Abstract
This was a randomized, controlled, parallel-group clinical trial with a blinded evaluator, designed to compare the efficacy and safety of the nail lacquer P-3051 with amorolfine 5% in the treatment of mild-to-moderate toenail onychomycosis. Patients were treated for 48 weeks with P-3051 daily, or twice weekly with amorolfine 5%. Out of 120 evaluable patients, 60 (50.0%) received P-3051 and 60 (50.0%) amorolfine 5%. At baseline, the two groups were homogeneous in terms of race, pathogens, number of affected toenails and severity of the infected target nail area. The statistical superiority of P-3051 versus amorolfine was achieved after 48 weeks (treatment success: 58.3% for P-3051 vs. 26.7% for amorolfine, p < 0.001; complete cure: 35.0% for P-3051 vs. 11.7% for amorolfine, p < 0.001). Mycological cure at week 48 was achieved in all patients treated with P-3051 compared to 81.7% of patients treated with amorolfine (p < 0.001). Moreover, fungal eradication by P-3051 was statistically superior at week 24. The results of this study, and of a previous pivotal study versus the insoluble formulation of ciclopirox 8%, led to consider P-3051 as the gold standard for the topical treatment of mild-to-moderate onychomycosis.
Key Words: Onychomycosis, Topical treatment, P-3051, Hydroxypropyl chitosan, Amorolfine, Controlled clinical trial
Introduction
Onychomycosis is a fungal nail infection, affecting overall 12-13% of the population [1,2,3,4,5,6,7]. The disease can lead to nail dystrophies if not treated, such as onycholysis, discoloration and thickening due to fungal colonization of the bed and matrix [8].
Whereas oral systemic therapy is widely used for the more severe cases of onychomycosis, while not considering the issue of side effects or drug interactions [9], topical nail lacquers are recommended for the treatment of mild-to-moderate onychomycosis as they minimize drug exposure, drug interactions and adverse events (AEs).
For many years, there have only been two topical products, available as nail lacquer formulations, used in most European countries: amorolfine 5% and ciclopirox 8%. Both products are applied in an insoluble vehicle, requiring nail filing before application and an organic solvent for removal.
In recent years, an innovative, water-soluble ciclopirox 8% formulation in hydroxypropyl chitosan (HPCH) technology (P-3051) has been approved and marketed in more than 40 countries. It does not require nail filing and is easily removed with water, thereby greatly improving patient compliance.
HPCH is able to form an invisible film on the nail surface, which may prevent fungal invasion [10]. Furthermore, HPCH allows the composition to remain in contact with the nail surface long enough for a substantial ciclopirox penetration into and through the nail [11].
P-3051 exhibited a better penetration and a higher predicted efficacy after in vivo multiple application to human fingernails when compared to the traditional insoluble ciclopirox 8% formulation and to amorolfine 5% [11].
In 2009, Baran et al. [12] showed a statistically significant superiority of P-3051, in terms of efficacy, versus the reference insoluble ciclopirox nail lacquer in 454 mild-to-moderate onychomycotic patients treated for 48 weeks.
Controversial data from controlled studies are available to assess amorolfine 5% efficacy. In a recent multicenter, randomized, open-label and controlled study conducted on 142 onychomycotic patients, amorolfine 5% showed complete cure in 12.7% [13].
In another controlled study aimed at evaluating the efficacy of a terbinafine nail lacquer, amorolfine 5% was chosen as comparator [14]; the study, conducted on 1,029 onychomycotic patients, showed a very poor efficacy of the product [0.96% complete cure, defined as a combination of both clinical and mycological cure, and 15.7% responsiveness, defined as a negative direct potassium hydroxide (KOH) microscopy and negative fungal pathogen culture, with an affected nail area of ≤10%].
In general, over recent decades, the deployment of a blinded evaluator has become a common practice in onychomycosis clinical trials in cases of different product features. This approach is unanimously regarded as scientifically valid and has become the standard practice when comparing topical products which cannot be made blind for obvious reasons. Therefore, Polichem SA decided to assess the clinical efficacy of P-3051 versus amorolfine 5%, in a comparative clinical trial, using a blinded evaluator.
