Abstract
Mutations in TREM2, which has been proposed to regulate the inflammatory responses and the clearance of apoptotic neurons and/or amyloid-β (Aβ), are genetically linked to increased risk for late-onset Alzheimer’s disease (AD). Interestingly, a missense variant in TREM-like transcript 2 (TREML2), a structurally similar protein encoded by the same gene cluster with TREM2 on chromosome 6, has been shown to protect against AD. However, the molecular mechanisms by which TREM2 and TREML2 regulate the pathogenesis of AD, and their functional relationship, if any, remain unclear. Here, we show that lipopolysaccharide (LPS) stimulation significantly suppressed TREM2 but increased TREML2 expression in mouse brain. Consistent with this in vivo result, LPS or oligomeric Aβ treatment down-regulated TREM2 but up-regulated TREML2 expression in primary microglia. Importantly, modulation of TREM2 or TREML2 levels had opposing effects on inflammatory responses with enhancement or suppression of LPS induced pro-inflammatory cytokine gene expression observed upon TREM2 or TREML2 down-regulation, respectively. In addition, the proliferation of primary microglia was significantly decreased when TREM2 was down-regulated, whereas it was increased upon TREML2 knockdown. Together, our results suggest that several microglial functions are strictly regulated by TREM2 and TREML2, whose dysfunctions likely contribute to AD pathogenesis by impairing brain innate immunity. Our findings provide novel mechanistic insights into the functions of TREM2 and TREML2 in microglia and have implications on designing new therapeutic strategies to treat AD.
Keywords: TREM2, TREML2, proliferation, microglia, inflammation
INTRODUCTION
Recently, genome-wide genetic studies performed on different populations identified a rare variant (R47H) in triggering receptor expressed on myeloid cells 2 (TREM2) as a strong genetic risk factor for late-onset Alzheimer’s disease (AD) (Benitez and Cruchaga, 2013, Guerreiro and Hardy, 2013, Guerreiro, et al., 2013, Jonsson, et al., 2013). As an important innate immune receptor in the brain, TREM2 is mainly expressed in microglia, coupling with DNAX-activating protein of 12 kDa (DAP12) for its signaling (Jiang, et al., 2013, Neumann and Takahashi, 2007). Emerging evidence has established the ability of TREM2 in promoting an anti-inflammatory response in microglia (Hamerman, et al., 2006, Painter, et al., 2015, Takahashi, et al., 2005, Turnbull, et al., 2006) and in animal disease models such as multiple sclerosis mouse model (Takahashi, et al., 2007) and senescence-accelerated mouse prone 8 (SAMP8) mice (Jiang, et al., 2014). TREM2 also binds to a wide variety of anionic and zwitterionic lipids known to associate with fibrillar Aβ and the surface of damaged cells, serving as a phagocytosis signaling receptor for bacteria, Aβ aggregates, and apoptotic neurons (Hsieh, et al., 2009, N’Diaye, et al., 2009, Stefano, et al., 2009, Takahashi, et al., 2005, Wang, et al., 2015). Moreover, studies showed that lack of TREM2 impairs the proliferation of osteoclast precursors in response to macrophage colony-stimulating factor (M-CSF) (Otero, et al., 2012, Takahashi, et al., 2005), suggesting that TREM2 may also be involved in the regulation of cell proliferation.
The TREM and TREM-like receptors belong to a structurally related transmembrane protein family encoded by genes clustered on chromosome 6p21.11 (Ford and McVicar, 2009). These receptors are expressed by a variety of innate cells of myeloid lineage including neutrophils, monocytes, osteoclasts, macrophages, dendritic cells, and microglia, and are emerging as important components in innate and adaptive immunity. Intriguingly, a recent study indicates that a missense variant of TREM like transcript-2 (TREML2, also named TLT2), p.S144G (rs3747742), plays a protective role in AD (Benitez, et al., 2014). TREML2 is a single-pass type I membrane protein which contains an Ig-like V-type (immunoglobulin-like) domain (Klesney-Tait, et al., 2006). In contrast to TREM2, TREML2 does not associate with DAP12, which contains a canonical immunoreceptor tyrosine-based activation motif (ITAM) in its cytoplasmic domain. Thus, TREML2 does not exhibit features for classical tyrosine-based signaling (Klesney-Tait, et al., 2006). Previous studies have shown that TREML2 is up-regulated in response to inflammation in macrophages, suggesting a potential role in pro-inflammatory responses (King, et al., 2006). However, the function of TREML2 in microglia has not been carefully demonstrated.
As TREM2 and TREML2 appear to differentially affect the risks of AD, in the present study we aim to explore the functional relationship between TREM2 and TREML2 in regulating microglial activation in AD pathogenesis. We found that lipopolysaccharide (LPS) stimulation significantly suppressed TREM2 but increased TREML2 levels in mouse brain and in primary microglia. Modulation of TREM2 or TREML2 levels affects inflammatory responses in the opposite directions. Our studies generated novel insights into the mechanisms of TREM2 and TREML2 in AD pathogenesis and should help to design targeted therapy for AD.
EXPERIMENTAL PROCEDURES
Reagents
LPS and TREML2 siRNA were purchased from Sigma Aldrich. Aβ42 peptide was purchased from AnaSpec. Oligomeric Aβ42 was prepared as previously described (Huang, et al., 2015) from the Proteomics Core at the Mayo Clinic. Briefly, aliquots of 100 μM Aβ monomer purified by size exclusion chromatography were incubated overnight at room temperature in 50 mM NaCl and 4 mM SDS. To remove the SDS and reduce the salt concentration, the sample was dialyzed against 20 mM sodium phosphate buffer at pH 7.0 (NaP) for 48–72 hours and then against 10 mM NaP. Sample quality was monitored and confirmed at each step of the preparation by circular dichroism (CD) and thioflavin T fluorescence. Residual or unconverted monomer was removed by filtering the dialyzed oligomer with an Amicon Ultra 4 centrifugal concentration/filtration device with a MW cutoff of 50 kDa.
Animals
All animal procedures were approved by the Mayo Clinic Institutional Animal Care and Use Committee (IACUC) and were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Wild-type (C57BL/6J) mice were purchased from Jackson Laboratory. TREM2 knockout mice (Trem2−/−; C57BL/6N) and the corresponding control C57BL/6N mice were obtained from the UC Davis Knockout Mouse Project (KOMP) repository. These Trem2−/− mice were originally generated by Velocigene as a “definitive null” and have a LacZ reporter cassette that replaces the entire coding region of the Trem2 locus. This line is identical to the line recently reported (Jay, et al., 2015).
LPS administration and tissue processing
C57BL/6N mice were intraperitoneally injected with LPS (2 μg/g body weight) at 9 weeks of age. Animals were deeply anesthetized with pentobarbital prior to cardiac perfusion with phosphate-buffered saline to expunge vascular components from the tissue at 4 and 24 hours post injection. Saline injection at 0 hour time-point was also conducted as a control. Hemi brain tissues were quickly isolated, frozen on dry ice and stored at −80°C until further processing. Tissues were briefly sonicated in Tris-buffered saline with EDTA (TBSE) (50 mM Tris, pH7.5, 150mM NaCl, 1mM EDTA) with 1× protease and phosphatase inhibitors (Thermo Scientific, Waltham, MA). An aliquot of sonicated tissue suspension was immediately placed into Trizol for RNA isolation using the Direct-zol RNA kit according to the manufacturer’s instructions (Zymo Research, Irvine, CA).
