82 women aged between 44 and 81 years (mean age, 63 years); 52.17% of them were affected by lung cancer, 17.39% by colon cancer, 8.7% by breast cancer, 8.7% by head and neck cancer, and 4.34% by thymus cancer.
80% of the patients treated with EGFRis developed a papulopustular rash. The severity of the rash was evaluated by CTCAE version 4.03.
According to the data reported in the literature, the papulopustular rash usually occurred within 2-3 weeks after the administration of the drug and reached the upper level after 1 month; this was also the case in our sample. Skin lesions started as erythematous follicular papules, but after a few days they evolved into pustules and then into crusts.
The lesions were above all localized on the face, neck, and retroauricular area, scalp and upper trunk, but the abdomen, arms, and legs were also involved. The majority of our patients developed a mild to moderate rash (grades 1 and 2), but 4 patients developed a severe rash (grade 3).
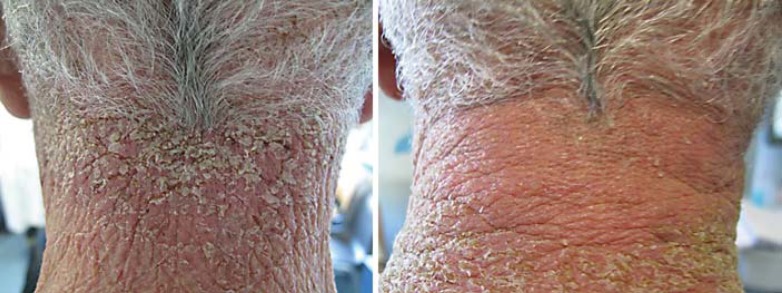
According to our experience, we developed a therapeutic protocol that recommends the application once or twice a day (depending on the severity of the rash) of a mixture of clindamycin 1% gel and gentamicin 0.1% unguent on the affected area. This leads to a complete resolution of the rash within 2 weeks for mild to moderate rash (fig. 5, 6).
Fig. 5.
Papulopustular rash before and after topical therapy.
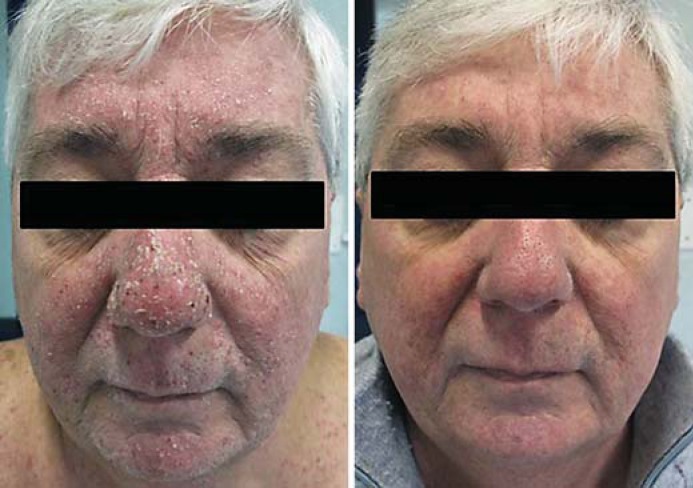
Fig. 6.
Papulopustular rash on the face before and after topical treatment.
For more severe rash, this topical application might not be sufficient. In these cases, we usually prescribe prednisone 12.5-25 mg/day for a week, progressively decreasing the dose. In 2 patients affected by lung cancer and treated with erlotinib, we prescribed doxycycline 40 mg bid for a month, however, obtaining excellent results only in 1 case. We decided to administer a lower dose of doxycycline in order to only benefit from the anti-inflammatory effect of this drug without its antibiotic action, with the aim to reduce the risk of antibiotic resistance and intestinal side effects [21,22].
Besides the pharmacological treatment, it is necessary to pay attention to the dermocosmetological care of the patients treated with EGFRis. They should be advised to use an alcohol-free highly occlusive moisturizing agent and lukewarm water, to avoid prolonged, hot showers, as well as to use a broad-spectrum sunscreen.
To cover grade 1 and 2 rash, a nonocclusive makeup could be used, which is usually tolerated well by patients. In order to protect the stratum corneum barrier, cosmetic formulations containing fatty acids and ceramides are recommended.
Conclusions
The increasing use of new targeted therapy for several oncological diseases can be determinant for the patients’ survival. Nevertheless, it can cause mild to moderate skin manifestations. Even if they might be considered as a biomarker for a good outcome, they highly affect the quality of life of these patients.
For these reasons, it is important for dermatologists to recognize the symptoms and to cure the manifestations in order to avoid the discontinuation of the patients’ cancer treatment.
With this aim, a strong collaboration among dermatologists and between dermatologists and oncologists will be necessary to create guidelines for the diagnosis and therapy of EGFRis-induced cutaneous side effects.