Patients and Methods
Study Design
This was a randomized, controlled, parallel-group study, under a blinded evaluator (PM1125 study, EudraCT No. 2011-003087-70). The trial was aimed at assessing the efficacy and safety of P-3051 (Ciclopoli® 8%, Taurus Pharma, Bad Homburg, Germany) versus amorolfine 5% (Loceryl® 5%, Galderma Laboratorium GmbH, Düsseldorf, Germany) in patients with mild-to-moderate toenail distal lateral subungual onychomycosis, caused by dermatophytes, yeasts and moulds, of at least one big toenail (target nail), without the presence of yellow spikes, dermatophytoma or lunula involvement.
According to the protocol, patients, 18-75 years old, had to sign an informed consent form before enrolment. Those patients with an infected target nail area ≥25 and ≤75%, and with both positive KOH and culture for fungal nail pathogens at screening, were enrolled in the trial.
Concomitant severe plantar tinea pedis, other nail abnormalities (such as psoriasis or lichen planus) and the use of any systemic or topical treatment were not allowed.
After a run-in period of 4-5 weeks, necessary to obtain the culture result of the nail specimens, patients were randomized at a 1:1 ratio to receive either P-3051 or amorolfine 5% nail lacquer (randomization visit). The investigator allocated the treatments, sequentially numbered, to each patient according to a randomization list.
Patients were instructed to perform a daily application of the nail lacquer for a 48-week treatment course with P-3051 or a twice-a-week application with amorolfine 5%, according to the instruction in the leaflet.
The study was performed in an open-label fashion, due to the different time schedules of administration and the technical characteristics of the two formulations (i.e. removal procedures). The accepted blinded evaluation methodology was used to avoid the potential bias of an open-label design. The patients underwent a further five clinical visits at 4, 8, 12, 24 and 48 weeks.
Efficacy and Safety Assessments
The main efficacy variables were complete cure rate, treatment success and mycological cure, evaluated at different time points in the intent-to-treat (ITT) population. Complete cure was defined as a composite of negative KOH microscopy and negative culture for fungal pathogens with no residual clinical involvement of the target toenail. Treatment success was defined as negative KOH microscopy and negative culture for fungal pathogens as well as ≤10% residual involvement of the target toenail. Mycological cure was defined as both negative direct microscopy and negative culture. Safety was assessed through the AE recording by the investigator.
Photographs and Planimetry
At the screening visit and at each study visit (except at the randomization visit), before the nail scraping for mycological testing, two digital photographs were taken by the investigator under standard conditions using a single-lens reflex camera. The first photograph showed the target big toenail as it appeared. Then, the local investigator directly traced the outline of the diseased area (if present) on the patient's nail, taking additional photographs.
The images related to visits at weeks 24 and 48, with the outline of the diseased area drawn by the local investigator, were reviewed by the blinded evaluator.
Statistical Analyses
A sample size of 120 patients (60 patients per group) was deemed necessary to detect a 14% difference in success rate between the two treatment groups based on the results of previous studies [12,15], where P-3051 and amorolfine achieved the efficacy parameter in 80 and 66% of patients, respectively. Assuming a screening failure rate of 30% of patients displaying negative fungal culture, about 160 patients had to be selected for screening. Statistical analyses were performed using SAS® 9.2 software (SAS Institute Inc., Cary, N.C., USA).
The significance level was equal to 5% for all tests, and no adjustments were made for multiple testing.
The main efficacy analysis was performed on the ITT population, i.e. on all patients enrolled and randomized and those who had received at least one dose of the study drug.
The z test was used to assess the difference between the two treatments with respect to the proportion of treatment success and the proportion of completely cured cases at weeks 12, 24 and 48.
The safety population was defined as all the patients randomized and treated at least once.
AEs were based on the safety population. They were coded using MedDRA version 16, and the incidence rates were tabulated by Preferred Term and by System Organ Class per treatment group. Safety analysis included tabulation of the type and frequency of all AEs. Results were summarized by their relationship to the study drugs and by their severity (i.e. mild, moderate or severe).
Number Needed to Treat Analysis
The number needed to treat (NNT) is an epidemiological measure used to assess the effectiveness of health care intervention, typically treatment with a medication. The NNT is the average number of patients who need to be treated to prevent one additional bad outcome (i.e. the number of patients who need to be treated for one to benefit compared with a control in a clinical trial). It is defined as the inverse of the absolute risk reduction (intended as a way of measuring the size of a difference between the efficacy of two treatments), as described in the milestone paper of Laupacis et al. [16]. The NNT is useful if several treatments are assessed for the same outcome measure in patients with similar baseline conditions and lengths of treatment [17]. The ideal NNT is 1, where everyone improves with treatment and no one improves with control. The higher the NNT, the less effective the treatment.
