Abstract
The heme oxygenase-1 (HO-1) enzyme system remains an attractive therapeutic target for the treatment of inflammatory conditions. HO-1, a cellular stress protein, serves a vital metabolic function as the rate-limiting step in the degradation of heme to generate carbon monoxide (CO), iron, and biliverdin-IXα (BV) which is converted to bilirubin-IXα (BR). HO-1 may function as a pleiotropic regulator of inflammatory signaling programs, through the generation of its biologically active end-products, namely CO and BV/BR. CO, when applied exogenously, can affect apoptotic, proliferative, and inflammatory cellular programs. Specifically, CO can modulate the production of pro- or anti-inflammatory cytokines and mediators. HO-1/CO may also have immunomodulatory effects with respect to regulating the functions of antigen-presenting cells, dendritic cells, and regulatory T-cells. Therapeutic strategies to modulate HO-1 in disease include the application of natural inducing compounds, as well as gene therapy approaches for the targeted genetic overexpression or knockdown of HO-1. Several compounds have been used therapeutically to inhibit HO activity, including competitive inhibitors of the metalloporphyrin series, or non-competitive isoform-selective derivatives of imidazole-dioxolanes. The end-products of HO activity, BV/BR and CO may be used therapeutically as pharmacological treatments. CO may be applied by inhalation, or through the use of CO releasing molecules (CORMs). This review will discuss HO-1 as a therapeutic target in diseases involving inflammation, including lung and vascular injury, sepsis, ischemia/reperfusion injury and transplant rejection.
INTRODUCTION
The heme oxygenase (HO) enzyme system continues to intrigue researchers across the spectrum of biological sciences, from those engaged in the study of basic metabolism and enzymology, to those investigating the pathogenesis of human disease with the ultimate goal of developing molecular medicine.1 HO provides an essential enzymatic activity by catalyzing the rate-limiting step in the oxidative catabolism of heme, in a reaction that generates carbon monoxide (CO), ferrous iron, and biliverdin-IXα (BV); the latter which is converted to bilirubin-IXα (BR) (Figure 1).2–3 Heme, the natural substrate and enzyme cofactor for HO, serves as a key mediator of many vital biological processes including oxygen transport and delivery to tissues, peroxide metabolism, cell signaling, xenobiotic detoxification, and mitochondrial bioenergetics. Thus, HO enzymes may fulfill a crucial metabolic function by regulating heme bioavailability and turnover in cells and tissues.4 In addition to this well-characterized metabolic function, heme oxygenase-1 (HO-1), the inducible form of HO, has gained recognition as a ubiquitous 32-kDa stress protein whose expression is highly upregulated in mammalian cells or tissues during cellular stress.5–6
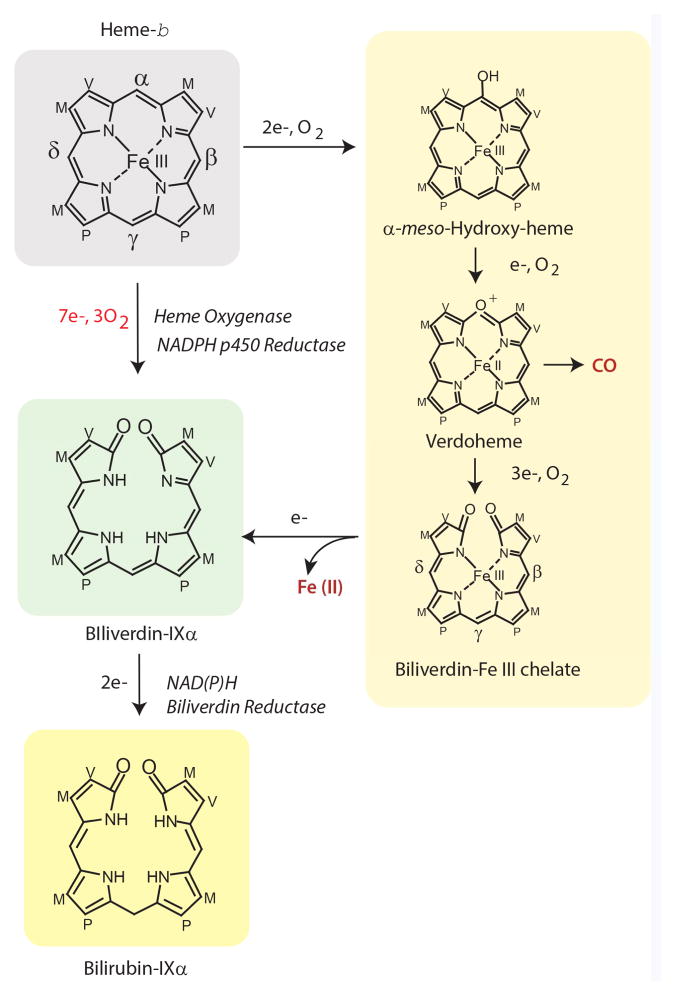
Figure 1.