Primary microglia culture
Primary microglial cells were prepared as described previously (Liu, et al., 1994, Zhu, et al., 2010) with minor modifications. Briefly, mixed glial cells from newborn (postnatal 1 to 3 day old) C57BL/6J pups were cultured in DMEM supplemented with 10% FBS and 100 U/ml penicillin/streptomycin in a poly-D-lysine (Sigma Aldrich)-coated cell culture flasks (Corning, Fisher). The medium was changed the next day with fresh DMEM medium plus 10% FBS and 25 ng/ml GM-CSF (R&D System). Microglia cells were harvested by shaking after 10–12 days in culture as described (Zhu, et al., 2010). The isolated microglia were subjected to TREM2 or TREML2 knockdown by electroporation, or plated for LPS or oligomeric Aβ treatments.
TREM2 or TREML2 knockdown by siRNA
Knockdown of TREM2 or TREML2 with TREM2 or TREML2 specific siRNAs in microglia was carried out by electroporation using an Amaxa Nucleofector, and a glial specific Nucleofector kit (Lonza) according to the manufacturer’s instructions. Each electroporation reaction contained 4 × 106 cells and 200 nM siRNA. Transfected cells were plated and used for LPS treatments or proliferation assays. The siRNA sequences for TREM2 were as follows: Trem2 siRNA1: 5′-CCAGUCCUUGAGGGUGUCAUGUACU-3′; Trem2 siRNA2: 5′-ACCCUUGCUGG AACCGUCACCAUCA-3′.
Reverse transcription and quantitative real-time PCR (qRT-PCR)
Total RNA was isolated from tissues or cells using Direct-zol RNA kit or NucleoSpin RNA II (Clontech) according to the manufacturer’s instructions. Total RNA was dissolved in nuclease-free water and stored at −80°C. Reverse transcription was performed using a SuperScript III First-Strand Synthesis System (Invitrogen), and the resulting cDNA was used for quantitative real-time PCR. The set of actin primers was used as an internal control for each specific gene amplification. The relative levels of expression were quantified and analyzed by using Bio-Rad iCycler iQ software (Bio-Rad). The real-time value for each sample was averaged and compared using the CT method, where the amount of target RNA (2–ΔΔCT) was first normalized to the endogenous actin reference (ΔCT) and then normalized against control levels. The primer sequences for TREM2, TREML2, interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α) and β-actin were as follows: Trem2-Forward: 5′-TCATAGGGGCAAGACACCT-3′; Trem2-Reverse: 5′-GCTGCTCATCTTACTCT TTGTC-3′; Trem12-Forward: 5′-TGGTGGTGGTGTTGACATTTCTTCC-3′; Trem12-Reverse: 5′-ATCCAGGGTTTAGCATAGTTGCTGC-3′; Il-1β-Forward: 5′-CCTGCAGCTGGAGAGTGTGGAT -3′; Il-1β-Reverse: 5′-TGTGCTCTGCTTGTGAGGTGCT-3′; TNF-α-Forward: 5′-AGCCCACGTCGTAG CAAACCAC-3′; TNF-α-Reverse: 5′-AGGTACAACCCATCGGCTGGCA-3′; β-actin-Forward: 5′-AGTGTGACGTTGACATCCGTA-3′; β-actin-Reverse: 5′-GCCAGAGCAGTAA TCTCCTTC-3′.
BrdU incorporation assay
BrdU incorporation assays were performed using a BrdU cell proliferation ELISA kit (Abcam) according to the manufacturer’s protocol. Briefly, microglia were seeded at a density of 1 × 104 cells/well, and BrdU was added to the cells for 18 hours for incorporation. Then the cells were fixed, permeabilized and denatured to enable the detection of incorporated BrdU with anti-BrdU antibody. Following incubation with horseradish peroxidase-conjugated secondary antibody, the colored reaction product was quantified using a spectrophotometer.
ELISA for Cytokines
The protein levels of murine IL-1β and TNF-α in the medium were detected by enzyme-linked immunosorbent assay (ELISA) using commercial ELISA kits (R&D). Experiments were performed according to the manufacturer’s guidelines and quantified by the microplate reader at 450 nm (Multimode Reader, Thermo scientific).
Fluorescence imaging of Aβ42
The intracellular accumulation of Aβ was visualized using confocal microscopy following treatment with fluorescent (FAM)-labeled Aβ42 (Anaspec, USA). Microglial cells (5 × 105) were seeded on poly-D-lysine coated glass cover slips and incubated with 500 nM of FAM-labeled Aβ42 in serum-free medium for 2 hr. The cells were then washed with PBS and fixed with 4% formaldehyde. The cells were washed three times with PBS and mounted on glass cover slips. The images were captured with ZEISS LSM 510 confocal microscope (Carl Zeiss, Jena, Germany).
MTT Assays
Cell viability was determined by the 3- (4,5)-dimethylthiahiazo (-z-y1)-3,5-di-phenytetrazoliumromide (MTT, Roche, Mannheim, Germany) assay. Briefly, cells were seeded in 96-well plates at a density of 3 × 104 cells per well. Following exposing to indicated concentrations of Aβ oligomers for 24 hr, 20 μl of 5 mg/ml MTT solution dissolved in PBS was added to each well and incubated at 37°C for 4 hr. The generated formazan was dissolved in dimethyl sulfoxide (DMSO) and the absorbance of the solution was measured at 490 nm to determine the cell viability.
Statistical analysis
All data are shown as mean ± SEM. Statistical significance was determined with an Unpaired t test, a one-way ANOVA test, or a two-way ANOVA test as specified in each experiment (GraphPad Prism 5), and p< 0.05 was considered significant.
RESULTS
Opposing effects on the expression of TREM2 and TREML2 in microglia and mouse brain in response to LPS
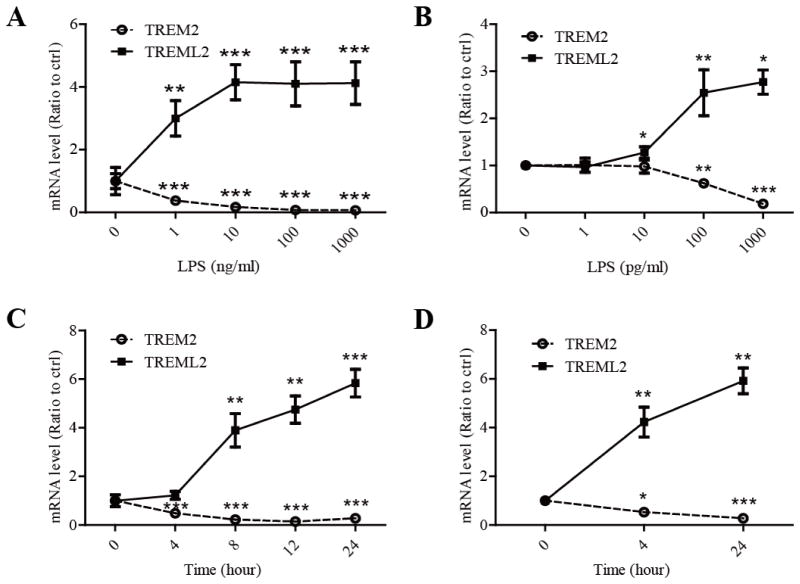
Neuro-inflammation is a prominent feature of AD (Heneka, et al., 2015). To explore the role of TREM2 and TREML2 in the regulation of neuro-inflammation, we first analyzed their expression levels by real-time PCR in primary microglia in response to LPS, an immuno-stimulator. Intriguingly, treatment of primary microglia with LPS significantly suppressed the expression of TREM2 but increased the expression of TREML2 in a dose-dependent manner (Fig. 1A). We further examined whether a lower dosage of LPS can induce such effects. We found that 100 pg/ml of LPS was sufficient to decrease TREM2 and increase TREML2 mRNA levels in microglia (Fig. 1B). Moreover, when microglia were treated with LPS (10 ng/ml) for different periods of time, TREM2 mRNA levels were decreased whereas TREML2 mRNA levels were increased in a time-dependent manner (Fig. 1C).