The NNT and the lower/upper 95% confidence limits are commonly obtained by inverting the absolute risk reduction of the active drug versus placebo and the associated lower/upper 95% confidence limits.
Results
Demography and Baseline Characteristics
Overall, 154 patients were screened, and 120 patients were randomized to one of the treatments. All patients were Caucasians, and there was a higher proportion of females compared to males. Both groups were homogeneous with respect to sex, age and weight, as well as to the number of affected toenails, causative pathogens and the percentage of the infected target nail area. This is consistent with a population of moderate onychomycosis. Baseline characteristics of the study population and mycological results at inclusion are summarized in table 1.
Table 1.
Baseline characteristics of randomized patients
| P-3051 (n = 60) | Amorolfine 5% (n = 60) | |
|---|---|---|
| Age, years | 51.45±11.75 | 53.85 ± 12.21 |
| Gender | ||
| Male | 6 (10.0) | 10 (16.7) |
| Female | 54 (90.0) | 50 (83.3) |
| Caucasian | 60 (100) | 60 (100) |
| Weight, kg | 73.00±10.21 | 73.00±10.21 |
| Fungal species | ||
| Dermatophytes | 43 (71.7) | 47 (78.3) |
| Yeast | 12 (20.0) | 11 (18.3) |
| Nondermatophyte moulds | 5 (8.3) | 2 (3.4) |
| Number of toenails with clinical | ||
| evidence of onychomycosis | 6.8±2.0 | 7.5±2.0 |
| Percentage of infected target | ||
| nail area | 44.62 ±12.93 | 46.20±13.58 |
Data are the mean ± SD, or n (%).
Efficacy
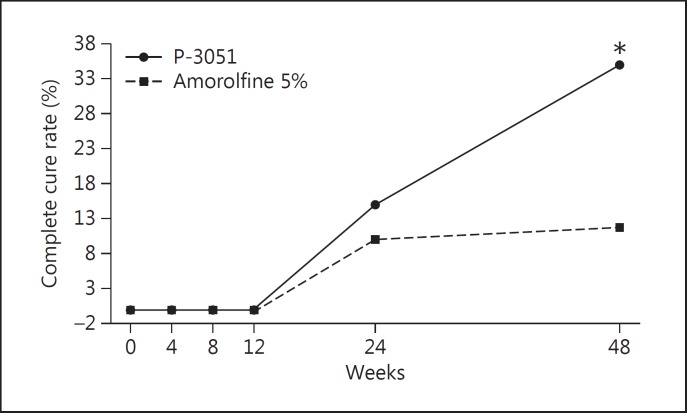
Figure 1 reports the results observed for the complete cure rate. The number of patients cured at week 48 was 21 (35.0%) in the P-3051 group and only 7 (11.7%) in the amorolfine 5% group, resulting in a very high statistical superiority (p < 0.001) in favor of P-3051. At week 24, 9 patients (15.0%) in the P-3051 group and 6 (10.0%) in the amorolfine 5% group were already cured. However, the difference between the groups was not statistically significant (p = 0.408) at this time point. The complete cure of nail infection treated with P-3051 at the end of treatment is documented in figure 2.
Fig. 1.
Complete cure rate in the ITT population. * p < 0.001.