The heme oxygenase (HO) reaction cleaves heme at the α-methene bridge carbon and generates carbon monoxide (CO), biliverdin-IXα. and ferrous iron (Fe II). The reaction proceeds through three sequential oxidation steps each requiring one mole of molecular oxygen (O2), and a total of seven electrons from NADPH: cytochrome p450 reductase. Three reaction intermediates have been proposed: α-meso-hydroxyheme, verdoheme, and the Fe (III)-biliverdin complex. Upon univalent reduction, the Fe (III)-biliverdin complex dissociates to form biliverdin-IXα and free Fe (II). The completion of enzymatic heme degradation involves the divalent reduction of biliverdin-IXα by NAD(P)H: biliverdin reductase (BVR; E.C. 1.3.1.24), which produces the lipid soluble pigment bilirubin-IXα. Heme side chains are designated: M=Methyl, V=Vinyl, P=Propionate.
In mammals, the gene(s) that encode HO-1 (HMOX1 in humans, Hmox1 in rodents), are highly transcriptionally-regulated by injurious stimuli. In additional to the natural substrate heme, and oxidizing cellular stress, such as generated by ultraviolet-A radiation, hydrogen peroxide (H2O2), and redox-cycling compounds, HO-1 responds to induction by a multiplicity of chemical and physical agents, including heat shock (in rodents), fluctuations in oxygen tension, nitric oxide, thiol-reactive substances, heavy metals, cytokines, and natural phytochemicals (see Table 1 for summary).5–22 Elevated HO-1 expression in tissue is commonly associated with increased inflammation or oxidative stress, as exemplified by models of acute lung injury (ALI) and ischemia/reperfusion (I/R) injury.22–24
Table 1.
Representative Chemical and Physical Inducers of HO-1 in cell culture models.
| Inducer Class | Chemical/Agent | Cell/Tissue Type | Refs |
|---|---|---|---|
|
| |||
| Metalloporphyrins | Heme Cobalt-Protoporphyrin |
Mouse hepatoma cells | 5 |
|
| |||
| Oxidants | H2O2 UVA (320–380 nm) radiation Menadione |
Human skin fibroblasts | 6–7 |
|
| |||
| Heavy Metals | CdCl2 | Mouse hepatoma cells | 5 |
|
| |||
| Thiol-Reactive Substances | Diethylmaleate | Chinese hamster ovary cells | 8 |
| Sodium arsenite | Human skin fibroblasts | 6 | |
| Nitric oxide | Vascular smooth muscle cells | 9 | |
|
| |||
| Pro-inflammatory Agents | LPS | RAW 264.7 Cells | 10 |
|
| |||
| Phytochemicals/Natural Antioxidants | Resveratol | PC-12 Cells | 11 |
| Curcumin | Endothelial cells, Renal epithelial cells, Astrocytes | 12–14 | |
| Quercetin | Rat aortic smooth muscle cells, Human hepatocytes | 15–17 | |
| Epigallocatchin gallate | Human aortic endothelial cells, B-lymphoblasts | 18–19 | |
|
| |||
| Physical Stress | Heat | Rat glioma, mouse melanoma | 20 |
|
| |||
| Oxygen tension | Hypoxia | Vascular smooth muscle cells | 21 |
| Hyperoxia | Epithelial cells, fibroblasts, macrophages, and smooth muscle cells. | 22 | |
The central importance of HO-1 in human physiology and tissue homeostasis is accentuated by studies of naturally occurring genetic deficiency of HO-1 in humans. A patient with human HO-1 deficiency presented with severe hemolytic anemia, endothelial degradation, reduced serum bilirubin, renal and hepatic iron accumulation, and a pro-inflammatory phenotype.25 Similarly, HO-1 gene-deleted mice (Hmox1−/−) displayed increased inflammation accompanied by tissue iron accumulation, whereas cells isolated from these animals displayed increased susceptibility to oxidative stress.26–27 Several studies, which have used Hmox1−/− mice or HO-1 transgenic mice, have demonstrated the tissue protective properties of HO-1 in mouse models of cardiovascular, pulmonary, cardiac, or skin injury and disease (see Table 2 for summary).28–37 Despite these observations, deleterious consequences of HO-1 or HO-2 overexpression have been reported in vitro and in vivo associated with toxic levels of iron accumulation.38–43
Table 2.