FIGURE 1. Opposing effects of LPS on TREM2 and TREML2 in microglia and mouse brains.
A and B, Primary mouse microglia were treated with or without LPS (at the indicated concentrations) for 8 hours. A, LPS dramatically suppressed TREM2, but enhanced TREML2 mRNA levels in a dose-dependent manner as measured by qRT-PCR. B, Low-dose of LPS significantly decreased TREM2 and increased TREML2 mRNA levels. C, LPS (10 ng/ml) decreased TREM2 and increased TREML2 mRNA levels in a time-dependent manner in mouse primary microglia. D, TREM2 mRNA levels were decreased whereas TREML2 mRNA levels were increased in the brains of mouse treated with LPS (2 μg/g body weight) for 4 or 24 hours. Data are plotted as mean ± SEM (n = 3). Compared to LPS untreated group *, p < 0.05; **, p < 0.01; ***, p < 0.001 (t-tests).
To further examine the effect of LPS on TREM2 and TREML2 levels in vivo, C57BL/6J mice were intraperitoneally injected with LPS (2 μg/g body weight) and the PBS perfused brains were harvested for RNA extraction. Consistent with our in vitro results, LPS stimulation significantly suppressed TREM2 mRNA levels but promoted TREML2 mRNA levels in the brain when compared with saline-treated control groups (Fig. 1D). The opposing expression patterns of TREM2 and TREML2 in response to LPS suggest that they may play different roles in microglial functions.
TREM2 down-regulation in primary microglia enhances the levels of TREML2 and pro-inflammatory cytokines in response to LPS
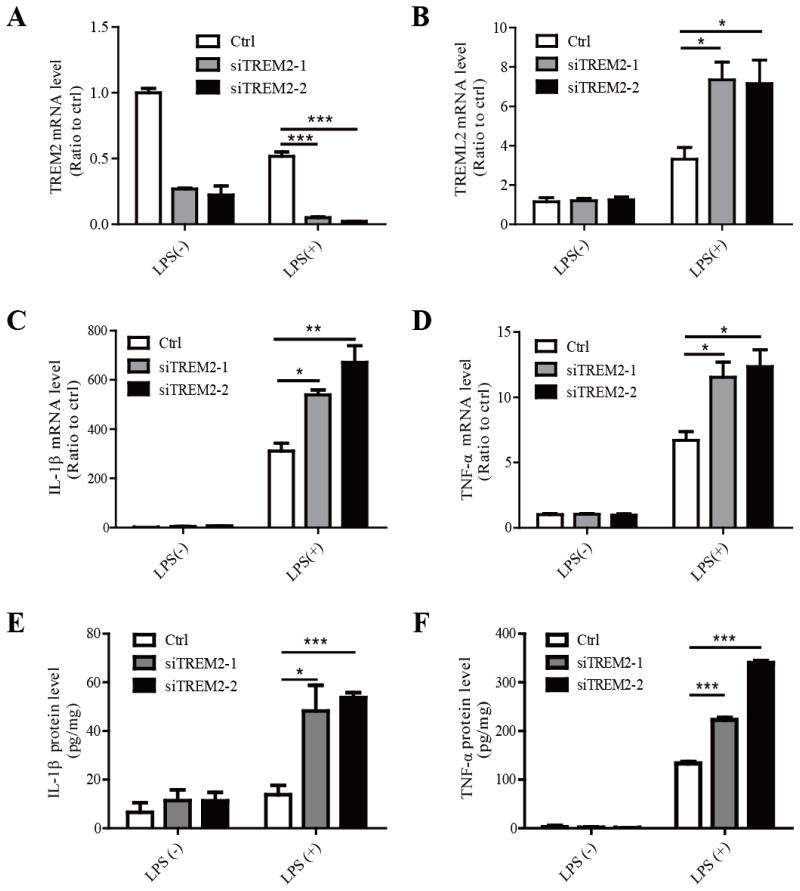
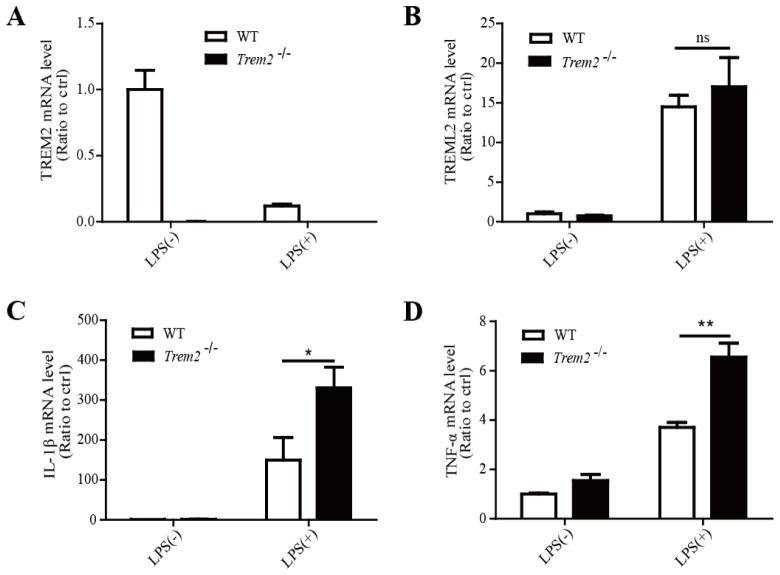
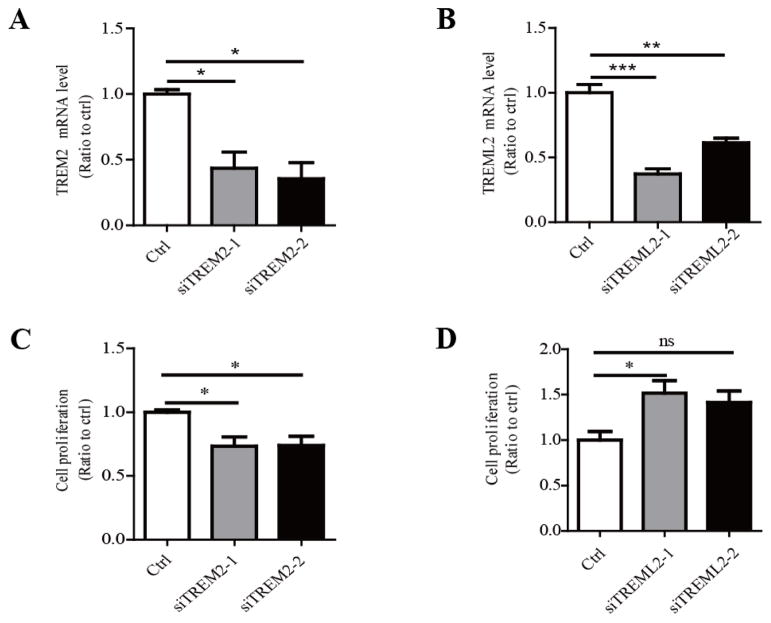
TREM2 has previously been shown to inhibit the production of inflammatory cytokines in macrophages (Takahashi, et al., 2005, Turnbull, et al., 2006). Thus, we examined the effects of TREM2 on the production of inflammatory cytokines in microglia. Using two independent siRNAs targeting distinct regions of TREM2, TREM2 expression levels were successfully reduced in microglia (Fig. 2A). Interestingly, knockdown of TREM2 significantly increased the expression of TREML2 in response to LPS compared to controls, suggesting that TREM2 might regulate microglial functions by modulating the expression of TREML2 (Fig. 2B). We found that the mRNA levels of two pro-inflammatory cytokines, IL-1β and TNF-α, were significantly increased in TREM2-knockdown (TREM2-KD) microglia treated with 10 ng/ml LPS, although no obvious change was observed in the absence of LPS (Fig. 2C and 2D). Moreover, the protein levels of IL-1β and TNF-α in the media were obviously increased in TREM2-KD microglia compared to those of the control group (Fig. 2E and 2F). To further confirm the observed effects of TREM2-KD microglia in response to LPS, primary microglia from wild-type (WT) control or Trem2−/− mice were treated with or without LPS (10 ng/ml) for 8 hours. The absence of TREM2 expression in the microglia from the Trem2−/− mice as well as the LPS-induced reduction of TREM2 mRNA in WT cells were verified by qRT-PCR (Fig. 3A). However, we did not observe a significant change in TREML2 mRNA level in Trem2−/− microglia compared to WT microglia either in the presence or absence of LPS treatment (Fig. 3B). Whether long-term deficiency of TREM2 compromises the responsiveness of TREML2 in Trem2−/− mice requires further investigation. Consistent with the results in TREM2-KD microglia, IL-1β and TNF-α were dramatically increased in Trem2−/− microglia treated with 10 ng/ml LPS (Fig. 3C and 3D). These results indicate that suppression of TREM2 in microglia amplifies the production of pro-inflammatory cytokines in response to LPS. In addition, TREM2 might modulate the function of microglia through regulating the expression of TREML2 in the presence of LPS stimulation.