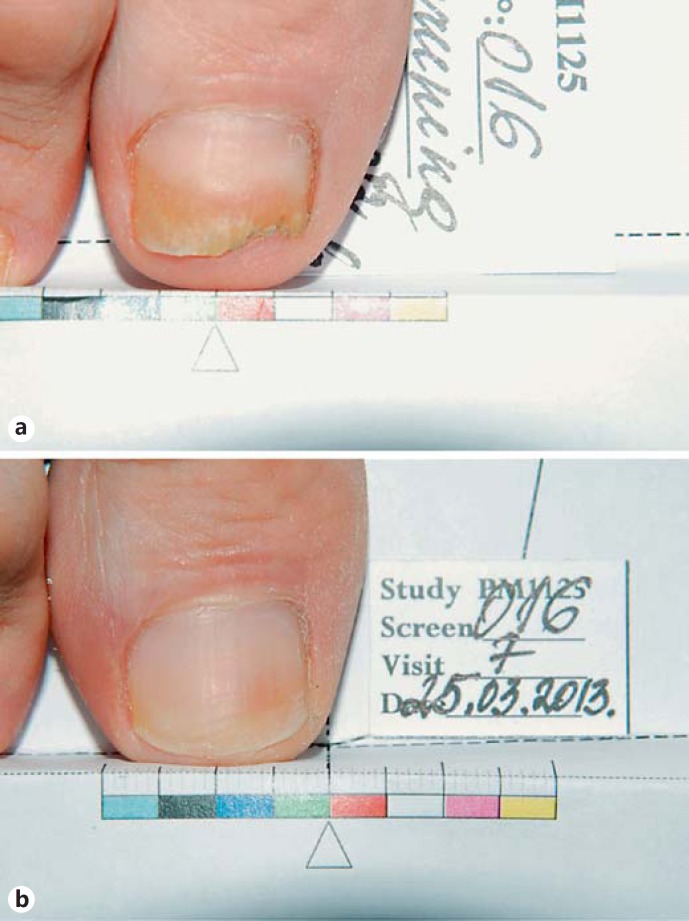
Fig. 2.
Complete cure with P-3051 (patient No. 16). a Before treatment (area affected by Trichophyton mentagrophytes). b After treatment (cured).
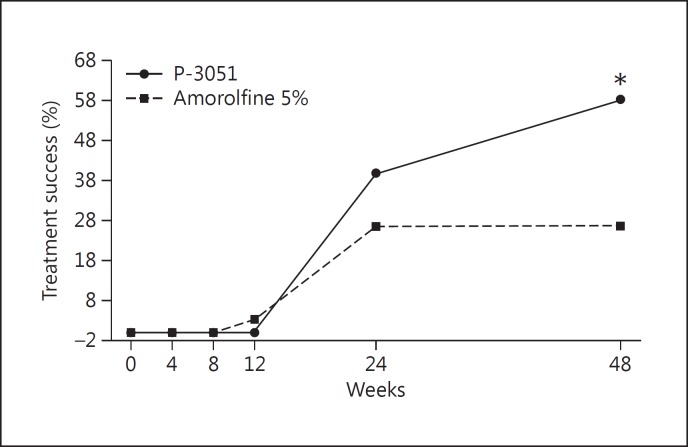
Figure 3 represents the results observed in the treatment success parameter: at week 48, 35 patients were considered successfully treated (58.3%) in the P-3051 group versus only 16 patients (26.7%) in the amorolfine 5% group (p < 0.001), resulting in a very high statistical superiority of P-3051. At week 24, 24 patients (40.0%) in the P-3051 group and 16 (26.7%) in the amorolfine 5% group showed a clinical benefit. The difference between the groups was not statistically significant (p = 0.121) at this time point.
Fig. 3.
Treatment success in the ITT population. * p < 0.001.
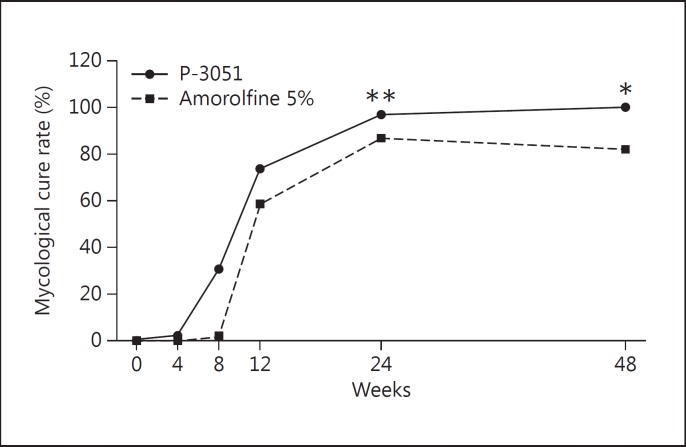
The results at week 48 showed that mycological cure was observed in all 60 patients (100.0%) in the P-3051 group and in 49 (81.7%) in the amorolfine 5% group, showing a very high statistically significant effect (p < 0.001) in favor of P-3051. Out of 11 (18.3%) mycological failures in the amorolfine 5% group, 8/47 (17%) were dermatophytes and 3/11 (27%) were Candida spp., while moulds were eradicated (2/2). The results are shown in figure 4.