Preclinical Studies Demonstrating the Importance of HO-1 in Disease
| Strain | Model | Phenotype | Refs |
|---|---|---|---|
|
| |||
| Hmox1−/− | Vascular Injury | Increased intimal hyperplasia | 29 |
| Pulmonary Ischemia/Reperfusion | Repression of fibrinolysis | 30 | |
| Hypoxia | Increased myocardial infarction, Right ventricular dilation | 31 | |
|
| |||
| Hmox1TG (Cardiac) | Ischemia/Reperfusion | Protection from I/R injury | 32 |
| Reduction of cardiomyocyte apoptosis | 33 | ||
| Hmox1TG (Cardiac, Inducible) | Cardiotoxicity (Cre-recombinase) | Protection from cardiotoxicity | 34 |
|
| |||
| Hmox1TG (Pulmonary) | Hypoxia-induced pulmonary hypertension | Reduced symptoms of hypertension | 35 |
| Anti-inflammatory protection | 36 | ||
|
| |||
| Hmox1TG (Keratinocyte) | Skin lesions | Improved wound healing and vascularization | 37 |
The mechanisms by which HO-1 expression is associated with context-specific cytoprotection remain incompletely clear, but may reside in the combined effects of the removal of heme (a pro-oxidant iron-chelate) with the enzymatic generation of biologically-active end-products from heme catabolism.43 This hypothesis has provided the basis for the development of new fields focused on the pharmacological delivery of HO-1 reaction products. In this regard, application of CO has demonstrated tissue protective effects in models of acute inflammation and organ injury.28, 44 These studies, using inhaled CO gas, include endotoxemia,45–47 hyperoxia-induced ALI,48–49 ventilator-induced lung injury (VILI),50–52 sepsis and pneumonia,53–55 I/R injury56–57, vascular injury and disease,58–60 and organ transplantation58,61–83 (see Table 3 for representative summary). The protective effects observed in these models were attributed to the effects of CO on apoptosis, cell proliferation, inflammation and immunomodulation.28,44 Similarly, the pharmacological applications of BV or BR, enzymatic products of heme metabolism, have shown protective effects in models of organ injury and transplantation.63,67,81,84–86
Table 3.
Representative studies demonstrating tissue protective effects of CO in pre-clinical models of inflammatory disease
| Model | Target Organ | Species | Phenotype | Ref |
|---|---|---|---|---|
|
| ||||
| Endotoxemia | Systemic | Mice | Increased survival, Reduced pro-inflammatory cytokine production, increased IL-10 | 45 |
| Pigs | Reduced coagulation. Reduced pro-inflammatory cytokine production, increased IL-10 | 46 | ||
| Monkeys (Macaques) | Reduced TNF, Reduced Neutropenia | 47 | ||
|
| ||||
| Hyperoxic Injury | Lung | Mice | Increased survival, Reduced pro-inflammatory cytokine production | 48–49 |
|
| ||||
| Mechanical Ventilation | Lung | Rats, Mice | Reduction of BAL protein and cell count, lung neutrophil recruitment, and edema | 50–52 |
|
| ||||
| Sepsis | Lung, Liver | Mice | Increased survival, increased bacterial clearance from organs and blood, induction of autophagy | 53–54 |
| Baboons | Normalization of pro-resolving mediator profiles | 55 | ||
|
| ||||
| Ischemia/Reperfusion | Lung Liver |
Mice | Inhibition of apoptosis Reduced inflammation |
56–57 |
|
| ||||