FIGURE 2. Knockdown of TREM2 in primary mouse microglia increases TREML2 expression and sensitizes microglia in response to LPS.
Primary microglia were transfected with two independent TREM2 siRNAs (siTREM2-1 and siTREM2-2) and treated with or without LPS (10 ng/ml) for 8 hours. A, The reduction of TREM2 mRNA levels upon siRNA knockdown was verified by qRT-PCR. B, TREML2 mRNA levels were increased in TREM2 knockdown microglia treated with LPS. C, IL-1β mRNA levels were significantly enhanced in TREM2 knockdown microglia in response to LPS treatments. D, TNF-α mRNA levels were significantly enhanced in TREM2 knockdown microglia in response to LPS treatments. E, The IL-1β protein levels were significantly increased in the medium of TREM2 knockdown microglia in response to LPS treatments. F, The TNF-α protein levels were significantly enhanced in the medium of TREM2 knockdown microglia in response to LPS treatments. Data are plotted as mean ± SEM (n = 3). Compared to the corresponding control (ctrl) group *, p < 0.05; **, p < 0.01; ***, p < 0.001 (Two-Way ANOVA with post-hoc Tukey’s t-test).
FIGURE 3. Primary microglia from TREM2-knockout mice are hypersensitive to LPS stimulation.
Primary microglia from WT control or Trem2−/− mice were treated with or without LPS (10 ng/ml) for 8 hours. A, The absence of TREM2 expression in the microglia from the Trem2−/− mice as well as the LPS-induced reduction of TREM2 mRNA in WT cells were verified by qRT-PCR. B, TREML2 mRNA levels were not changed in Trem2−/− microglia compared to WT cells with or without LPS treatment. C, IL-1β mRNA levels were significantly increased in Trem2−/− microglia compared to WT cells in response to LPS treatment. D, TNF-α mRNA levels were significantly enhanced in Trem2−/− microglia compared to WT cells in response to LPS treatment. Data are plotted as mean ± SEM (n = 3). Compared to WT microglia treated with LPS *, p < 0.05; ***, p < 0.001; ns, not significant (Two-Way ANOVA with post-hoc Tukey’s t-test).
TREML2 knockdown leads to decreased cytokine levels in response to LPS
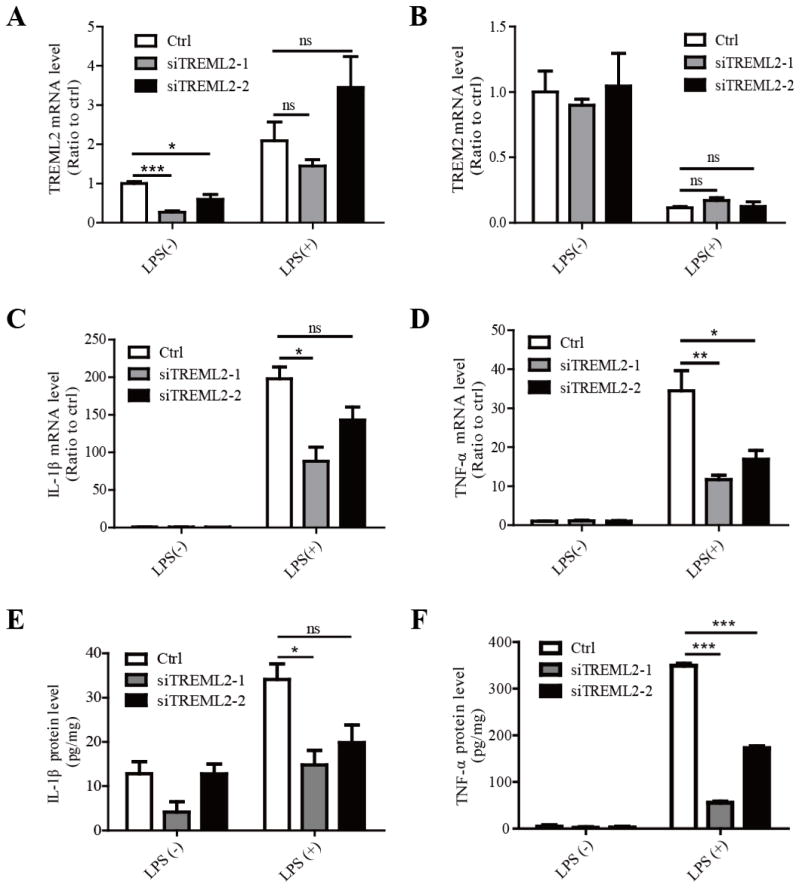
Inspired by the opposite expression patterns of TREM2 and TREML2 in microglia in response to LPS stimulation, we next examined the effects of TREML2 down-regulation on inflammatory responses. TREML2 expression was successfully knocked down in microglia using two independent siRNAs targeting distinct regions of TREML2 (Fig. 4A). TREML2 knockdown did not alter the mRNA levels of TREM2 in the presence or absence of LPS treatment (Fig. 4B). In addition, knockdown of TREML2 in microglia significantly suppressed the mRNA levels of IL-1β and TNF-α in the presence of LPS compared to the control group, whereas there was no significant effect in the absence of LPS treatment (Fig. 4C and 4D). The protein levels of IL-1β and TNF-α released from TREML2-KD microglia were dramatically decreased compared to the control group (Fig. 4E and 4F). Together, our results demonstrated that TREM2 and TREML2 have differential functions in modulating immune responses in microglia.