Fig. 4.
Mycological cure in the ITT population. * p < 0.001; ** p < 0.05.
These results are also consistent with the two parameters (KOH microscopy and culture) when taken singly (data not shown).
The results at week 24 showed that mycological cure was observed in 58 patients (96.7%) in the P-3051 group and in 52 (86.7%) in the amorolfine 5% group: in this case, the difference between the groups was statistically significant (p < 0.05) in favor of P-3051, also at week 24.
Safety
None of the patients in either group had serious AEs, AEs of severe intensity, treatment-related AEs or any AEs that led to definite discontinuation of the treatment. One patient (1.6%) in the P-3051 group temporary discontinued treatment due to AEs, consisting of trauma of the right foot. As no serious or treatment-related AEs leading to temporary or definitive discontinuation of the study drugs were reported in any patient in either group, it is possible to confirm that both treatments were well tolerated without any safety concern.
NNT Analysis in PM1125
As PM1125 did not include a placebo arm, a ‘putative’ estimate of placebo effect was computed using data gathered from vehicle-controlled studies (without P-3051) reported in table 2 [14,18,19]. The pooled estimate of the cure rate in the vehicle group (2.11%), obtained by combining the five studies, was used as a historical vehicle control group.
Table 2.
Reported cure rates in vehicle control groups in recent studies
| First author [Ref.], year | Test regimen | Treatment duration, weeks | Cure rate in the vehicle group |
|---|---|---|---|
| Elewski [19], 2013 | Efinaconazole 10% solution once a day | 48 | 7/214 (3.3%) |
| Elewski [19], 2013 | Efinaconazole 10% solution once a day | 48 | 11/202 (5.5%) |
| Elewski [14], 2011 | Terbinafine solution | 48 | 0/256 (0.0%) |
| Gupta [18], 2000 | Insoluble ciclopirox 8% lacquer once a day | 48 | 1/109 (0.9%) |
| Gupta [18], 2000 | Insoluble ciclopirox 8% lacquer once a day | 48 | 0/117 (0.0%) |
| All 5 studies combined | 19/898 (2.11%) | ||
The NNTs computed using complete cure data are displayed in table 3. The NNT data clearly show that P-3051 is almost 4 times more effective than amorolfine 5%. In other words, while it is necessary to treat only 3 patients with P-3051 to get on average 1 cure, one needs to treat 11 patients with amorolfine 5% to obtain the same clinical results. Moreover, the nonoverlapping 95% confidence interval indicates a statistically significant superiority of P-3051 versus amorolfine 5%.
Table 3.
NNTs computed for complete cure
| Treatment | NNT | NNT lower 95% CL | NNT upper 95% CL |
|---|---|---|---|
| P-3051 | 3 | 2 | 5 |
| Amorolfine 5% | 11 | 6 | 88 |
CL = Confidence limit.
NNT Analysis in Previous RCT European Studies
A further analysis was performed comparing data gathered from the pivotal studies of Baran et al. [12] (P-3051 pivotal trial), Gupta et al. [18] and Elewski et al. [14] (amorolfine 5% as reference, calculated using placebo of the test drug, and terbinafine nail solution). The NNTs computed using complete cure data from pivotal studies are displayed in table 4.
Table 4.
NNTs computed for complete cure of pivotal trials
| Treatment | NNT | NNT lower 95% CL | NNT upper 95% CL |
|---|---|---|---|
| P-3051a | 17 | 10 | 43 |
| Amorolfine 5%b | 104 | 55 | 817 |
| CPX 8%c | 21 | 13 | 60 |
The NNT data clearly show that P-3051 is almost 6 times more effective than amorolfine 5% in complete cure. The nonoverlapping 95% confidence intervals indicate a statistically significant superiority of P-3051 versus amorolfine 5%. Moreover, P-3051 appears clinically superior compared to the standard insoluble ciclopirox 8% formulation.
Discussion
This clinical trial compared two of the topical treatments most widely used in Europe in the management of onychomycosis, P-3051 (ciclopirox 8% nail hydrolacquer) and amorolfine 5%.
Although encouraging results had already been obtained in in vitro as well as in in vivo studies using HPCH technology, in terms of a better active permeation [11] of P-3051 compared to amorolfine, a comparative clinical study between the two compounds was still missing, and for this reason it has been performed.