| Vascular Injury | Vascular Tissue | Mice, Rats | Reduced intimal hyperplasia Reduced inflammation |
58 |
|
| ||||
| Sickle Cell Disease | Vascular Tissue | Mice | Reduced vascular stasis Reduced lung NF-κB activation |
59–60 |
|
| ||||
| Organ Transplantation | Lung | Rat | Inhibition of apoptosis and inflammation, reduced macrophage infiltration | 61–63 |
| Swine | Graft tolerance, Inhibition of inflammation | 64 | ||
| Vascular | Rat | Reduced intimal hyperplasia Reduced inflammation |
58, 65 | |
| Heart | Mouse-to-Rat, Rat | Inhibition of platelet aggregation, thrombosis, apoptosis, infarction | 66–70 | |
| Kidney | Rat | Improved renal function, survival | 67; 71–74 | |
| Swine | Improved graft function, reduced I/R injury | 75–76 | ||
| Liver | Rat | Reduced I/R injury | 77–78 | |
| Intestine | Rat | Reduced I/R injury | 79–80 | |
| Pancreas | Rats, Mice | Improved graft survival, Islet function | 81–83 | |
In addition to pleiotropic cellular effects of HO-1, including reported effects on the regulation of programmed cell death and proliferation programs,87 current research points to profound anti-inflammatory and immunomodulatory properties of HO-1 and its reaction products (e.g., CO)88 (Figure 2). This review will focus on the crucial impact of HO-1/CO in inflammation and the underlying mechanisms, in human diseases. Emphasis will be placed on the modulation of HO-1 expression and activity as a potential therapeutic strategy in human diseases that implicate inflammation as a key mediator of pathogenesis. Such strategies may include natural inducing compounds and gene therapy approaches to elevate HO-1 expression, the pharmacological delivery of reaction products such as CO or BV/BR, as well as gene silencing approaches and chemical inhibitors to reduce HO expression and activity in a context-specific fashion. (Figure 3). 1,28,44,89
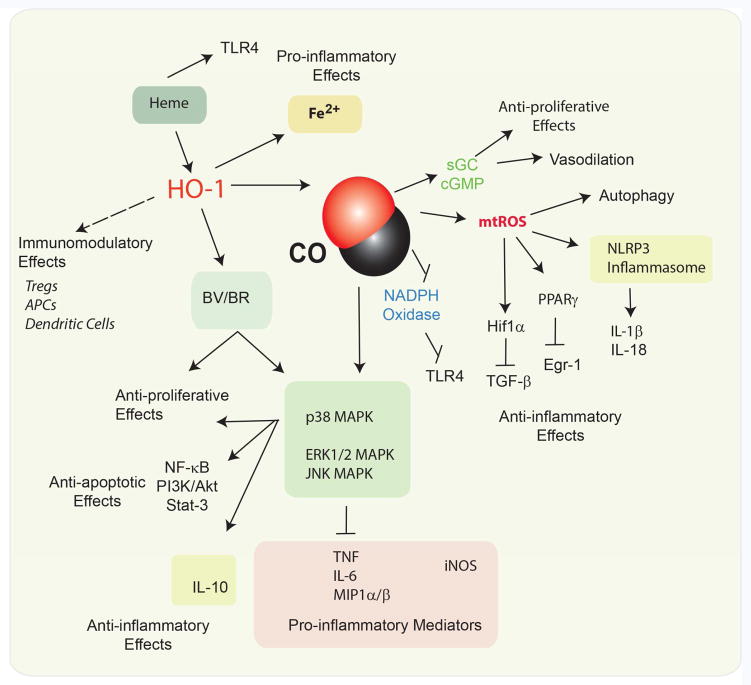
Figure 2.
Pivotal Functions of HO-1 in inflammation. HO-1 may have immunomodulatory effects with respect to regulating the functions of antigen presenting cells, dendritic cells, and regulatory T-cells. Heme may exert pro-inflammatory effects. HO-1 end products generated from heme degradation may modulate inflammation. Iron release from HO activity may be pro-inflammatory in the case of excess activation, and has been associated with neurodegenerative diseases. CO whether endogenously produced or applied as a pharmacological treatment, has been shown to modulate apoptotic, proliferative, and inflammatory cellular programs. In particular, CO can downregulate the production of pro-inflammatory cytokines (e.g., IL-1β, IL-6, TNFα, Mip1α/β, and upregulate the anti-inflammatory cytokines (IL-10). These effects were attributed to alterations of MAPK activities including p38 MAPK. CO can stimulate mitochondrial ROS production, which can promote the autophagy program, activate HIF-1α, and downregulate pro-inflammatory transcription factor Egr1. Recent evidence also suggests that CO can modulate the activation of the NLRP3 inflammasome, which regulates the production of IL-1β, and IL-18. BR, a product of heme degaradtion, also may exert anti-inflammatory and anti-proliferative effects.