FIGURE 4. Knockdown of TREML2 in primary mouse microglia does not affect TREM2 expression but decreases the microglial sensitivity to LPS.
Primary mouse microglia were transfected with two independent TREML2 siRNAs (siTREML2-1 and siTREML2-2) and treated with or without LPS (10 ng/ml) for 8 hours. A, Reduction of TREML2 mRNA upon siRNA-mediated knockdown was verified by qRT-PCR. B, TREM2 mRNA levels were not changed in TREML2-knockdown microglia treated with LPS. C, IL-1β mRNA levels were significantly decreased in TREML2 knocked down microglia in response to LPS treatments. D, TNF-α mRNA levels were significantly decreased in TREML2-knockdown microglia in response to LPS treatments. E, The IL-1β protein levels were significantly down-regulated in the medium of TREML2-knockdown microglia in response to LPS treatments. F, The TNF-α protein levels were significantly decreased in the medium of TREML2-knockdown microglia in response to LPS treatments. Data are plotted as mean ± SEM (n = 3). Compared to the corresponding control (ctrl) group *, p < 0.05; **, p < 0.01; ***, p < 0.001; ns, not significant (Two-Way ANOVA with post-hoc Tukey’s t-test).
Opposite effects of decreased TREM2 or TREML2 expression on the proliferation of microglia
In addition to the immuno-regulatory effects in the central nervous system (CNS), microglia can quickly proliferate to defend against insults (Kettenmann, et al., 2011). To investigate whether TREM2 and TREML2 differentially regulate microglial proliferation, we knocked down TREM2 or TREML2 in primary microglia with specific siRNAs (Fig. 5A and 5B) and examined their effects on the proliferation of microglia (Fig. 5C and 5D) with a BrdU incorporation assay. We found that TREM2 knockdown significantly suppressed microglia proliferation (Fig. 5B), whereas TREML2 knockdown increased microglia proliferation (Fig. 5D). We further confirmed this effect on the growth of microglia by directly counting the cell numbers upon TREM2 or TREML2 down-regulation (Supplementary Fig. 1). These results demonstrate that TREM2 and TREML2 play opposing roles in microglia proliferation.
FIGURE 5. TREM2 and TREML2 regulate the proliferation of microglia in the opposite direction.
A, TREM2 knockdown with two independent siRNAs in mouse primary microglia was confirmed by qRT-PCR. B, TREML2 knockdown with two independent siRNAs in mouse primary microglia was confirmed by qRT-PCR. C, TREM2 knockdown suppressed proliferation of microglia examined by the BrdU incorporation assay. D, TREML2 knockdown resulted in increased proliferation of microglia examined by the BrdU incorporation assay. Data are plotted as mean ± SEM (n = 3). Compared to control (ctrl) group **, p < 0.01; ***, p < 0.001; ns, not significant (One-Way ANOVA with post-hoc Tukey’s t-test).
Opposite changes in the expression of TREM2 and TREML2 upon treatment of microglia with oligomeric Aβ42
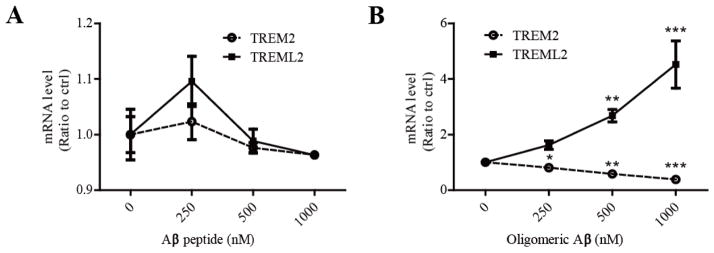
It has been shown that microglia numbers surrounding the amyloid plaques are increased in AD and in the APPV717F AD mouse model (Mitrasinovic and Murphy, 2002). Aβ is one of the crucial pathological molecules that trigger microglial activation in AD. We thus examined whether Aβ treatment affects TREM2 and TREML2 expression in microglia. The phagocytosis of Aβ by microglia was visualized using fluorescent (FAM)-labeled Aβ42 (Supplementary Fig. 2). As controls, we did not observe significant differences in the cell viability between the control and the Aβ-treated groups (Supplementary Fig. 3). We treated primary microglia with Aβ42 peptide (which contains primarily monomers) or oligomeric Aβ42 for 8 hours and examined the levels of TREM2 and TREML2 by real-time PCR. Interestingly, we found that the oligomeric Aβ42, the species that was shown to be more toxic to synapses (Nimmrich and Ebert, 2009, Tu, et al., 2014), significantly decreased TREM2 mRNA levels but increased TREML2 mRNA levels in a dose-dependent manner, whereas Aβ42 peptide had no significant effects on their levels (Fig. 6A and 6B). These results demonstrate that oligomeric Aβ differentially affects TREM2 and TREML2 levels, suggesting that oligomeric Aβ induced alterations in microglial TREM2 and TREML2 expression may contribute to AD pathogenesis.
FIGURE 6. Oligomeric Aβ, but not monomeric Aβ, alters the mRNA levels of TREM2 and TREML2.
A, Monomeric Aβ did not affect the mRNA levels of TREM2 or TREML2 analyzed by qRT-PCR. B, Oligomeric Aβ significantly decreased TREM2 but increased TREML2 mRNA levels in a dose-dependent manner. Data are plotted as mean ± SEM (n = 3). Compared to the untreated group **, p < 0.01. ***, p < 0.001 (One-Way ANOVA with post-hoc Tukey’s t-test).
DISCUSSION
Microglia, which account for approximately 20% of the glial cell population and around 5% of the total cell population in the cerebral cortex of mice, are the primary innate immune effector cells in the CNS (Aguzzi, et al., 2013, Block, et al., 2007, Lawson, et al., 1990, Polazzi and Monti, 2010). Microglia are constantly surveying their immediate environment for pathogens, foreign materials, and apoptotic cells (Streit, 2004). Upon injury, microglia rapidly proliferate, migrate to the lesion sites, recognize the pathogen, ramify, and mount an immune response (Ransohoff and Perry, 2009). In the brains of both AD patients and amyloid model mice, microglia are found to be closely associated with the amyloid plaques and exhibit an ‘activated’ pro-inflammatory phenotype (Frautschy, et al., 1998, Landel, et al., 2014, Lee and Landreth, 2010, Perlmutter, et al., 1990). As such, investigating the biological mechanisms that regulate microglial functions is essential for understanding the potential etiology and for developing future therapeutic strategies for AD.