Mycological cure was chosen as an objective independent endpoint for evaluating antifungal agents [20]. In this clinical study, a large spectrum of nail pathogens has been treated, revealing a similar effect both of P-3051 and of amorolfine 5% on mycological findings until the 24th week of treatment. In the following 6 months of treatment, the antifungal activity of P-3051 increased until 100%, in terms of conversion to negative culture and KOH microscopy analysis, confirming the antifungal activity of P-3051, as already shown by Baran et al. [12].
On the other hand, amorolfine 5% exerted the maximum effect at 24 weeks, but surprisingly, its antifungal activity decreased after 48 weeks of treatment.
This issue is explained by the occurrence of strains resistant to amorolfine 5% and the well-known lack of tolerance or resistance of fungi to ciclopirox, probably due to its multiple mechanisms of action; this fact may contribute to the reduction of the relapse rate in the P-3051 group in comparison to the amorolfine 5% group, due to the possible selection of drug-resistant strains [21] in the latter case.
After a 48-week treatment, even the clinical efficacy of amorolfine 5% showed a modest improvement in comparison to the results obtained after 6 months of treatment. This finding is in contrast with the recommendations of experts, suggesting that the length of treatment with topical drugs in mild-to-moderate onychomycosis should not be less than 1 year. It was even suggested that an extension of the duration of treatment to 18 months could produce a better clinical outcome that reflects true nail pathology [10].
In our experience, the further clinical benefits expected in the following 24 weeks of treatment and related to the normal growth of the nail seemed to be almost zero, due to the possible occurrence of relapses or resistance.
However, in the present trial, the efficacy of amorolfine 5% nail lacquer (with a complete cure rate of about 12%) is higher than the results given in the study of Elewski et al. [14] (where the complete cure rate was 0.96%) and substantially in line with the data obtained in the study of Paul et al. [13] (12.7%).
The efficacy rate shown by P-3051 in this trial, with respect to the previous pivotal study [12], could be a reference to a population at baseline in terms of milder nail pathogen infections.
Therefore, it is not surprising that higher efficacy rates were reported with criteria which are less restrictive but certainly closer to the day-by-day management of the disease.
Finally, the results obtained in this head-to-head study between P-3051 and amorolfine 5% are not unexpected. In fact, the comparison of the NNT analysis indicated a better clinical efficacy of P-3051 versus amorolfine 5%, ranging from 4 to 6 times.
Both P-3051 and amorolfine 5% were very well tolerated, confirming an excellent safety profile.
Conclusion
The results of this comparative clinical study versus amorolfine 5% and of the previous pivotal study versus the insoluble formulation of ciclopirox 8% led to P-3051 being considered as the most effective topical drug for the treatment of mild-to-moderate onychomycosis.
Its efficacy, different from that of amorolfine 5%, is maintained in the long term by the well-documented capability of ciclopirox, which does not induce fungal resistance.
As a consequence, from now on, P-3051 should be considered as the gold standard for the topical treatment of onychomycosis.
Statement of Ethics
The study was reviewed and approved by the relevant Institutional Review Boards/Independent Ethics Committees and conducted between February 2012 and June 2014 following the Good Clinical Practices (CPMP/ICH/135/95), the Declaration of Helsinki and its subsequent amendments, as well as the national regulations.
Disclosure Statement
None of the authors have affiliations with or are involved in any organization or entity with any financial or nonfinancial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in the paper.
Supplementary Material
Supplementary data
Acknowledgment
This project was funded by Polichem SA, which designed and conducted the study, collected the data, performed the data management, data analysis and the interpretation of the data.
Erratum
In the article by Iorizzo et al., entitled ‘Ciclopirox 8% HPCH nail lacquer in the treatment of mild-to-moderate onychomycosis: a randomized, double-blind amorolfine controlled study using a blinded evaluator’ [Skin Appendage Disord 2015;1:134–140, DOI: 10.1159/000441569], the following corrections need to be made:
1. The title should be amended to ‘Ciclopirox 8% HPCH nail lacquer in the treatment of mild-to-moderate onychomycosis: a randomized, amorolfine controlled study using a blinded evaluator’.
2. The text in table 1, p. 136, line 6, should read as follows:
3. The Acknowledgment on p. 139 should be amended to read as follows: ‘This project was funded by Polichem SA. Data management and the statistical analysis were carried out independently by Sintesi Research SRL, Milan, Italy.’
Table 1.