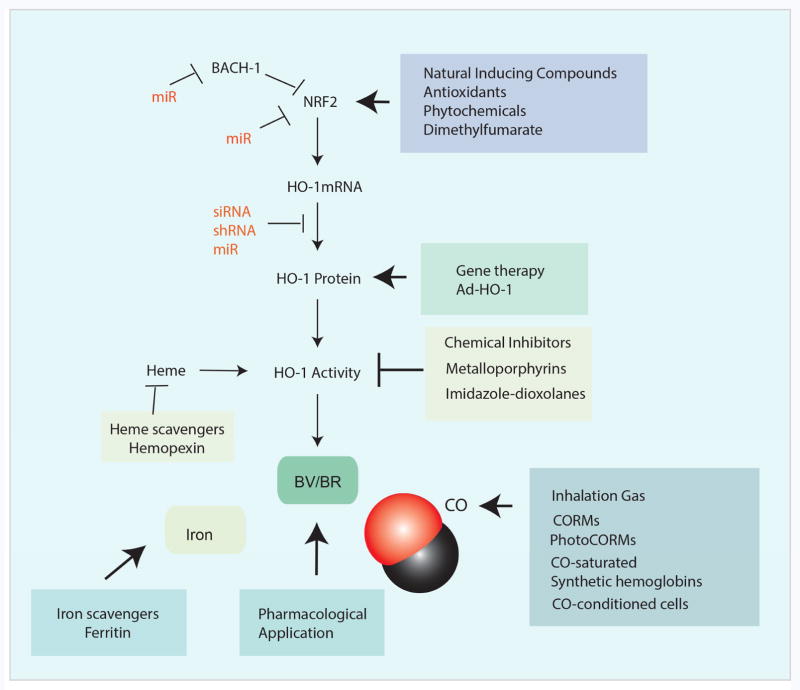
Figure 3.
Therapeutic modulation of the HO-1/CO system. HO-1 can be upregulated by natural antioxidants which act upon the NRF2/Keap1 system to upregulate the transcription of the Hmox1 gene. The application of antioxidant compounds may represent a possible strategy for therapeutic manipulation of HO-1. Gene therapy approaches using retroviral vectors may be used to upregulate HO-1. HO-1 is subject to natural up or downregulation through mIRs that target Hmox1 or other genes involved in its regulation (Bach1, Nrf2). The targeted expression of specific mIRs may also be combined with gene therapy approaches. Several compounds have been used therapeutically to inhibit HO activity. These include competitive inhibitors of the metaloporphyrin series, or non-competitive isoform selective derivatives of imidazole-dioxolanes. The end-products of HO activity, namely BV or BR, and CO may be used therapeutically as pharmological reagents. CO may be applied by inhalation, or through the use of CO releasing molecules (CORMs and PhotoCORMs). Additional therapeutic strategies involving CO include CO-saturated hemoglobins, or the use of CO preconditioned cells for cell based therapy. Furthermore, the removal of hemoglobin/heme or iron by scavenger compounds or chelation, respectively, have been proposed as intervention strategies.
HEME OXYGENASE ACTIVITY AND ISOZYMES
HO activity [heme, hydrogen-donor:oxygen oxidoreductase (α-methene-oxidizing, hydroxylating), EC: 1:14:99:3] catalyzes the rate-limiting step in the oxidative catabolism of heme-b. The HO reaction cleaves heme at the α-methene bridge carbon which is liberated as carbon monoxide (CO), with stoichiometric generation of the open-chain tetrapyrrole biliverdin-IXα (BV), and ferrous iron (Fe II).2–3, 90–91 The reaction proceeds through three sequential oxidation steps each requiring one mole of molecular oxygen (O2).92–94 Each oxidation cycle requires reducing equivalents from NADPH: cytochrome p450 reductase (CPR; E.C. 1.6.2.4).95–97 In the first oxidation step, which converts heme to α-meso-hydroxyheme, one electron from NADPH is utilized for the reduction of the ferric heme iron to its ferrous form, which accepts the binding of O2. A second electron is utilized for the activation of O2 to form the hydroxylating species, Fe-OOH.93–94, 98–100 Additional electron transfer steps are required for the conversion of α-meso-hydroxyheme to verdoheme, which releases CO, and the subsequent conversion of verdoheme the Fe (III)-biliverdin complex90, 94, 101–103 Upon univalent reduction, the Fe (III)-biliverdin complex dissociates to release BV and free Fe (II).99 The completion of enzymatic heme degradation involves the divalent reduction of BV by NAD(P)H: biliverdin reductase (BVR; E.C. 1.3.1.24), which produces the lipid soluble pigment bilirubin-IXα (BR). 2–3, 104 HO activity occurs highest in the spleen, testes, and brain, with moderately high levels reported in the liver, kidney and lung.4 HO activity is represented by two major molecular species, HO-1 and HO-2.105–106 HO-1 expression is highest in the spleen, the site of erythrocyte turnover. In most tissues HO-1 is undetectable under basal conditions, but highly inducible under conditions of stress or inflammation.107 The constitutive isoform HO-2 is expressed highly in many tissues including testes, spleen, liver, kidney, cardiovascular and nervous systems.4,107 HO-1 and HO-2 represent the products of distinct genes and differ in primary amino acid sequence and biochemical/biophysical properties.108–109 HO-1 and HO-2 share a highly conserved heme catalytic domain as well as hydrophobic regions at the carboxyl-terminus.110–111 HO-2 contains additional non-catalytic heme binding sites termed heme regulatory domains, with unclear functional significance.112