Recent studies identified rare variants of TREM2 that increased the risk for AD, whereas a missense variant in TREML2 protected against AD (Benitez, et al., 2014, Guerreiro, et al., 2013, Jonsson, et al., 2013). Both TREM2 and TREML2 are expressed on the cells of myeloid lineages including microglia. They belong to the TREM receptor family, which modulates the innate immune response by either amplifying or dampening Toll-like receptor (TLR)-induced signals and thereby playing a critical role in the regulation of inflammatory responses (Ford and McVicar, 2009, King, et al., 2006, Klesney-Tait, et al., 2006). As an important innate immune receptor in the brain, TREM2 couples with DAP12 to initiate signaling (Jiang, et al., 2013, Neumann and Takahashi, 2007). Distinct from TREM2, TREML2 is capable of recruiting SH3 domain-containing effector proteins with its cytoplasmic tail to mediate signal transduction (Klesney-Tait, et al., 2006). However, the specific roles of TREM2 and TREML2 in microglia and their functional relationship to the pathogenesis of AD remain unclear. In the current study, we demonstrated that Aβ and LPS substantially decreased TREM2 mRNA levels but increased TREML2 mRNA levels in microglia. This effect is consistent with a previous study in which microglia were treated with IL-1α (Benitez, et al., 2014). The opposing mRNA expression patterns of TREM2 and TREML2 in response to stimuli suggest their critical but different functions in microglia.
Studies concerning the function of the TREM gene cluster that encodes a group of trans-membrane proteins have revealed that they are important components in the innate and adaptive immunity (Colonna, 2003). Only four of the trans-membrane receptors, TREM1, TREM2, TREML1, and TREML2, have direct homologs in mice and humans. TREM2 was down-regulated after LPS treatment in mouse macrophages (Gao, et al., 2013, Turnbull, et al., 2006). TREM2 deletion in murine alveolar macrophages resulted in enhanced expression of TLR-4, TNF-α and IL-10 in response to LPS (Gao, et al., 2013). In contrast, TREM2 over-expression in microglia leads to a reduced production of TNF-α and inducible nitric oxide synthase (iNOS) (Takahashi, et al., 2005, Turnbull, et al., 2006). Interestingly, TREML2 up-regulation has been shown in both neutrophils and macrophages in response to inflammatory stimuli (King, et al., 2006). As TREML2 and TREM2 respond differently to the stimuli, it is critical to understand the function of TREML2 in microglia. Here, we demonstrated opposing roles of TREM2 and TREML2 in the regulation of microglial functions. Knockdown of TREM2 in microglia led to an increase of pro-inflammatory cytokines such as IL-1β and TNF-α in the presence of LPS, indicating the ability of TREM2 to promote an anti-inflammatory phenotype in microglia. In contrast, TREML2 down-regulation resulted in a reduction of pro-inflammatory responses in microglia. Intriguingly, knockdown of TREM2 enhanced TREML2 levels in the presence of LPS, suggesting that TREM2 may modulate the inflammatory response at least partially by altering the expression of TREML2. A previous study showed that TREM and TREM-like receptors modulate the innate immune response by either amplifying or dampening TLR-induced signals (Ford and McVicar, 2009). In addition, TREM2-deficient dendritic cells showed increased inflammation in response to TLR agonists such as LPS, CpG, DNA, and Zymosan (Ito and Hamerman, 2012). Whether TREM2 and TREML2 affect the activation of TLR-mediated signaling in microglia in an opposite manner requires further investigation.
It is known that microglia proliferate and actively migrate to lesions by following chemotactic gradients in response to injury signals (Kettenmann, et al., 2011). In the AD brain and amyloid mouse models, microglia have been shown to proliferate and accumulate around amyloid plaques likely for the purpose of limiting plaque expansion (Mosher and Wyss-Coray, 2014). Our results demonstrated that TREM2 and TREML2 modulated the proliferation of microglia in an opposite manner in which knockdown of TREM2 suppressed but knockdown of TREML2 promoted microglial proliferation. Dysregulation of TREM2 and TREML2 may result in aberrant microglial proliferation and compromise the ability of microglia to response to immune stimuli.
Microglia secretes cytokines, such as TNF-α and IL-1β, which have both been shown to promote neuronal damage when present in excessive amounts (Monif, et al., 2010, Rossi, et al., 2014). Our results showed that LPS suppressed TREM2 but increased TREML2 mRNA levels, thus promoting microglia to pro-inflammatory status. Furthermore, down-regulation of TREM2 sensitized microglia, enhancing the production of pro-inflammatory cytokines in response to LPS. Thus, aberrant expression and functions of TREM2 and/or TREML2 may contribute to abnormal neuro-inflammatory responses in AD. It is possible that the inflammatory cytokines in AD brains further down-regulate TREM2 expression, thus initiating a vicious cycle in which abnormal neuro-inflammation and impaired microglial functions synergistically promote AD pathogenesis.
TREM2 regulates cell proliferation, differentiation, survival and inflammation by signaling through its adaptor protein DAP12 to activate various signaling pathways (Otero, et al., 2012, Peng, et al., 2010, Sun, et al., 2013). Our study demonstrated that TREML2, which does not require DAP12 for its signaling, promotes the activation of microglia. TREML2 has been suggested to interact with SH3 or WW domain-containing effector proteins either constitutively or in response to ligand binding (Ford and McVicar, 2009, Klesney-Tait, et al., 2006). Distinct from TREM2, we found that TREML2 suppresses cell proliferation and promotes the inflammation of microglia. Thus, it will be interesting to further understand the molecular pathways by which TREML2 modulates the functions of microglia.
Taken together, our findings demonstrate the opposing roles of TREM2 and TREML2 in the critical functions of microglia. TREM2 and TREML2 are likely fine-tuning the inflammatory responses in response to stimuli and regulating the proliferation of microglia. Our studies provide novel insights into the pathological mechanisms of TREM2 and TREML2 in microglia-related functions and might inspire novel approaches for ameliorating the chronic inflammation induced by microglia activation in neurodegenerative diseases such as AD.
Supplementary Material
Highlights.
LPS suppressed TREM2 but stimulated TREML2 in microglia.
TREM2 and TREML2 regulate microglial proliferation and neuro-inflammation in opposite directions.
TREM2 and TREML2 play opposing roles in regulating microglial functions.
Acknowledgments
This work was supported by the Education Department of Fujian Province, China (to H.Z.) and NIH grants R01AG027924, R01AG035355, R01AG046205 (to G.B.) and grants (81100842 to H.Z., U1505227 to G.B.) from the National Natural Science Foundation of China.
