Abstract
Mutants of presenilin1 (PS1) increase neuronal cell death causing autosomal dominant Familial Alzheimer’s disease (FAD). Recent literature shows that treatment of neuronal cultures with low concentrations of trypsin, a member of the serine family of proteases, protects neurons from toxic insults by binding to the Proteinase-Activated Receptor-2 (PAR2) and stimulating survival kinase ERK1/2. Other studies show that PS1 is necessary for the neuroprotective activity of specific neurotrophic factors, such as BDNF, against excitotoxicity and oxidative stress. Here we show that treatment of mouse cortical neuronal cultures with trypsin activates ERK1/2 and protects neurons against glutamate excitoxicity. The trypsin-dependent ERK activation and neuroprotection requires both alleles of PS1, because neither PS1 knockout nor PS1 hemizygous neuronal cultures can use exogenous trypsin to activate ERK1/2 or increase neuronal survival. The protective effect of PS1 does not depend on its γ-secretase activity since inhibitors of γ-secretase have no effect on trypsin-mediated neuroprotection. Importantly, cortical neuronal cultures either heterozygous or homozygous for PS1 FAD mutants are unable to use trypsin to activate ERK1/2 and rescue neurons from excitotoxicity indicating that FAD mutants inhibit trypsin-dependent neuroprotection in an autosomal dominant manner. Furthermore, our data support the theory that PS FAD mutants increase neurodegeneration by inhibiting the ability of neurons to use cellular factors as protective agents against toxic insults.
Keywords: Familial Alzheimer’s Disease, Presenilin 1, neurotoxicity, neuroprotection, Proteinase-Activated Receptor 2
INTRODUCTION
Alzheimer’s disease (AD) is a neurodegenerative disorder and the most common form of dementia. Most AD cases are sporadic, although a small fraction (about 5%) of all cases are caused by genetic mutations and are classified as familial AD (FAD). FADs are caused by specific mutations in the genes of the amyloid precursor protein (APP), presenilin1 (PS1), and presenilin2 (PS2) and are transmitted in an autosomal dominant manner. Most known FAD cases map on the gene of PS1. Presenilins (PSs) belong to a family of homologous multi-pass transmembrane proteins that are found as part of the γ-secretase complex (1, 2) and have been shown to have γ-secretase-dependent and -independent functions (3). Furthermore, FAD mutants of PS1 have been linked to increased neurotoxicity in animal models of AD (4, 5).
In addition to deposition of protein aggregates and inflammation, the hallmarks of neurodegenerative diseases include increased neuronal loss in selective brain regions suggesting that potential therapies against neurodegeneration may include factors able to protect neuronal cells against toxic insults such as excitotoxicity and oxidative stress. Recent studies have shown that trypsinogen, the inactive precursor of trypsin, enhances white matter neurogenesis (6) while others identified trypsin as a neuroprotective factor against kainate toxicity (7). Trypsin belongs to the serine family of proteases and has been shown to activate matrix metalloproteinases (MMPs) and to function as a signaling agent by activating the Proteinase-Activated Receptor-2 (PAR2), a seven transmembrane domain G protein-coupled receptor expressed in neurons and glia (8). Specifically, low concentrations of trypsin activate PAR2 by promoting cleavage of its extracellular N-terminus thus creating a new N-terminus which acts as a “tethered-ligand” by binding the extracellular sequence of PAR2 and activating its signaling (9, 10). Trypsin-activated PAR2 stimulates the phosphorylation/activation of mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK1/2) (9, 11, 12), a key factor in cell growth and survival (13–15). However, other studies have shown that astrocytic PAR2 activation can inhibit ERK phosphorylation (7). Furthermore, PAR2 has been implicated in neurodegenerative diseases as neuronal PAR2 activation protects neuronal cells against Aβ 1-42 toxicity (16), while in multiple sclerosis (MS), patients show increased activation of PAR2 in white matter (17). In the present study, we show that trypsin protects primary cortical neuronal cultures against glutamate excitoxicity, a function that requires PAR2 and phosphorylation/activation of MAP kinase ERK1/2. Importantly, PS1 null neurons are unable to use trypsin in neuroprotection while PS2 has no effects on the neuroprotective functions of trypsin. Finally we present data that FAD mutations of PS1 abolish the trypsin-mediated neuroprotection and the trypsin-induced phosphorylation of ERK1/2 in an autosomal dominant matter.
MATERIALS AND METHODS
Reagents
Common reagents, MEK/ERK inhibitor UO126 and soybean trypsin inhibitor (SBTI) were from Sigma (St. Louis, MO) and matrix metalloproteinase inhibitor GM6001 was from Millipore. Neurobasal medium and B27 supplement were from Invitrogen (Eugene, OR), anti-ERK1/2 and pERK Thr202/Tyr204 from Cell Signaling Technology (Beverly, MA, USA) and anti-c-myc was from SantaCruz Biotechnology, Inc. γ-secretase inhibitor (DAPT) was from EMD Millipore-Calbiochem (CA).
Neuronal cultures
Primary neuronal cultures were prepared from E15.5 PS1+/+ (WT), PS1−/− (KO) PS1+/− (KO/+), PS1+/I213T, I213T/I213T, PS1+/M146V and M146V/M146V embryonic brains and genotypes were determined as described previously (5, 18, 19). Neocortices were dissected out, treated with 0.025% of trypsin in Krebs-Ringer buffer [containing (in mM) 120 NaCl, 5.0 KCl, 25 NaHCO3, 1.2 KH2PO4, 1.5 MgCl2, 10 glucose, pH 7.4] for 10 min at 37 °C and, subsequently, were mechanically dissociated in DNase I-containing (0.008%) Krebs-Ringer buffer. After centrifugation, the dissociated cells were resuspended in Neurobasal medium containing B27 and plated on Poly-D-Lysine coated 24-well dishes at 2 × 105 cells/well and used at 7DIV. PAR2 KO neurons from the crossing of B6.CG-F2rl1 tm1Mslb/J mice (Jackson labs) were prepared as described above.
Cell survival assays
Hoechst staining assay (Sigma) was used to measure neuronal viability as previously described (20, 21). Briefly, neurons on poly-D-lysine-coated 24-well plates were pre-treated with 5.25nM trypsin (Sigma) for 1 hour followed by 50μM glutamate for 3 hours. Cells were then fixed in 4% paraformaldehyde for 20 minutes at room temperature and stained with Hoechst 33342 for 10 minutes. Neurons were observed under a fluorescence microscope on ultraviolet illumination. Numbers of viable neurons were counted in 9 fields per well with at least 20 neurons per field, and cell counting was performed by at least two independent researchers using different preparations Results are expressed as percent of control value. Progranulin was added to the cultures 24h before glutamate treatment at 35nM concentration.