Baseline characteristics of randomized patients
| P-3051 (n = 60) | Amorolfine 5% (n = 60) | |
|---|---|---|
| Age, years | 51.45±11.75 | 53.85±12.21 |
| Gender | ||
| Male | 6 (10.0) | 10 (16.7) |
| Female | 54 (90.0) | 50 (83.3) |
| Caucasian | 60 (100) | 60 (100) |
| Weight, kg | 71.80±12.70 | 73.20±10.30 |
| Fungal species | ||
| Dermatophytes | 43 (71.7) | 47 (78.3) |
| Yeast | 12 (20.0) | 11 (18.3) |
| Nondermatophyte moulds | 5 (8.3) | 2 (3.4) |
| Number of toenails with clinical | ||
| evidence of onychomycosis | 6.8±2.0 | 7.5±2.0 |
| Percentage of infected target | ||
| nail area | 44.62 ±12.93 | 46.20±13.58 |
Data are the mean ± SD, or n (%).
References
- 1.Roberts DT. Prevalence of dermatophyte onychomycosis in the United Kingdom: results of an omnibus survey. Br J Dermatol. 1992;126:23–27. doi: 10.1111/j.1365-2133.1992.tb00005.x. [DOI] [PubMed] [Google Scholar]
- 2.Elewski BE, Charif MA. Prevalence of onychomycosis in patients attending a dermatology clinic in northeastern Ohio for other conditions. Arch Dermatol. 1997;133:1172–1173. [PubMed] [Google Scholar]
- 3.Sais G, Jucgl A, Peyr J. Prevalence of dermatophyte onychomycosis in Spain: a cross-sectional study. Br J Dermatol. 1995;132:758–761. doi: 10.1111/j.1365-2133.1995.tb00722.x. [DOI] [PubMed] [Google Scholar]
- 4.Ghannoum MA, Hajjeh RA, Scher R, Konnikov N, Gupta AK, Summerbell R, Sullivan S, Daniel R, Krusinski P, Fleckman P, Rich P, Odom R, Aly R, Pariser D, Zaiac M, Rebell G, Lesher J, Gerlach B, Ponce-De-Leon GF, Ghannoum A, Warner J, Isham N, Elewski B. A large-scale North American study of fungal isolates from nails: the frequency of onychomycosis, fungal distribution, and antifungal susceptibility patterns. J Am Acad Dermatol. 2000;43:641–648. doi: 10.1067/mjd.2000.107754. [DOI] [PubMed] [Google Scholar]
- 5.Sigurgeirsson B, Steingrímsson O, Sveinsdóttir S. Prevalence of onychomycosis in Iceland: a population-based study. Acta Derm Venereol. 2002;82:467–469. doi: 10.1080/000155502762064665. [DOI] [PubMed] [Google Scholar]
- 6.Gupta AK, Jain HC, Lynde CW, Watteel GN, Summerbell RC. Prevalence and epidemiology of unsuspected onychomycosis in patients visiting dermatologists’ offices in Ontario, Canada - a multicenter survey of 2001 patients. Int J Dermatol. 1997;36:783–787. doi: 10.1046/j.1365-4362.1997.00349.x. [DOI] [PubMed] [Google Scholar]
- 7.Svejgaard EL, Nilsson J. Onychomycosis in Denmark: prevalence of fungal nail infection in general practice. Mycoses. 2004;47:131–135. doi: 10.1111/j.1439-0507.2004.00968.x. [DOI] [PubMed] [Google Scholar]
- 8.Welsh O, Vera-Cabrera L, Welsh E. Onychomycosis. Clin Dermatol. 2010;28:151–159. doi: 10.1016/j.clindermatol.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Shear N, Drake L, Gupta AK, Lambert J, Yaniv R. The implications and management of drug interactions with itraconazole, fluconazole and terbinafine. Dermatology. 2000;201:196–203. doi: 10.1159/000018488. [DOI] [PubMed] [Google Scholar]
- 10.Ghannoum MA, Long L, Isham N, Bulgheroni A, Setaro M, Caserini M, Palmieri R, Mailland F. Ability of hydroxypropyl chitosan nail lacquer to protect against dermatophyte nail infection. Antimicrob Agents Chemother. 2015;59:1844–1848. doi: 10.1128/AAC.04842-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monti D, Herranz U, Dal Bo L, Subissi A. Nail penetration and predicted mycological efficacy of an innovative hydrosoluble ciclopirox nail lacquer vs a standard amorolfine lacquer in healthy subjects. J Eur Acad Dermatol Venereol. 2013;27:e153–e158. doi: 10.1111/j.1468-3083.2012.04529.x. [DOI] [PubMed] [Google Scholar]