HO-1 and CPR localize to the smooth ER, though recent evidence suggests an association of these proteins with other intracellular membranes, including the inner mitochondrial membrane,113–114 and plasma membrane caveolae.115 Immunological evidence supports the existence of intramolecular interactions between HO-1, CPR, BVR, and between HO-1, BVR, and caveolin-1.115–117 Recent studies suggest that the plasma membrane scaffolding caveolin-1 can act as a natural inhibitor of HO activity.115–116 Additional studies suggest the occurrence of immunoreactive forms of HO-1 in the nucleus which lack catalytic activity. A putative role for nuclear forms of HO-1 in transcriptional regulation has been proposed.118
REGULATION OF HEME OXYGENASE GENE EXPRESSION
Transcriptional Regulation
The mechanisms by which HO-1 is regulated at a transcriptional level have been elucidated from functional analyses of the 5′ regulatory regions of the corresponding genes in rodents and humans. Studies of the mouse Hmox1 gene promoter have revealed two major regulatory enhancer regions which are located at −4 kb and −10 kb upstream of the transcriptional start site. These enhancer elements are essential for the transcriptional regulation of the Hmox1 gene in response to many inducing chemicals including bacterial LPS, and heavy metals such as CdCl2.119–121 The mouse proximal and distal enhancer regions contain tandem repeats of the stress-responsive element/antioxidant response element (StRE/ARE) motif.121
The nuclear factor erythroid 2 (NFE2)-related factor-2 (NRF2) recognizes and binds to the StRE/ARE motifs, and represents the major transcriptional regulator of the Hmox1 gene.122 Nrf2 is anchored in the cytoplasm by the Kelch-like ECH-associated protein (Keap1), which inhibits its transcriptional activity. Keap1 facilitates the targeted ubiquitination of Nrf2 by the Cullin 3-based E3 ubiquitin ligase complex, which marks Nrf2 for proteasomal degradation.123–125 Under basal conditions, Keap1 forms a complex with Nrf2 and prevents its nuclear translocation. Due to the reversible oxidation state of critical cysteine residues, Keap1 has been proposed to act as a redox sensor.126 The selective autophagy cargo adaptor protein p62 interacts with Keap1 at the Nrf2 binding site, thus promoting the displacement of Keap-1 from Nrf2 to enhance Nrf2 transcriptional activity.127 Keap1 has also been found to undergo constitutive degradation through the lysosome-dependent autophagy pathway.128 When cells are exposed to inducing stimuli, such as heme, electrophiles and oxidants, Nrf2 dissociates from Keap1 and then translocates to the nucleus where its transcription factor activity requires the formation of stable heterodimers with small Maf proteins (i.e., MafF, MafG).122–124 The heme binding protein BTB and CNC homologue 1 (Bach1) has been identified as a negative transcriptional regulator of Hmox1. Bach1 forms a complex with small Maf proteins and competes against Nrf2 for binding at the StRE. The heme complex with Bach1 impairs its DNA binding activity and promotes its nuclear export.129–130 Although LPS acts as a positive inducer of HO-1 in many cell types, including murine monocytes and macrophages, the paradoxical inhibition of HO-1 by LPS in primary human monocytes and macrophages was attributed to activation of Bach1.131–132
In addition to the proximal and distal enhancer regions, the Hmox1 gene contains other functional cis-acting elements that contribute to transcriptional regulation, including the heat shock and hypoxia-responsive elements identified in rodents.20–21 The human HMOX1 gene also contains regions analogous to the upstream enhancer regions described in rodents, but differs from the rodent genes in sequence-specific variation in the regulatory elements at −4 kb, and the presence of an early growth response-1 protein (Egr-1) binding site at −9.5 kb.133–134 The multiplicity of functional as well as putative regulatory sequences occurring in the genes encoding HO-1 in rodents or humans suggests that the regulation of these genes involves complex transcription factor interactions that may vary in an inducer-specific fashion.135–137
HMOX1 Promoter Polymorphisms