Treatment with inhibitors and cell lysates
Cells were treated with MEK/ERK inhibitor UO126 (10 μM), soybean trypsin inhibitor (SBTI, 11nM) or matrix metalloproteinase inhibitor GM6001 (2.5 μM) 3.5 hours prior to glutamate addition and with γ-secretase inhibitor DAPT (4 μm) 6 hours before glutamate addition. Cell lysates for Western blotting were prepared in SDS lysis buffer [(in mM) 100 Tris-HCl, 20 NaCl, 10 EGTA, 10 EDTA, 1% SDS, phosphatase inhibitors: 20 NaF, 5 sodium orthovanadate, 1 sodium pyrophosphate, and 200 microcystin, containing complete protease inhibitor mixture (Boehringer Mannheim, Indianapolis, IN)]. The following antibodies were used for blotting analysis: anti-ERK1/2 and pERKThr202/Tyr204 (as above), anti-PAR2 (Enzo Life Sciences Inc.), anti-GFP and anti c-myc (as above).
Statistical analysis
Statistical analysis was based on densitometric data from several independent experiments. Results are expressed as mean ± SEM. Statistical significance was determined using one-way ANOVA followed by Tukey’s post hoc where applicable.
RESULTS
Trypsin protects neurons from glutamate excitotoxicity in a PAR2- and ERK1/2-dependent manner
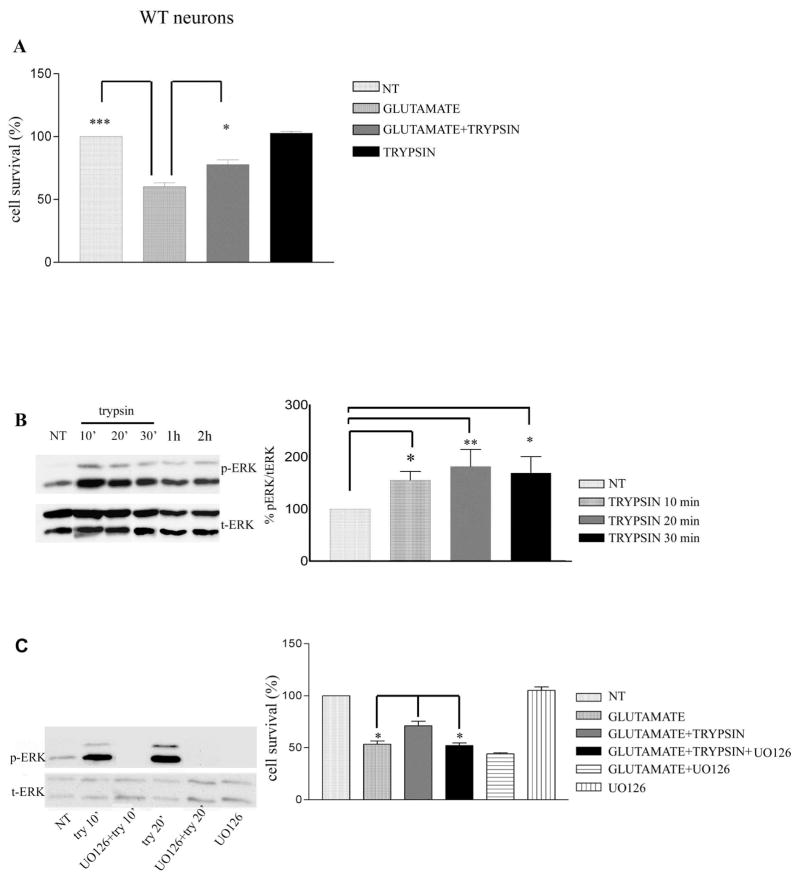
Previous studies have shown that low concentrations of trypsin protect organotypic hippocampal slice cultures against kainate insult (7). As shown in Fig. 1A, following glutamate treatment, 60.2± 3.12% of neurons survive compared to untreated neurons. In contrast, pre-treatment with trypsin increases the percentage of surviving neurons to 77.8±3.74% (n=4, P<0.05), showing that trypsin protects neurons from glutamate toxicity. To elucidate the signaling pathway through which trypsin decreases glutamate-induced neuronal death, we examined ERK1/2 phosphorylation that has been reported to be induced by trypsin in cell lines (11, 22, 23). Fig. 1B shows that trypsin causes a significant and sustained increase in the phosphorylation of ERK1/2 Thr202/Tyr204 (Fig. 1B). Furthermore, UO126, a specific inhibitor of the MEK/ERK1/2 pathway (24), blocks the trypsin-induced ERK1/2 phosphorylation and neuroprotection (Fig. 1C), suggesting that trypsin-mediated neuroprotection against glutamate depends on ERK1/2 phosphorylation. In addition, we have performed western blots that show that concomitant application of glutamate and trypsin in wt neurons increases pERK activation relatively to untreated and glutamate-treated neurons 4h post treatment (Sup Fig 1).
Figure 1. Trypsin induces neuroprotection via ERK1/2 phosphorylation.
(A) WT mouse cortical neurons at 7 days in vitro (DIV) were pretreated with 5.25 nM trypsin for 60 minutes followed by incubation with 50 μM glutamate for 3 hours. Cells were then fixed with 4% paraformaldehyde and stained with Hoechst 33342 as described (20). Nine pictures were taken from each well and each condition represented the average of 3–4 wells. Cell survival was measured by counting the number of cells with normal nuclear morphology (wt vs glutamate, P<0.001, glutamate vs glutamate+trypsin P<0.05, glutamate+trypsin vs glutamate+trypsin+UO126 P=0.05). Results (mean ± standard error [SE]) were calculated from 4 independent experiments. * p<0.05, ** p <0.005, *** p<0.001 comparing cultures treated with glutamate in the presence or absence of trypsin (One-way Anova, Tukey’s post-hoc). (B) Mouse cortical neurons were cultured and treated with trypsin as above for the indicated time periods. Untreated cultures were used as controls (NT). Following incubation, cells were collected and assayed on Western blots (WBs) for the indicated proteins. (Left) A representative blot out of 7 independent experiments is shown. (Right) Densitometric analysis of p-ERK 1/2 in the presence of trypsin at different time points expressed as percentage of phospho-ERK 1/2 (p-ERK) to total ERK 1/2 (t-ERK) ratio that was set as 100% for control (NT). Bars represent phospho-protein to protein ratios relative to control. (C) ERK 1/2 inhibitor U0126 (10 μM) blocked trypsin-induced ERK1/2 phosphorylation. Inhibitor was added to cultures 30 minutes prior to addition of 5.25 nM trypsin. Neurons were subsequently collected at the indicated times and subjected to SDS-PAGE and WBs as above (Left). (Right) Quantitative data show that UO126 inhibits trypsin-induced neuroprotection against glutamate. Results (mean ± standard error [SE]) were calculated from 4 independent experiments. *p<0.05, ** p <0.005 (Tukey’s post-hoc). UO126 shows no toxicity when administered in culture.