References
- 1.Wagner L, Lai SE, Aneja M, et al. Development of a functional assessment of side-effects to therapy (FAST) questionnaire to assess dermatology-related quality of life in patients treated with EGFR inhibitors (EGFRI): the FAST-EGFRI (abstract) J Clin Oncol. 2007;25:19532. [Google Scholar]
- 2.Hecht JR, Patnaik A, Berlin J, et al. Panitumumab monotherapy in patients with previously treated metastatic colorectal cancer. Cancer. 2007;110:980–988. doi: 10.1002/cncr.22915. [DOI] [PubMed] [Google Scholar]
- 3.Fukuoka M, Yano S, Giaccone G, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected] J Clin Oncol. 2003;21:2237–2246. doi: 10.1200/JCO.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 4.Fabbrocini G, Romano MC, Cameli N, et al. ‘Il corpo ritrovato’: dermocosmetological skin care project for the oncologic patient. ISRN Oncol. 2011;2011:650482. doi: 10.5402/2011/650482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lynch TJ, Jr, Kim ES, Eaby B, et al. Epidermal growth factor receptor inhibitor-associated cutaneous toxicities: an evolving paradigm in clinical management. Oncologist. 2007;12:610–621. doi: 10.1634/theoncologist.12-5-610. [DOI] [PubMed] [Google Scholar]
- 6.Boucher J, Olson L, Piperdi B. Preemptive management of dermatologic toxicities associated with epidermal growth factor receptor inhibitors. Clin J Oncol Nurs. 2011;15:501–508. doi: 10.1188/11.CJON.501-508. [DOI] [PubMed] [Google Scholar]
- 7.Chanprapaph K, Vachiramon V, Rattanakaemakorn P. Epidermal growth factor receptor inhibitors: a review of cutaneous adverse events and management. Dermatol Res Pract. 2014;2014:734249. doi: 10.1155/2014/734249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heidary N, Naik H, Burgin S. Chemotherapeutic agents and the skin: an update. J Am Acad Dermatol. 2008;58:545–570. doi: 10.1016/j.jaad.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Reyes-Habito CM, Roh EK. Cutaneous reactions to chemotherapeutic drugs and targeted therapies for cancer. Part II. Targeted therapies. J Am Acad Dermatol. 2014;71:217e1–217e11. doi: 10.1016/j.jaad.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 10.Hu JC, Sadeghi P, Pinter-Brown LC, Yashar S, Chiu MW. Cutaneous side effects of epidermal growth factor receptor inhibitors: clinical presentation, pathogenesis, and management. J Am Acad Dermatol. 2007;56:317–326. doi: 10.1016/j.jaad.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Peuvrel L, Bachmeyer C, Reguiai Z, Bachet JB, André T, Bensadoun RJ, Bouché O, Ychou M, Dréno B. Semiology of skin toxicity associated with epidermal growth factor receptor (EGFR) inhibitors. Support Care Cancer. 2012;20:909–921. doi: 10.1007/s00520-012-1404-0. [DOI] [PubMed] [Google Scholar]
- 12.Lacouture ME. Mechanisms of cutaneous toxicities to EGFR inhibitors. Nat Rev Cancer. 2006;6:803–812. doi: 10.1038/nrc1970. [DOI] [PubMed] [Google Scholar]
- 13.Eaby-Sandy B, Lynch K. Side effects of targeted therapies: RASH Seminars in Oncology. Nursing. 2014;30:147–154. doi: 10.1016/j.soncn.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Gridelli C, Maione P, Amoroso D, Baldari M, Bearz A, Bettoli V, Cammilluzzi E, Crinò L, De Marinis F, Di Pietro FA, Grossi F, Innocenzi D, Micali G, Piantedosi FV, Scartozzi M. Clinical significance and treatment of skin rash from erlotinib in non-small cell lung cancer patients: results of an Experts Panel Meeting. Crit Rev Oncol Hematol. 2008;66:155–162. doi: 10.1016/j.critrevonc.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Tanusree P, Schumann C, Rüdiger S, Boeck S, Heinemann V, Kächele V, Steffens M, Scholl C, Hichert V, Seufferlein T, Stingl JC. Cytokine regulation by epidermal growth factor receptor inhibitors and epidermal growth factor receptor inhibitor associated skin toxicity in cancer patients. Eur J Cancer. 2014;50:1855–1863. doi: 10.1016/j.ejca.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 16.Lichtenberger BM, Gerber PA, Holcmann M, Buhren BA, Amberg N, Smolle V, et al. Epidermal EGFR controls cutaneous host defense and prevents inflammation. Sci Transl Med. 2013;5:199ra111. doi: 10.1126/scitranslmed.3005886. [DOI] [PubMed] [Google Scholar]
- 17.Herbst RS, LoRusso PM, Purdom M, et al. Dermatologic side effects associated with gefitinib therapy: clinical experience and management. Clin Lung Cancer. 2003;4:366–369. doi: 10.3816/clc.2003.n.016. [DOI] [PubMed] [Google Scholar]
- 18.Jacot W, Bessis D, Jorda E, et al. Acneiform eruption induced by epidermal growth factor receptor inhibitors in patients with solid tumours. Br J Dermatol. 2004;151:238–241. doi: 10.1111/j.1365-2133.2004.06026.x. [DOI] [PubMed] [Google Scholar]
- 19.Tomková H, Pospíšková M, Zábojníková M, Kohoutek M, Serclová M, Gharibyar M, Sternberský J. Phytomenadione pre-treatment in EGFR inhibitor-induced folliculitis. J Eur Acad Dermatol Venereol. 2013;27:514–519. doi: 10.1111/j.1468-3083.2011.04324.x. [DOI] [PubMed] [Google Scholar]
- 20.Micantonio T, Fargnoli MC, Ricevuto E, et al. Efficacy of treatment with tetracyclines to prevent acneiform eruption secondary to cetuximab therapy. Arch Dermatol. 2005;141:1173–1174. doi: 10.1001/archderm.141.9.1173. [DOI] [PubMed] [Google Scholar]
- 21.Sapadin AN, Fleischmajer R. Tetracyclines: nonantibiotic properties and their clinical implications. J Am Acad Dermatol. 2006;54:258–265. doi: 10.1016/j.jaad.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Skidmore R, Kovach R, Walker C, Thomas J, Bradshaw M, Leyden J, et al. Effects of subantimicrobial-dose doxycycline in the treatment of moderate acne. Arch Dermatol. 2003;139:459–464. doi: 10.1001/archderm.139.4.459. [DOI] [PubMed] [Google Scholar]