Microsatellite (GT)n dinucleotide length polymorphisms have been identified in the regulatory regions of the human HMOX1 gene (located on chromosome 22q13.1) which may result in the impaired transcriptional regulation and decreased expression of HO-1 in individuals that carry the long (L) allele [(GT)n ≥30] of this polymorphism. A number of studies have described positive correlation between the incidence of HMOX1 promoter polymorphisms with disease severity, mortality, or risk in various human diseases.138–139 These studies include associations of HMOX1 promoter polymorphisms with diseases such as atherosclerosis and cardiovascular disease,140 chronic kidney disease,141 coronary artery disease,141 necrotizing acute pancreatitis,142 and preeclampsia.143 Additional studies reported associations of the long allele of (GT)n with chronic obstructive pulmonary disease (COPD), acute respiratory distress syndrome, vascular restenosis, rheumatoid arthritis and outcome of renal transplantation.144–150 In contrast, short [(GT)n ≥20] HMOX1 promoter polymorphisms were associated with susceptibility to neonatal hyperbilirubinemia.151 Microsatellite polymorphisms can affect alternative splicing in the HMOX1 5′ untranslated region, thus providing an additional regulatory mechanism for HMOX1 translation.152 Additional SNPs in HMOX1 have been identified, including rs2071746 T/A which has been associated with increased risk of Parkinson’s disease, and with protection from cardiovascular disease.153–154
In contrast, various negative studies have reported no associations between HMOX1 polymorphisms in the outcome of cardiac or renal transplantation,155–156 coronary artery disease,157 and alcoholic liver disease.158 Tanaka et al. recently reported no association between five SNPs (i.e., rs3761439, rs2071746 and (GT)n) and HMOX1 gene expression or lung functional decline in COPD patients.159 In conclusion, association studies, when positive, have suggested that a genetically-dependent downregulation of HO-1 expression may arise in subpopulations, possibly linked to increased susceptibility to oxidative stress, inflammation and associated diseases. This hypothesis remains inconclusive and obscured by negative studies. Further research will be needed to validate these observations.
Regulation of HO-1 by MicroRNA
MicroRNAs (miR) are small non-coding RNAs that function in post-transcriptional regulation of gene expression. Emerging studies suggest the critical involvement of miRs in the regulation of HO-1 gene expression either directly by decreasing mRNA stability and/or translation, or indirectly by modulating the expression of upstream regulatory factors (e.g., Nrf2/Keap1, Bach1, etc.).160 A number of miRs may regulate the expression of Nrf2, which may result in the altered regulation of HO-1.160–163 Several miRs identified in direct HO-1 translational regulation include miR-24164–166 miR-200c,162 miR-204/miR-211,167 miR-155,168 miR-378,169 miR-377,170 and miR-217.170 Additional indirect mechanisms for miR-dependent regulation of HO-1 have been proposed. For example, several miRs (e.g., miR-155) can potentially activate HO-1 expression through the downregulation of the transcriptional repressor Bach1.171–173 Additionally, miR-101 promoted HO-1 expression by targeting the Cullin 3: E3 ubiquitin ligase, resulting in Nrf2 stabilization,174 whereas miR-200a promoted Nrf2 activation by targeting its cytoplasmic anchor Keap-1.175
HO-1 can also regulate miR networks, implicating downstream miR regulation in the functional effects of HO-1 in differentiation, angiogenesis, proliferation, inflammation and tumorigenesis.176 For example, expression of HO-1 in myoblasts downregulated specific miRs (e.g., miR-1, miR-133a/b, and miR-206) associated with the inhibition of myoblast differentiation.177 Expression of HO-1 in tumor cells was associated with downregulation of miR-378.169 These observations point to an emerging complexity of HO-1 regulation and function that may involve a network of miR-dependent regulatory events.