It is has been reported that trypsin activates PAR2 receptor by cleaving its N-terminus (10, 11, 25) and that activated PAR2 initiates a signaling cascade that results in increased phosphorylation/activation of survival kinase ERK1/2 (11, 22). To examine whether neuroprotection and presumed PAR2 activation are trypsin-dependent, we used Soybean trypsin inhibitor (SBTI) that specifically inhibits the proteolytic activity of trypsin (26). Fig. 2 (A and B) shows that treatment of neuronal cultures with SBTI eliminated both, the trypsin-induced neuroprotection and ERK1/2 phosporylation indicating that the proteolytic activity of trypsin is necessary for trypsin-induced ERK1/2 activation and neuroprotection. To examine whether PAR2 receptor is necessary for the neuroprotective function of trypsin, we used cortical neurons from PAR2 knockout (KO) mice. Figure 2C shows that neuronal cultures from PAR2 null mice are unable to use trypsin as a neuroprotective factor against glutamate-induced death supporting the suggestion that PAR2 mediates the neuroprotective function of trypsin. In contrast, Fig. 2D shows that absence of PAR2 has no effect on the neuroprotective function of Progranulin (PGRN), a protein known to protect against glutamate toxicity (21). Furthermore, Fig. 2E shows that, in contrast to wild type (WT) neurons, there is no significant upregulation of phospho-ERK1/2 (pERK) following trypsin treatment of PAR2 KO neurons. Together, these data indicate that trypsin-induced neuroprotection depends on PAR2 and is mediated by the ERK1/2 survival signaling pathway.
Figure 2. Trypsin activity and PAR2 receptor are necessary for trypsin-induced neuroprotection against glutamate excitotoxicity.
(A) Treatment of 7DIV cortical neurons with 11 nM SBTI 30 minutes prior to trypsin administration inhibits trypsin-induced neuroprotection against glutamate toxicity (glutamate vs glutamate+trypsin P<0.05, glutamate+trypsin vs glutamate+trypsin+SBTI P<0.05). Results (Tukey’s post-hoc, mean ± standard error [SE]) were calculated from 4 independent experiments. SBTI shows no neurotoxicity. (B) SBTI (11 nM) blocks trypsin-induced ERK1/2 phosphorylation. Inhibitor was added to cultures 30 minutes prior to the addition of 5.25 nM trypsin. Neurons were subsequently collected at the indicated times after trypsin administration and subjected to SDS-PAGE and WB as above. (C) PAR2 KO mouse cortical neurons were treated at 7DIV with 5.25 nM trypsin for 1 hour, followed by 3 hours of exposure to glutamate. Cells were fixed in 4% paraformaldehyde, stained with Hoechst and neuronal survival was measured as described in Fig. 1. Trypsin treatment does not protect against glutamate in cortical neurons lacking PAR2 (percentage of survival for glutamate treated neurons 59.7±2.2, for glutamate +trypsin treated neurons 56.94±2.3). Results (mean ± standard error) were calculated from 5 independent experiments. (D) PAR2 deficiency has no effect on progranulin-induced neuroprotection against glutamate. Eight DIV mouse PAR2 KO cortical neurons were treated overnight with 35nM progranulin, fixed as described above and healthy Hoechst stained nuclei were counted. Progranulin is neuroprotective even in the absence of PAR2 (P<0.05, n=4). (E) Densitometric analysis of p-ERK 1/2 in the presence of trypsin at different time points expressed as percentage of phospho-ERK 1/2 (p-ERK) to total ERK 1/2 (t-ERK) ratio that was set as 100% for control (NT). Bars represent phospho-protein to total protein ratios relative to control. *p<0.05, (Tukey’s post hoc). (F) A representative blot out of 5 experiments shows no ERK activation after trypsin treatment in the absence of PAR2.
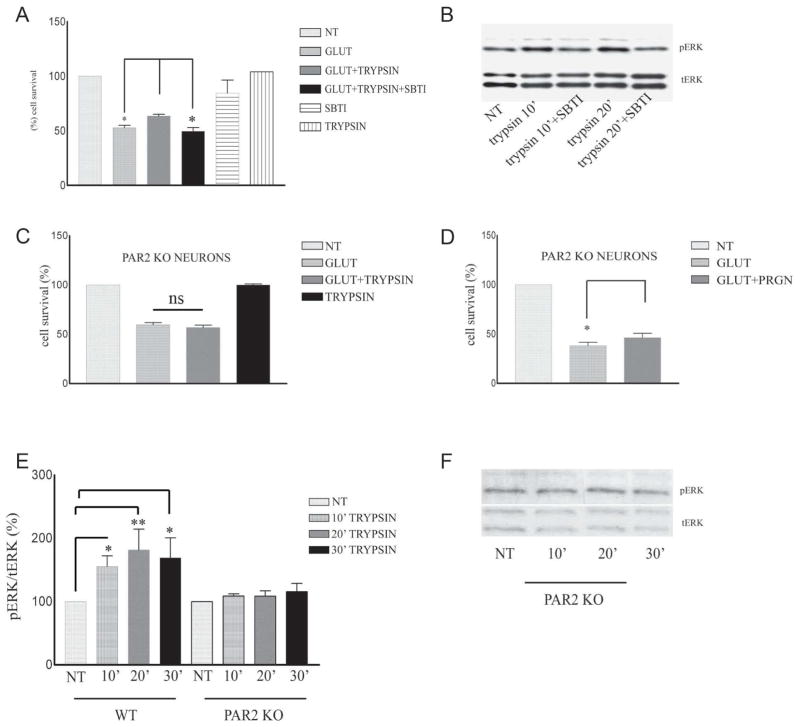
Trypsin-dependent neuroprotection requires both PS1 alleles, but is independent of PS2 and γ-secretase
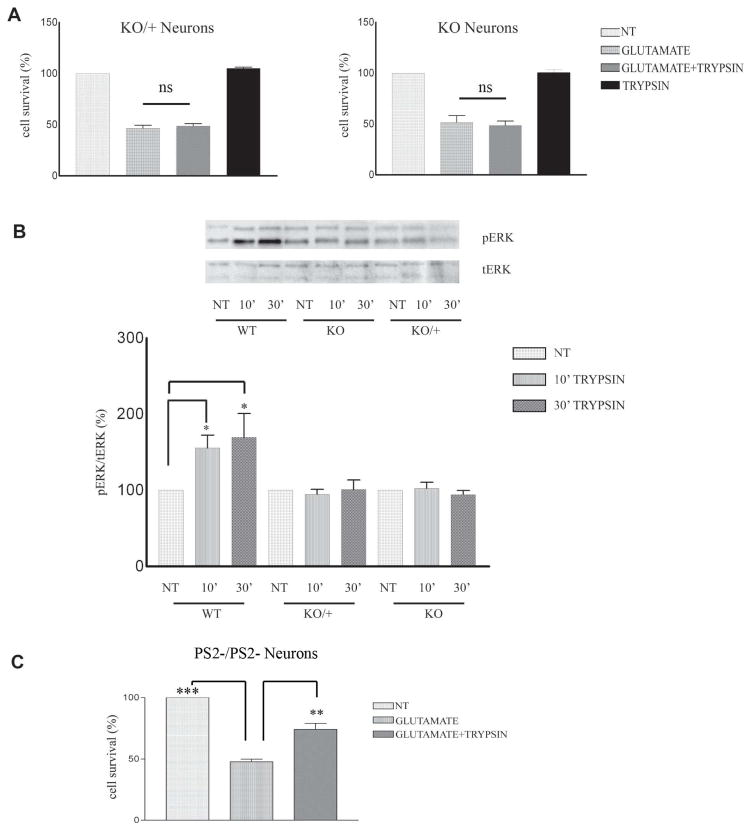
It was recently shown that PS1 is necessary for the neuroprotective functions of BDNF and ephrinB1 (20). To examine whether PS1 plays a role in the trypsin-dependent neuroprotection we used mouse cortical neuronal cultures missing either one (KO/+) or both (KO) PS1 alleles prepared as described (20, 21). Fig. 3A shows that in contrast to WT neurons (Fig. 1), neuronal cultures that lack either one or both PS1 alleles are not able to use trypsin as a neuroprotective factor against glutamate excitotocity. These data indicate that absence of even one PS1 allele results in decreased trypsin-dependent neuroprotection, a result similar to that reported for the ephrinB- and BDNF-dependent neuroprotection (21). We then examined whether PS1 affects the trypsin-induced phosphorylation of neuronal ERK1/2. As shown in Fig. 3B, trypsin fails to induce a significant increase of p-ERK1/2 in neurons prepared from either hemizygous (KO/+) or homozygous (KO) PS1 knockout mice suggesting that both PS1 alleles are necessary for trypsin-dependent phosphorylation of survival kinase ERK1/2. Similarly, simutaneous treatment with trypsin and glutamate does not increase ERK phosphorylation in PS1 KO neurons (Supp Fig 1). In contrast, absence of neuronal PS2 has no effect on the trypsin-dependent neuroprotection against glutamate (Fig. 3C).