The abbreviations used are
- AD
Alzheimer’s disease
- TREM2
Triggering receptor expressed on myeloid cells 2
- TREML2
TREM-like transcript 2
- DAP12
DNAX-activating protein of 12 kDa
Footnotes
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Author contributions
H.Z and CC.L designed, performed, analyzed the experiments and wrote the paper. YW.Z, X.X and G.B conceived the study. S.S.K, J.D.F, L.J, W.H and X.Z coordinated the study. T.L.R and Y.A provided technical assistance. L.J and L.Y contributed to the preparation of the figures. X.C revised the paper. All authors reviewed the results and approved the final version of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguzzi A, Barres BA, Bennett ML. Microglia: scapegoat, saboteur, or something else? Science. 2013;339(6116):156–61. doi: 10.1126/science.1227901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitez BA, Cruchaga C. TREM2 and neurodegenerative disease. N Engl J Med. 2013;369(16):1567–8. doi: 10.1056/NEJMc1306509#SA4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitez BA, Jin SC, Guerreiro R, Graham R, Lord J, Harold D, Sims R, Lambert JC, Gibbs JR, Bras J, Sassi C, Harari O, Bertelsen S, Lupton MK, Powell J, Bellenguez C, Brown K, Medway C, Haddick PC, van der Brug MP, Bhangale T, Ortmann W, Behrens T, Mayeux R, Pericak-Vance MA, Farrer LA, Schellenberg GD, Haines JL, Turton J, Braae A, Barber I, Fagan AM, Holtzman DM, Morris JC, Williams J, Kauwe JS, Amouyel P, Morgan K, Singleton A, Hardy J, Goate AM, Cruchaga C. Missense variant in TREML2 protects against Alzheimer’s disease. Neurobiol Aging. 2014;35(6):1510, e19–26. doi: 10.1016/j.neurobiolaging.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8(1):57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- Colonna M. TREMs in the immune system and beyond. Nat Rev Immunol. 2003;3(6):445–53. doi: 10.1038/nri1106. [DOI] [PubMed] [Google Scholar]
- Ford JW, McVicar DW. TREM and TREM-like receptors in inflammation and disease. Current opinion in immunology. 2009;21(1):38–46. doi: 10.1016/j.coi.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frautschy SA, Yang F, Irrizarry M, Hyman B, Saido TC, Hsiao K, Cole GM. Microglial response to amyloid plaques in APPsw transgenic mice. Am J Pathol. 1998;152(1):307–17. [PMC free article] [PubMed] [Google Scholar]
- Gao X, Dong Y, Liu Z, Niu B. Silencing of triggering receptor expressed on myeloid cells-2 enhances the inflammatory responses of alveolar macrophages to lipopolysaccharide. Mol Med Rep. 2013;7(3):921–6. doi: 10.3892/mmr.2013.1268. [DOI] [PubMed] [Google Scholar]
- Guerreiro R, Hardy J. TREM2 and neurodegenerative disease. N Engl J Med. 2013;369(16):1569–70. doi: 10.1056/NEJMc1306509. [DOI] [PubMed] [Google Scholar]
- Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, Cruchaga C, Sassi C, Kauwe JS, Younkin S, Hazrati L, Collinge J, Pocock J, Lashley T, Williams J, Lambert JC, Amouyel P, Goate A, Rademakers R, Morgan K, Powell J, St George-Hyslop P, Singleton A, Hardy J Alzheimer Genetic Analysis G. TREM2 variants in Alzheimer’s disease. N Engl J Med. 2013;368(2):117–27. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamerman JA, Jarjoura JR, Humphrey MB, Nakamura MC, Seaman WE, Lanier LL. Cutting edge: inhibition of TLR and FcR responses in macrophages by triggering receptor expressed on myeloid cells (TREM)-2 and DAP12. J Immunol. 2006;177(4):2051–5. doi: 10.4049/jimmunol.177.4.2051. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, Jacobs AH, Wyss-Coray T, Vitorica J, Ransohoff RM, Herrup K, Frautschy SA, Finsen B, Brown GC, Verkhratsky A, Yamanaka K, Koistinaho J, Latz E, Halle A, Petzold GC, Town T, Morgan D, Shinohara ML, Perry VH, Holmes C, Bazan NG, Brooks DJ, Hunot S, Joseph B, Deigendesch N, Garaschuk O, Boddeke E, Dinarello CA, Breitner JC, Cole GM, Golenbock DT, Kummer MP. Neuroinflammation in Alzheimer’s disease. The Lancet Neurology. 2015;14(4):388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh CL, Koike M, Spusta SC, Niemi EC, Yenari M, Nakamura MC, Seaman WE. A role for TREM2 ligands in the phagocytosis of apoptotic neuronal cells by microglia. Journal of neurochemistry. 2009;109(4):1144–56. doi: 10.1111/j.1471-4159.2009.06042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Zimmerman MI, Martin PK, Nix AJ, Rosenberry TL, Paravastu AK. Antiparallel beta-Sheet Structure within the C-Terminal Region of 42-Residue Alzheimer’s Amyloid-beta Peptides When They Form 150-kDa Oligomers. Journal of molecular biology. 2015;427(13):2319–28. doi: 10.1016/j.jmb.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H, Hamerman JA. TREM-2, triggering receptor expressed on myeloid cell-2, negatively regulates TLR responses in dendritic cells. Eur J Immunol. 2012;42(1):176–85. doi: 10.1002/eji.201141679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay TR, Miller CM, Cheng PJ, Graham LC, Bemiller S, Broihier ML, Xu G, Margevicius D, Karlo JC, Sousa GL, Cotleur AC, Butovsky O, Bekris L, Staugaitis SM, Leverenz JB, Pimplikar SW, Landreth GE, Howell GR, Ransohoff RM, Lamb BT. TREM2 deficiency eliminates TREM2+ inflammatory macrophages and ameliorates pathology in Alzheimer’s disease mouse models. The Journal of experimental medicine. 2015;212(3):287–95. doi: 10.1084/jem.20142322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T, Yu JT, Zhu XC, Tan L. TREM2 in Alzheimer’s disease. Mol Neurobiol. 2013;48(1):180–5. doi: 10.1007/s12035-013-8424-8. [DOI] [PubMed] [Google Scholar]
- Jiang T, Yu JT, Zhu XC, Tan MS, Gu LZ, Zhang YD, Tan L. Triggering receptor expressed on myeloid cells 2 knockdown exacerbates aging-related neuroinflammation and cognitive deficiency in senescence-accelerated mouse prone 8 mice. Neurobiol Aging. 2014;35(6):1243–51. doi: 10.1016/j.neurobiolaging.2013.11.026. [DOI] [PubMed] [Google Scholar]
- Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J, Bjornsson S, Huttenlocher J, Levey AI, Lah JJ, Rujescu D, Hampel H, Giegling I, Andreassen OA, Engedal K, Ulstein I, Djurovic S, Ibrahim-Verbaas C, Hofman A, Ikram MA, van Duijn CM, Thorsteinsdottir U, Kong A, Stefansson K. Variant of TREM2 associated with the risk of Alzheimer’s disease. N Engl J Med. 2013;368(2):107–16. doi: 10.1056/NEJMoa1211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91(2):461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- King RG, Herrin BR, Justement LB. Trem-like transcript 2 is expressed on cells of the myeloid/granuloid and B lymphoid lineage and is up-regulated in response to inflammation. J Immunol. 2006;176(10):6012–21. doi: 10.4049/jimmunol.176.10.6012. [DOI] [PubMed] [Google Scholar]
- Klesney-Tait J, Turnbull IR, Colonna M. The TREM receptor family and signal integration. Nature immunology. 2006;7(12):1266–73. doi: 10.1038/ni1411. [DOI] [PubMed] [Google Scholar]