- 12.Baran R, Tosti A, Hartmane I, Altmeyer P, Hercogova J, Koudelkova V, Ruzicka T, Combemale P, Mikazans I. An innovative water-soluble biopolymer improves efficacy of ciclopirox nail lacquer in the management of onychomycosis. J Eur Acad Dermatol Venereol. 2009;23:773–781. doi: 10.1111/j.1468-3083.2009.03164.x. [DOI] [PubMed] [Google Scholar]
- 13.Paul C, Coustou D, Lahfa M, Bulai-Livideanu C, Doss N, Mokthar I, Turki H, Nouira R, Fazaa B, Ben Osman A, Zourabichvili O, Cazeau C, Coubetergues H, Picot S, Bienvenu AL, Voisard JJ. A multicenter, randomized, open-label, controlled study comparing the efficacy, safety and cost-effectiveness of a sequential therapy with RV4104A ointment, ciclopiroxolamine cream and ciclopirox film-forming solution with amorolfine nail lacquer alone in dermatophytic onychomycosis. Dermatology. 2013;227:157–164. doi: 10.1159/000353667. [DOI] [PubMed] [Google Scholar]
- 14.Elewski BE, Ghannoum MA, Mayser P, Gupta AK, Korting HC, Shouey RJ, Baker DR, Rich PA, Ling M, Hugot S, Damaj B, Nyirady J, Thangavelu K, Notter M, Parneix-Spake A, Sigurgeirsson B. Efficacy, safety and tolerability of topical terbinafine nail solution in patients with mild-to-moderate toenail onychomycosis: results from three randomized studies using double-blind vehicle-controlled and open-label active-controlled designs. J Eur Acad Dermatol Venereol. 2013;27:287–294. doi: 10.1111/j.1468-3083.2011.04373.x. [DOI] [PubMed] [Google Scholar]
- 15.Reinel D, Clarke C. Comparative efficacy and safety of amorolfine nail lacquer 5% in onychomycosis, once-weekly versus twice-weekly. Clin Exp Dermatol. 1992;17(suppl):44–49. doi: 10.1111/j.1365-2230.1992.tb00278.x. [DOI] [PubMed] [Google Scholar]
- 16.Laupacis A, Sackett DL, Roberts RS. An assessment of clinically useful measures of the consequences of treatment. N Engl J Med. 1988;318:1728–1733. doi: 10.1056/NEJM198806303182605. [DOI] [PubMed] [Google Scholar]
- 17.McQuay HJ, Moore RA. Using numerical results from systematic reviews in clinical practice. Ann Intern Med. 1997;126:712–720. doi: 10.7326/0003-4819-126-9-199705010-00007. [DOI] [PubMed] [Google Scholar]
- 18.Gupta AK, Fleckman P, Baran R. Ciclopirox nail lacquer topical solution 8% in the treatment of toenail onychomycosis. J Am Acad Dermatol. 2000;43(suppl):S70–S80. doi: 10.1067/mjd.2000.109071. [DOI] [PubMed] [Google Scholar]
- 19.Elewski BE, Rich P, Pollak R, Pariser DM, Watanabe S, Senda H, Ieda C, Smith K, Pillai R, Ramakrishna T, Olin JT. Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase III multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013;68:600–608. doi: 10.1016/j.jaad.2012.10.013. erratum in J Am Acad Dermatol 2014;70:399. [DOI] [PubMed] [Google Scholar]
- 20.Ghannoum M, Isham N, Catalano V. A second look at efficacy criteria for onychomycosis: clinical and mycological cure. Br J Dermatol. 2014;170:182–187. doi: 10.1111/bjd.12594. [DOI] [PubMed] [Google Scholar]
- 21.Ghelardi E, Celandroni F, Gueye SA, Salvetti S, Senesi S, Bulgheroni A, Mailland F. Potential of Ergosterol synthesis inhibitors to cause resistance or cross-resistance in Trichophyton rubrum. Antimicrob Agents Chemother. 2014;58:2825–2829. doi: 10.1128/AAC.02382-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data