Figure 3. PS1 specifically promotes trypsin-induced neuroprotection and ERK1/2 phosphorylation.
(A) Seven DIV mouse PS1 KO/+ (left) and PS1 KO (right) neuronal cultures were treated with 5.25 nM trypsin for 60 minutes before 3h exposure to glutamate (50μM) as described in Fig. 1. Cell survival assay showed that absence of one PS1 allele is sufficient to inhibit trypsin-mediated neuroprotection. Results (mean ± standard error [SE]) were calculated from 4 independent experiments. (B) (Upper) WT, PS1 KO/+ and PS1 KO neuronal cultures prepared as in Fig. 1 from the same littermate were treated at 7 DIV with 5.25 nM trypsin for the indicated time periods. Untreated cultures (NT) were used as controls. Following incubation, cells were collected and assayed on WB for the indicated proteins. A representative blot out of 5–7 independent experiments is shown. (Lower) Densitometric analysis of p-ERK 1/2 in the presence of trypsin at different time points expressed as percentage of phospho-ERK 1/2 (pERK) to total ERK 1/2 (t-ERK) ratio that was set as 100% for control (NT). Bars represent phospho-protein to total protein ratios relative to control. *p=0.05, ** p<0.005 (Tukey’s post-hoc). (C) PS2 −/− 7DIV neuronal cultures were treated with 5.25nM trypsin 60 minutes prior to the addition of 50 μM glutamate for 3h. Cell survival assay shows that trypsin rescued cortical neurons from glutamate excitotoxicity in the absence of PS2, n=3 **p<0.005, *** p<0.001 (Tukey’s post hoc).
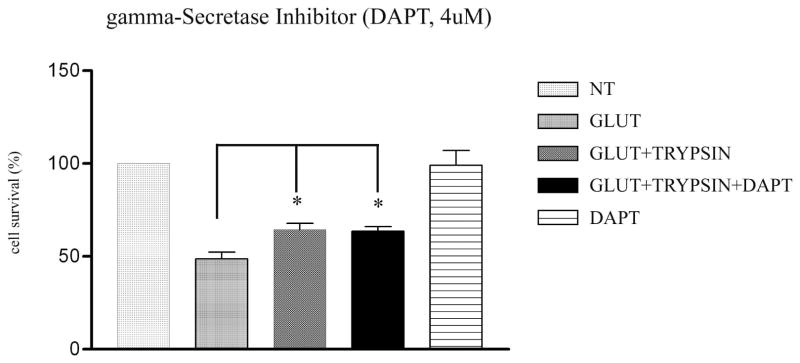
Since PS1 is part of the γ-secretase complex (27–29), we asked whether inhibition of γ-secretase activity interferes with the trypsin-dependent neuronal survival. To this end, we used the γ-secretase inhibitor DAPT extensively used to study γ-secretase function (30, 31). As shown in Fig. 4, DAPT has no effect on the trypsin neuroprotection, suggesting that γ-secretase activity does not play a role in this function of trypsin. This result is in accordance with previous studies showing that the PS1-mediated neuroprotective activities of BDNF and ephrinB1 are independent of γ-secretase (20).
Figure 4. Trypsin neuroprotection does not depend on γ-secretase activity.
DAPT, a γ-secretase inhibitor, was added in 7DIV WT neuronal cultures at a concentration of 4 μM, 2 hours before treatment with trypsin. Healthy Hoechst-stained nuclei were counted and paired t-test statistical analysis showed that DAPT does not affect trypsin-mediated neuroprotection. Results (mean ± standard error [SE]) were calculated from 4 independent experiments. *p<0.05, (Tukey’s post hoc). DAPT shows no neurotoxicity
PS1 FAD mutations inhibit trypsin-induced neuroprotection against glutamate toxicity
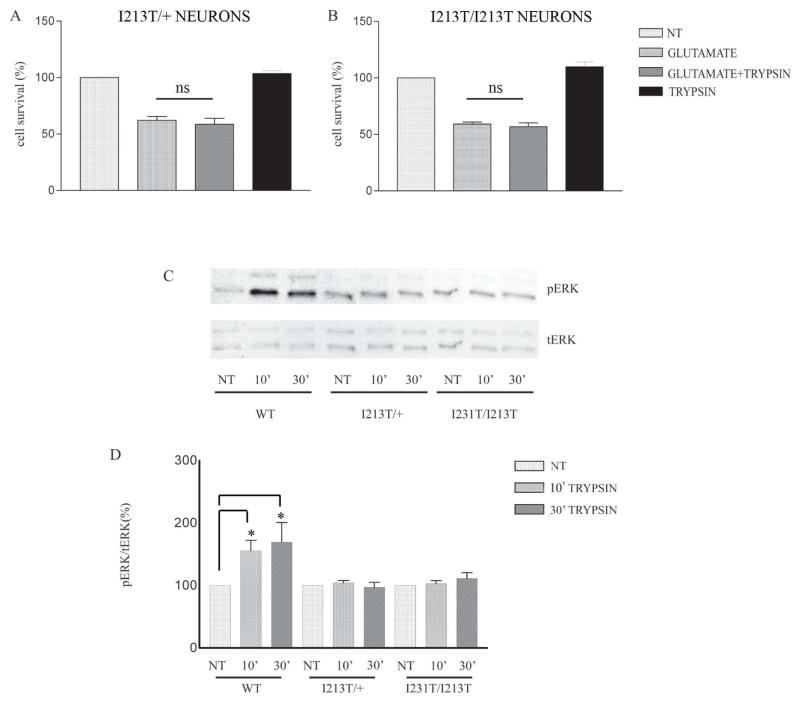
To examine whether PS1 FAD mutations affect the trypsin-mediated neuroprotection against glutamate, we used cortical neurons from knockin (KI) transgenic mice carrying FAD mutant PS1I213T available in our laboratory (18). This mouse model has the same genotype as human patients carrying this PS1 FAD mutation (18) and should closely recapitulate in vivo effects of this mutation on neuronal survival. Heterozygous animals carrying a WT and a FAD mutant allele of PS1 have been considered to resemble more the human disease compared to overexpression or knockout models. Our results indicate that the presence of even one FAD mutant allele of PS1 is sufficient to inhibit the trypsin-dependent neuroprotection from glutamate toxicity (Fig. 5A, B). We next asked whether the PS1 FAD allele I213T has any effects on the trypsin-induced phosphorylation of ERK1/2. Fig. 5C, D shows that the trypsin-induced increase in p-ERK1/2 is abolished in the presence of either one or two alleles of this FAD mutant, a result similar to that observed in KO/+ and KO animals (Fig. 3). These data suggest that PS1 FAD mutation I213T inhibits trypsin-mediated neuroprotection by inhibiting trypsin-induced ERK1/2 phosphorylation/activation.