- Landel V, Baranger K, Virard I, Loriod B, Khrestchatisky M, Rivera S, Benech P, Feron F. Temporal gene profiling of the 5XFAD transgenic mouse model highlights the importance of microglial activation in Alzheimer’s disease. Molecular neurodegeneration. 2014;9:33. doi: 10.1186/1750-1326-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson LJ, Perry VH, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39(1):151–70. doi: 10.1016/0306-4522(90)90229-w. [DOI] [PubMed] [Google Scholar]
- Lee CY, Landreth GE. The role of microglia in amyloid clearance from the AD brain. J Neural Transm. 2010;117(8):949–60. doi: 10.1007/s00702-010-0433-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Brosnan CF, Dickson DW, Lee SC. Macrophage colony-stimulating factor mediates astrocyte-induced microglial ramification in human fetal central nervous system culture. Am J Pathol. 1994;145(1):48–53. [PMC free article] [PubMed] [Google Scholar]
- Mitrasinovic OM, Murphy GM., Jr Accelerated phagocytosis of amyloid-beta by mouse and human microglia overexpressing the macrophage colony-stimulating factor receptor. J Biol Chem. 2002;277(33):29889–96. doi: 10.1074/jbc.M200868200. [DOI] [PubMed] [Google Scholar]
- Monif M, Burnstock G, Williams DA. Microglia: proliferation and activation driven by the P2X7 receptor. The international journal of biochemistry & cell biology. 2010;42(11):1753–6. doi: 10.1016/j.biocel.2010.06.021. [DOI] [PubMed] [Google Scholar]
- Mosher KI, Wyss-Coray T. Microglial dysfunction in brain aging and Alzheimer’s disease. Biochem Pharmacol. 2014;88(4):594–604. doi: 10.1016/j.bcp.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- N’Diaye EN, Branda CS, Branda SS, Nevarez L, Colonna M, Lowell C, Hamerman JA, Seaman WE. TREM-2 (triggering receptor expressed on myeloid cells 2) is a phagocytic receptor for bacteria. J Cell Biol. 2009;184(2):215–23. doi: 10.1083/jcb.200808080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann H, Takahashi K. Essential role of the microglial triggering receptor expressed on myeloid cells-2 (TREM2) for central nervous tissue immune homeostasis. J Neuroimmunol. 2007;184(1–2):92–9. doi: 10.1016/j.jneuroim.2006.11.032. [DOI] [PubMed] [Google Scholar]
- Nimmrich V, Ebert U. Is Alzheimer’s disease a result of presynaptic failure? Synaptic dysfunctions induced by oligomeric beta-amyloid. Reviews in the neurosciences. 2009;20(1):1–12. doi: 10.1515/revneuro.2009.20.1.1. [DOI] [PubMed] [Google Scholar]
- Otero K, Shinohara M, Zhao H, Cella M, Gilfillan S, Colucci A, Faccio R, Ross FP, Teitelbaum SL, Takayanagi H, Colonna M. TREM2 and beta-catenin regulate bone homeostasis by controlling the rate of osteoclastogenesis. J Immunol. 2012;188(6):2612–21. doi: 10.4049/jimmunol.1102836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter MM, Atagi Y, Liu CC, Rademakers R, Xu H, Fryer JD, Bu G. TREM2 in CNS homeostasis and neurodegenerative disease. Molecular neurodegeneration. 2015;10:43. doi: 10.1186/s13024-015-0040-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Q, Malhotra S, Torchia JA, Kerr WG, Coggeshall KM, Humphrey MB. TREM2- and DAP12-dependent activation of PI3K requires DAP10 and is inhibited by SHIP1. Science signaling. 2010;3(122):ra38. doi: 10.1126/scisignal.2000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter LS, Barron E, Chui HC. Morphologic association between microglia and senile plaque amyloid in Alzheimer’s disease. Neurosci Lett. 1990;119(1):32–6. doi: 10.1016/0304-3940(90)90748-x. [DOI] [PubMed] [Google Scholar]
- Polazzi E, Monti B. Microglia and neuroprotection: from in vitro studies to therapeutic applications. Prog Neurobiol. 2010;92(3):293–315. doi: 10.1016/j.pneurobio.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–45. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- Rossi S, Motta C, Studer V, Macchiarulo G, Volpe E, Barbieri F, Ruocco G, Buttari F, Finardi A, Mancino R, Weiss S, Battistini L, Martino G, Furlan R, Drulovic J, Centonze D. Interleukin-1beta causes excitotoxic neurodegeneration and multiple sclerosis disease progression by activating the apoptotic protein p53. Molecular neurodegeneration. 2014;9:56. doi: 10.1186/1750-1326-9-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefano L, Racchetti G, Bianco F, Passini N, Gupta RS, Panina Bordignon P, Meldolesi J. The surface-exposed chaperone, Hsp60, is an agonist of the microglial TREM2 receptor. Journal of neurochemistry. 2009;110(1):284–94. doi: 10.1111/j.1471-4159.2009.06130.x. [DOI] [PubMed] [Google Scholar]
- Streit WJ. Microglia and Alzheimer’s disease pathogenesis. J Neurosci Res. 2004;77(1):1–8. doi: 10.1002/jnr.20093. [DOI] [PubMed] [Google Scholar]
- Sun M, Zhu M, Chen K, Nie X, Deng Q, Hazlett LD, Wu Y, Li M, Wu M, Huang X. TREM-2 promotes host resistance against Pseudomonas aeruginosa infection by suppressing corneal inflammation via a PI3K/Akt signaling pathway. Investigative ophthalmology & visual science. 2013;54(5):3451–62. doi: 10.1167/iovs.12-10938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Prinz M, Stagi M, Chechneva O, Neumann H. TREM2-transduced myeloid precursors mediate nervous tissue debris clearance and facilitate recovery in an animal model of multiple sclerosis. PLoS Med. 2007;4(4):e124. doi: 10.1371/journal.pmed.0040124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Rochford CD, Neumann H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. The Journal of experimental medicine. 2005;201(4):647–57. doi: 10.1084/jem.20041611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu S, Okamoto S, Lipton SA, Xu H. Oligomeric Abeta-induced synaptic dysfunction in Alzheimer’s disease. Molecular neurodegeneration. 2014;9:48. doi: 10.1186/1750-1326-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull IR, Gilfillan S, Cella M, Aoshi T, Miller M, Piccio L, Hernandez M, Colonna M. Cutting edge: TREM-2 attenuates macrophage activation. J Immunol. 2006;177(6):3520–4. doi: 10.4049/jimmunol.177.6.3520. [DOI] [PubMed] [Google Scholar]
- Wang Y, Cella M, Mallinson K, Ulrich JD, Young KL, Robinette ML, Gilfillan S, Krishnan GM, Sudhakar S, Zinselmeyer BH, Holtzman DM, Cirrito JR, Colonna M. TREM2 lipid sensing sustains the microglial response in an Alzheimer’s disease model. Cell. 2015;160(6):1061–71. doi: 10.1016/j.cell.2015.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Zheng H, Shao X, Wang W, Yao Q, Li Z. Excitotoxicity of TNFalpha derived from KA activated microglia on hippocampal neurons in vitro and in vivo. Journal of neurochemistry. 2010;114(2):386–96. doi: 10.1111/j.1471-4159.2010.06763.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.