Figure 5. Trypsin-induced neuroprotection and ERK1/2 phosphorylation are inhibited by PS1 FAD mutation I213T.
Mouse cortical neuronal cultures prepared from brains expressing one (A, I213T/+) or both (B, I213T/I213T) alleles of PS1 FAD mutation I213T were treated at 7 DIV with 5.25nM trypsin for 60 minutes followed by 50μM glutamate as above. Quantification of Hoechst positive nuclei shows that trypsin does not protect neurons from glutamate in either case (A, B). Results are expressed as mean ± standard error and were calculated from four independent experiments. (C) WT, I213T/+, and I213T/I213T cortical neurons from the same littermate were treated at 7 DIV with 5.25 nM trypsin for the indicated time periods. Untreated cultures were used as controls (NT). Following incubation, cells were collected and assayed on WB for the indicated proteins. A representative blot out of 5–7 independent experiments is shown. (D) Densitometric analysis of p-ERK in the presence of trypsin at different time points expressed as percentage of phospho-ERK 1/2 (p-ERK) to total ERK 1/2 (t-ERK) ratio that was set as 100% for control (NT). Bars represent phospho-protein to total protein ratios relative to control. *p=0.05, ** p<0.005 (Tukey’s post-hoc).
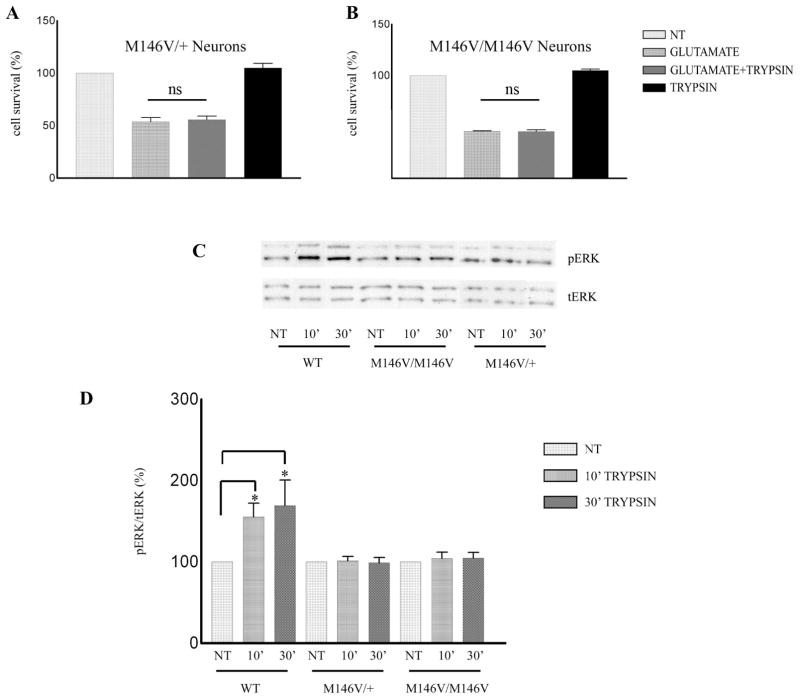
To examine whether inhibition of trypsin-induced neuroprotection is specific to the I213T PS1 allele, we examined neurons derived from a second KI mouse model expressing the FAD mutant allele PS1 M146V (5). Similar to neurons carrying the I213T allele, primary cortical neuronal cultures from mouse brains carrying either one or two PS1 M146V alleles showed no trypsin-dependent neuroprotection against glutamate excitotoxicity (Fig. 6A, B). Furthermore, p-ERK1/2 was not upregulated by trypsin in neuronal cultures prepared from brains carrying either one or two alleles of FAD mutant PS1 M146V (Fig. 6C, D), a result similar to that obtained in neurons carrying the PS1 I213T mutant. Taken together, our studies show that even in the heterozygous state, PS1 FAD mutants inhibit the trypsin-dependent phosphorylation of ERK1/2 kinase and abolish trypsin-dependent neuroprotection.
Figure 6. Trypsin-induced neuroprotection and ERK1/2 phosphorylation are inhibited by PS1 FAD mutation M146V.
Mouse cortical neuronal cultures prepared from brains expressing one (A, M146V/+) or both (B, M146V/M146V) alleles of PS1 FAD mutation M146V were treated at 7 DIV with 5.25nM trypsin for 60 minutes followed by 50μM glutamate as above. Quantification of Hoechst positive nuclei shows that trypsin does not protect neurons from glutamate in either case (A, B). Results are expressed as mean ± standard error and were calculated from four independent experiments. (C) WT, M146V/+ and M146V/M146V cortical neurons from the same littermate were treated at 7 DIV with 5.25 nM trypsin for the indicated time periods. Untreated cultures were used as controls (NT). Following incubation, cells were collected and assayed on WB for the indicated proteins. A representative blot out of 4–7 independent experiments is shown. (D) Densitometric analysis of p-ERK 1/2 in the presence of trypsin at different time points expressed as percentage of phospho-ERK 1/2 (p-ERK) to total ERK 1/2 (t-ERK) ratio that was set as 100% for control (NT). Bars represent phospho-protein to total protein ratios relative to control. *p=0.05, ** p<0.005 (Tukey’s post-hoc)
DISCUSSION
PS1 plays pivotal roles in the neurodegeneration of FAD as most mutations linked to this disorder map on the gene of PS1. PS1 is an important component of the γ-secretase enzyme that processes many transmembrane proteins, including APP, Notch1 and cadherins, and plays critical roles in the production of Aβ (2). In addition, PS1 has γ-secrertase independent functions in neuroprotection and electrophysiological properties (20, 32–34). The mechanism however by which PS mutations promote neurodegeneration is still unclear and both Aβ-dependent and -independent mechanisms have been proposed (35). Trypsin belongs to the superfamily of serine proteases such as kallikrein and activated protein C (36, 37), which participate in cell proliferation, migration and differentiation and has recently been implicated in neurogenic (6) and neuroprotective (7) functions. Studies in organotypic hippocampal slice cultures showed that trypsin affects cell survival (7) by activating PAR2, a G-coupled receptor that is specifically activated by this protease (9, 10). The protease activity of trypsin cleaves the N-terminal sequence of PAR2 receptor (see Introduction), an event that initiates a signaling cascade resulting in the phosphorylation/activation of survival kinase ERK1/2 (10, 21, 22). However, the role of trypsin against excitotoxicity in pure neuronal cultures that are devoid of other cell types, and the involvelment of PS1 FAD mutants have not been demonstrated.
Excitotoxicity, a neuronal insult due to excessive activation of glutamate receptors, has been implicated in the pathogenesis of many neurodegenerative disorders including AD (38, 39). Thus, agents that protect neurons from excitotoxicity are considered potential treatments of neurodegenerative disorders (40). Here we show that trypsin is able to rescue brain primary neuronal cultures from glutamate excitotoxicity. Furthermore, we found that trypsin-induced protection of primary neurons depends on PAR2 as PAR2 KO neurons are not rescued by trypsin from glutamate-iduced death and they do not show trypsin-induced increase of ERK1/2 phosphorylation. SBTI, a specific inhibitor of the trypsin protease activity, inhibits trypsin-induced neuroprotection and ERK1/2 phosphorylation suggesting that trypsin exerts its neuroprotective function by proteolytically activating the PAR2 receptor. Interestingly, PAR2 has no effect on the PGRN-dependent neuroprotection against glutamate (27), indicating that this receptor specifically mediates the neuroprotective functions of trypsin. Since several studies show that trypsin activates matrix metalloproteinases (MMP) (41–43), we used the general MMP inhibitor GM6001 (44), to ask whether trypsin-mediated neuroprotection depends on activation of MMPs. We found that this inhibitor has no effect on the neuroprotective function of trypsin (data not shown), suggesting that activation of MMPs is not involved in trypsin neuroprotection.
Our data show that absence of either one (haploinsufficiency) or both PS1 alleles significantly reduces trypsin-dependent neuroprotection. The haploinsufficiency effect of PS1 is similar to reports showing that the neuroprotective function of BDNF in vitro depends on both PS1 alleles and that neurons expressing reduced levels of PS1 show reduced BDNF-dependent neuroprotection against excitotoxicity (20). In addition, we found that neither PS2, a protein homologous to PS1, nor γ-secretase activity affect the trypsin-induced neuroprotection against glutamate, indicating that this function of trypsin is specifically mediated by PS1 in a non-γ-secretase-dependent manner. In summary, our data suggest that PS1 is needed for the trypsin-induced activation of PAR2 signaling and ERK1/2 activation. The mechanism, however, by which PS1 mediates PAR2 activation is unknown. Since both PS1 and PAR2 are located at the plasma membrane it is reasonable to speculate that PS1 may modulate the mechanism by which trypsin activity cleaves PAR2.
Our data showing that the absence of one PS1 allele results in significant reduction of the trypsin-dependent neuroprotection supports the hypothesis that inactivation of even one PS1 allele by genetic mutations decreases neuronal protection from toxic insults. Such a mechanism may result in increased rates of neuronal cell death in vivo, a process that over many years may lead to significant neuronal loss and dementia. This observation seems analogous to familial forms of frontotemporal dementia (FTD) where loss/inactivation of one allele of PRGN (haploinsufficiency) results in dominant transmission of FTD (45) and raises the possibility that loss of PS function by allele interference (35) may be involved in FAD neurodegeneration caused by PS mutants. Thus, we tested the effects of PS1 FAD mutants on the survival of primary cortical neuronal cultures carrying the same genotype as human FAD patients. Our results show that the presence of either one (heterozygous state) or both (homozygous state) PS1 FAD mutant alleles abolishes trypsin-dependent neuroprotection. Thus cortical neuronal cultures either heterozygous or homozygous for FAD mutant PS1M146V show decreased trypsin neuroprotection against excitotoxicity compared to WT. Importantly, we obtained similar data with neurons carrying FAD mutant PS1I213T providing further support to the hypothesis that PS1 FAD mutations may interfere with the function of PS1 in trypsin-induced neuroprotection. Together, our observations suggest that FAD mutants may increase the rate of neuronal cell loss by interfering with PS-dependent neuroprotective mechanisms against toxic insults in the brain. Over time the increased rate of neuronal loss may lead to dementia.
In conclusion, our studies using cortical neuronal cultures in vitro indicate that trypsin acts as a neuroprotective factor against glutamate excitotoxicity and that trypsin-induced neuroprotection depends on both PS1 and PAR2 receptor. In addition, the presence of both neuronal PS1 alleles is necessary for trypsin-dependent neuroprotection. Furthermore, neurons carrying either heterozygous or homozygous PS1 FAD mutants are unable to use trypsin as a neuroprotective agent against toxic insults. These data support the theory that PS1 FAD mutations may increase the rate of neurodegeneration in vivo by inhibiting neuroprotective function of PS1 against toxic insults.
Supplementary Material
WT and PS1 KO neuronal cultures prepared from the same littermate were treated at 7 DIV with 5.25 nM trypsin and 50 μM of glutamate for 4 hours. Untreated cultures were used as controls. Following incubation, cells were collected and assayed on WB for the indicated proteins. A representative blot is showing a decrease in ERK activation after glutamate addtion, however concomitant treatment of wt neurons with glutamate and trypsin increases ERK phosphorylation when compared to untreated neurons. In contrast, there is no effect of trypsin treatment on ERK activation in PS1 KO neurons.
Highlights.
The highlights of this study are:
Trypsin shows neuroprotective properties against glutamate toxicity in cortical neurons.
Trypsin-induced neuroprotection depends on ERK activation, inhibition of ERK abolishes trypsin-mediated neuroprotection
PS1 is necessary for trypsin-induced neuronal survival against glutamate-induced toxicity, absence of PS1 inhibits trypsin-induced ERK activation and trypsin-mediated neuroprotection.
PS1 mutations found in FAD also inhibit trypsin-induced neuroprotection and ERK activation.
Trypsin neuroprotection depends on activation of its receptor, PAR2; PAR2 knockout neurons are not rescued by trypsin from glutamate neurotoxicity and this effect is specific since progranulin-dependent neuroprotection is not affected by the absence of PAR2.
Acknowledgments
This work was supported by NIH grants R37 AG-017926, AG-008200, and R01-NS047229 to NKR.
Footnotes
The authors declare no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wolfe MS, Xia W, Ostaszewski BL, Diehl TS, Kimberly WT, Selkoe DJ. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and gamma-secretase activity. Nature. 1999;398:513–517. doi: 10.1038/19077. [DOI] [PubMed] [Google Scholar]
- 2.Barthet G, Georgakopoulos A, Robakis NK. Cellular mechanisms of gamma-secretase substrate selection, processing and toxicity. Progress in neurobiology. 2012;98:166–175. doi: 10.1016/j.pneurobio.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pimplikar SW, Nixon RA, Robakis NK, Shen J, Tsai LH. Amyloid-independent mechanisms in Alzheimer’s disease pathogenesis. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:14946–14954. doi: 10.1523/JNEUROSCI.4305-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grilli M, Diodato E, Lozza G, Brusa R, Casarini M, Uberti D, Rozmahel R, Westaway D, St George-Hyslop P, Memo M, Ongini E. Presenilin-1 regulates the neuronal threshold to excitotoxicity both physiologically and pathologically. Proc Natl Acad Sci U S A. 2000;97:12822–12827. doi: 10.1073/pnas.97.23.12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo Q, Fu W, Sopher BL, Miller MW, Ware CB, Martin GM, Mattson MP. Increased vulnerability of hippocampal neurons to excitotoxic necrosis in presenilin-1 mutant knock-in mice. Nature medicine. 1999;5:101–106. doi: 10.1038/4789. [DOI] [PubMed] [Google Scholar]
- 6.Nikolakopoulou AM, Dutta R, Chen Z, Miller RH, Trapp BD. Activated microglia enhance neurogenesis via trypsinogen secretion. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1218856110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenwood SM, Bushell TJ. Astrocytic activation and an inhibition of MAP kinases are required for proteinase-activated receptor-2-mediated protection from neurotoxicity. J Neurochem. 2010;113:1471–1480. doi: 10.1111/j.1471-4159.2010.06737.x. [DOI] [PubMed] [Google Scholar]
- 8.Bushell TJ, Plevin R, Cobb S, Irving AJ. Characterization of proteinase-activated receptor 2 signalling and expression in rat hippocampal neurons and astrocytes. Neuropharmacology. 2006;50:714–725. doi: 10.1016/j.neuropharm.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 9.Macfarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R. Proteinase-activated receptors. Pharmacol Rev. 2001;53:245–282. [PubMed] [Google Scholar]
- 10.Nystedt S, Emilsson K, Wahlestedt C, Sundelin J. Molecular cloning of a potential proteinase activated receptor. Proc Natl Acad Sci U S A. 1994;91:9208–9212. doi: 10.1073/pnas.91.20.9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeFea KA, Zalevsky J, Thoma MS, Dery O, Mullins RD, Bunnett NW. beta-arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J Cell Biol. 2000;148:1267–1281. doi: 10.1083/jcb.148.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin G, Hayashi T, Kawagoe J, Takizawa T, Nagata T, Nagano I, Syoji M, Abe K. Deficiency of PAR-2 gene increases acute focal ischemic brain injury. J Cereb Blood Flow Metab. 2005;25:302–313. doi: 10.1038/sj.jcbfm.9600021. [DOI] [PubMed] [Google Scholar]
- 13.Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999;286:1358–1362. doi: 10.1126/science.286.5443.1358. [DOI] [PubMed] [Google Scholar]
- 14.Han BH, Holtzman DM. BDNF protects the neonatal brain from hypoxic-ischemic injury in vivo via the ERK pathway. J Neurosci. 2000;20:5775–5781. doi: 10.1523/JNEUROSCI.20-15-05775.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boscia F, Esposito CL, Di Crisci A, de Franciscis V, Annunziato L, Cerchia L. GDNF selectively induces microglial activation and neuronal survival in CA1/CA3 hippocampal regions exposed to NMDA insult through Ret/ERK signalling. PLoS One. 2009;4:e6486. doi: 10.1371/journal.pone.0006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Afkhami-Goli A, Noorbakhsh F, Keller AJ, Vergnolle N, Westaway D, Jhamandas JH, Andrade-Gordon P, Hollenberg MD, Arab H, Dyck RH, Power C. Proteinase-activated receptor-2 exerts protective and pathogenic cell type-specific effects in Alzheimer’s disease. J Immunol. 2007;179:5493–5503. doi: 10.4049/jimmunol.179.8.5493. [DOI] [PubMed] [Google Scholar]
- 17.Noorbakhsh F, Tsutsui S, Vergnolle N, Boven LA, Shariat N, Vodjgani M, Warren KG, Andrade-Gordon P, Hollenberg MD, Power C. Proteinase-activated receptor 2 modulates neuroinflammation in experimental autoimmune encephalomyelitis and multiple sclerosis. J Exp Med. 2006;203:425–435. doi: 10.1084/jem.20052148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakano Y, Kondoh G, Kudo T, Imaizumi K, Kato M, Miyazaki JI, Tohyama M, Takeda J, Takeda M. Accumulation of murine amyloidbeta42 in a gene-dosage-dependent manner in PS1 ‘knock-in’ mice. Eur J Neurosci. 1999;11:2577–2581. doi: 10.1046/j.1460-9568.1999.00698.x. [DOI] [PubMed] [Google Scholar]
- 19.Shen J, Bronson RT, Chen DF, Xia W, Selkoe DJ, Tonegawa S. Skeletal and CNS defects in Presenilin-1-deficient mice. Cell. 1997;89:629–639. doi: 10.1016/s0092-8674(00)80244-5. [DOI] [PubMed] [Google Scholar]
- 20.Barthet G, Dunys J, Shao Z, Xuan Z, Ren Y, Xu J, Arbez N, Mauger G, Bruban J, Georgakopoulos A, Shioi J, Robakis NK. Presenilin mediates neuroprotective functions of ephrinB and brain-derived neurotrophic factor and regulates ligand-induced internalization and metabolism of EphB2 and TrkB receptors. Neurobiology of aging. 2013;34:499–510. doi: 10.1016/j.neurobiolaging.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu J, Xilouri M, Bruban J, Shioi J, Shao Z, Papazoglou I, Vekrellis K, Robakis NK. Extracellular progranulin protects cortical neurons from toxic insults by activating survival signaling. Neurobiology of aging. 2011;32:2326 e2325–2316. doi: 10.1016/j.neurobiolaging.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stalheim L, Ding Y, Gullapalli A, Paing MM, Wolfe BL, Morris DR, deTrejo J. Multiple independent functions of arrestins in the regulation of protease-activated receptor-2 signaling and trafficking. Mol Pharmacol. 2005;67:78–87. doi: 10.1124/mol.104.006072. [DOI] [PubMed] [Google Scholar]
- 23.Ramachandran R, Mihara K, Mathur M, Rochdi MD, Bouvier M, Defea K, Hollenberg MD. Agonist-biased signaling via proteinase activated receptor-2: differential activation of calcium and mitogen-activated protein kinase pathways. Mol Pharmacol. 2009;76:791–801. doi: 10.1124/mol.109.055509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, Copeland RA, Magolda RL, Scherle PA, Trzaskos JM. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. The Journal of biological chemistry. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- 25.Dery O, Thoma MS, Wong H, Grady EF, Bunnett NW. Trafficking of proteinase-activated receptor-2 and beta-arrestin-1 tagged with green fluorescent protein. beta-Arrestin-dependent endocytosis of a proteinase receptor. J Biol Chem. 1999;274:18524–18535. doi: 10.1074/jbc.274.26.18524. [DOI] [PubMed] [Google Scholar]
- 26.De Vonis Bidlingmeyer U, Leary TR, Laskowski M., Jr Identity of the tryptic and alpha-chymotryptic reactive sites on soybean trypsin inhibitor (Kunitz) Biochemistry. 1972;11:3303–3310. doi: 10.1021/bi00767a028. [DOI] [PubMed] [Google Scholar]
- 27.De Strooper B. Aph-1, Pen-2, and Nicastrin with Presenilin generate an active gamma-Secretase complex. Neuron. 2003;38:9–12. doi: 10.1016/s0896-6273(03)00205-8. [DOI] [PubMed] [Google Scholar]
- 28.Takasugi N, Tomita T, Hayashi I, Tsuruoka M, Niimura M, Takahashi Y, Thinakaran G, Iwatsubo T. The role of presenilin cofactors in the gamma-secretase complex. Nature. 2003;422:438–441. doi: 10.1038/nature01506. [DOI] [PubMed] [Google Scholar]
- 29.Edbauer D, Winkler E, Regula JT, Pesold B, Steiner H, Haass C. Reconstitution of gamma-secretase activity. Nat Cell Biol. 2003;5:486–488. doi: 10.1038/ncb960. [DOI] [PubMed] [Google Scholar]
- 30.Esler WP, Kimberly WT, Ostaszewski BL, Diehl TS, Moore CL, Tsai JY, Rahmati T, Xia W, Selkoe DJ, Wolfe MS. Transition-state analogue inhibitors of gamma-secretase bind directly to presenilin-1. Nat Cell Biol. 2000;2:428–434. doi: 10.1038/35017062. [DOI] [PubMed] [Google Scholar]
- 31.Geling A, Steiner H, Willem M, Bally-Cuif L, Haass C. A gamma-secretase inhibitor blocks Notch signaling in vivo and causes a severe neurogenic phenotype in zebrafish. EMBO Rep. 2002;3:688–694. doi: 10.1093/embo-reports/kvf124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tu H, Nelson O, Bezprozvanny A, Wang Z, Lee SF, Hao YH, Serneels L, De Strooper B, Yu G, Bezprozvanny I. Presenilins form ER Ca2+ leak channels, a function disrupted by familial Alzheimer’s disease-linked mutations. Cell. 2006;126:981–993. doi: 10.1016/j.cell.2006.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang C, Wu B, Beglopoulos V, Wines-Samuelson M, Zhang D, Dragatsis I, Sudhof TC, Shen J. Presenilins are essential for regulating neurotransmitter release. Nature. 2009;460:632–636. doi: 10.1038/nature08177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baki L, Shioi J, Wen P, Shao Z, Schwarzman A, Gama-Sosa M, Neve R, Robakis NK. PS1 activates PI3K thus inhibiting GSK-3 activity and tau overphosphorylation: effects of FAD mutations. The EMBO journal. 2004;23:2586–2596. doi: 10.1038/sj.emboj.7600251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robakis NK. Mechanisms of AD neurodegeneration may be independent of Abeta and its derivatives. Neurobiology of aging. 2011;32:372–379. doi: 10.1016/j.neurobiolaging.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thiyagarajan M, Fernandez JA, Lane SM, Griffin JH, Zlokovic BV. Activated protein C promotes neovascularization and neurogenesis in postischemic brain via protease-activated receptor 1. J Neurosci. 2008;28:12788–12797. doi: 10.1523/JNEUROSCI.3485-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ling L, Hou Q, Xing S, Yu J, Pei Z, Zeng J. Exogenous kallikrein enhances neurogenesis and angiogenesis in the subventricular zone and the peri-infarction region and improves neurological function after focal cortical infarction in hypertensive rats. Brain Res. 2008;1206:89–97. doi: 10.1016/j.brainres.2008.01.099. [DOI] [PubMed] [Google Scholar]
- 38.Greenamyre JT, Young AB. Excitatory amino acids and Alzheimer’s disease. Neurobiol Aging. 1989;10:593–602. doi: 10.1016/0197-4580(89)90143-7. [DOI] [PubMed] [Google Scholar]
- 39.Mattson MP, Zhu H, Yu J, Kindy MS. Presenilin-1 mutation increases neuronal vulnerability to focal ischemia in vivo and to hypoxia and glucose deprivation in cell culture: involvement of perturbed calcium homeostasis. J Neurosci. 2000;20:1358–1364. doi: 10.1523/JNEUROSCI.20-04-01358.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choi DW. Ischemia-induced neuronal apoptosis. Curr Opin Neurobiol. 1996;6:667–672. doi: 10.1016/s0959-4388(96)80101-2. [DOI] [PubMed] [Google Scholar]
- 41.Sorsa T, Salo T, Koivunen E, Tyynela J, Konttinen YT, Bergmann U, Tuuttila A, Niemi E, Teronen O, Heikkila P, Tschesche H, Leinonen J, Osman S, Stenman UH. Activation of type IV procollagenases by human tumor-associated trypsin-2. J Biol Chem. 1997;272:21067–21074. doi: 10.1074/jbc.272.34.21067. [DOI] [PubMed] [Google Scholar]
- 42.Lauhio A, Sorsa T, Srinivas R, Stenman M, Tervahartiala T, Stenman UH, Gronhagen-Riska C, Honkanen E. Urinary matrix metalloproteinase -8, -9, -14 and their regulators (TRY-1, TRY-2, TATI) in patients with diabetic nephropathy. Ann Med. 2008;40:312–320. doi: 10.1080/07853890801923746. [DOI] [PubMed] [Google Scholar]
- 43.Moilanen M, Sorsa T, Stenman M, Nyberg P, Lindy O, Vesterinen J, Paju A, Konttinen YT, Stenman UH, Salo T. Tumor-associated trypsinogen-2 (trypsinogen-2) activates procollagenases (MMP-1, -8, -13) and stromelysin-1 (MMP-3) and degrades type I collagen. Biochemistry. 2003;42:5414–5420. doi: 10.1021/bi020582s. [DOI] [PubMed] [Google Scholar]
- 44.Georgakopoulos A, Litterst C, Ghersi E, Baki L, Xu C, Serban G, Robakis NK. Metalloproteinase/Presenilin1 processing of ephrinB regulates EphB-induced Src phosphorylation and signaling. Embo J. 2006;25:1242–1252. doi: 10.1038/sj.emboj.7601031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goedert M, Spillantini MG. Frontotemporal lobar degeneration through loss of progranulin function. Brain. 2006;129:2808–2810. doi: 10.1093/brain/awl291. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
WT and PS1 KO neuronal cultures prepared from the same littermate were treated at 7 DIV with 5.25 nM trypsin and 50 μM of glutamate for 4 hours. Untreated cultures were used as controls. Following incubation, cells were collected and assayed on WB for the indicated proteins. A representative blot is showing a decrease in ERK activation after glutamate addtion, however concomitant treatment of wt neurons with glutamate and trypsin increases ERK phosphorylation when compared to untreated neurons. In contrast, there is no effect of trypsin treatment on ERK activation in PS1 KO neurons.