HEME OXYGENASE/CARBON MONOXIDE AS MODULATORS OF INFLAMMATION AND THE IMMUNE RESPONSE
Acute Inflammation Models
HO-1 has been identified as a major modulator of the acute inflammatory response, as demonstrated in in vitro and in vivo models of inflammation and acute lung injury.45, 178–179. Early studies by Willis et al. suggested the involvement of HO-1 in the resolution of acute inflammation in vivo.178 In a model of carageenin-induced inflammation of the pleural cavity associated with neutrophil influx, the expression of HO-1 in pleural macrophages was highest at the time of resolution. Inhibition of HO activity by treatment with the HO inhibitor Sn-protoporphyrin-IX (SnPPIX) increased inflammatory exudates in this model whereas hemin-dependent upregulation of HO-1 reduced inflammation.178 The enhanced gene expression of HO-1 by intratracheal adenoviral-mediated gene transfer protected against LPS-induced lung injury in mice involving the increased production IL-10.179 Anti-inflammatory effects of low-dose inhaled CO (i.e., 250 ppm) were also demonstrated in vivo using a mouse model of endotoxemia.45 CO preconditioning reduced the production of serum TNF-α, IL-1β, IL-6, whereas increased the production of the anti-inflammatory cytokine IL-10; reduced organ injury and prolonged survival following LPS challenge.45, 180 The anti-inflammatory potential of CO with respect to modulation of pro- or anti-inflammatory cytokines production was reduced in heat shock factor-1 knockout (hsf1−/−) mice, implicating a role of the heat shock response in vivo.181 Similar anti-inflammatory effects were observed in mice treated with inhalation CO prior to hyperoxia exposure, a model of ALI.48–49 The anti-inflammatory protection against LPS-induced organ injury conferred by CO was also observed in association with inhibition of inducible nitric oxide synthase (iNOS) expression and activity in the lung, and enhancement of iNOS expression and activity in the liver.182 Pharmacological application of BV, an enzymatic by-product of HO activity, also decreased pro-inflammatory cytokine production, upregulated IL-10 levels, and reduced lung injury markers in LPS-treated rats.183
In cultured RAW 264.7 macrophages challenged with LPS, HO-1 gene transfer or application of low concentrations of CO (e.g., 250 ppm) inhibited the production of pro-inflammatory cytokines, including TNF-α, IL-1β, and macrophage inflammatory protein-1β (MIP-1β), and simultaneously increased the expression of the anti-inflammatory cytokine IL-10.45 The effect of CO on the downregulation of pro-inflammatory cytokines production was associated with the modulation of mitogen activated protein kinase (MAPK) activities, including p38 MAPK and c-Jun NH2-terminal kinase (JNK).45, 180 Lee et al. demonstrated that HO-1 can be upregulated by IL-10, and proposed that the anti-inflammatory effect of this cytokine depended on HO-1 activation.184 The anti-inflammatory effect of CO in macrophages involved the downregulation of reactive oxygen species (ROS)-dependent recruitment of TLR4 to plasma membrane lipid rafts.185 Pretreatment of RAW 264.7 macrophages with heme, or transfection of HO-1 significantly inhibited the production and nuclear translocation of the pro-inflammatory factor high-mobility group box-1 (HMGB1), and impaired the release of pro-inflammatory cytokines in response to LPS challenge. These effects were mimicked by CO donor compounds and reversed by CO scavengers.54 Additional studies have associated the anti-inflammatory effect of CO with activation of the heat shock factor-1 (HSF-1), and the upregulation of the immunoresponsive gene-1 protein in macrophages.181,186
Anti-inflammatory effects of CO have also been demonstrated in pre-clinical models employing higher mammals. In swine, CO reduced the development of disseminated intravascular coagulation and inhibited serum levels of the pro-inflammatory cytokine IL-1β in response to LPS, whereas upregulated IL-10 levels.46 Similar, though less pronounced anti-inflammatory effects were observed in a non-human primate model of Cynomolgous macaques subjected to LPS challenge.47 CO exposure following LPS inhalation decreased TNF-α release in broncoalveolar lavage (BAL) fluid, but had no apparent effect on IL-6 and IL-8 release, in addition to reducing pulmonary neutrophilia at the highest concentration (500 ppm). The therapeutic efficacy of CO in this model required relatively high doses that resulted in elevated carboxyhemoglobin (CO-Hb) levels (>30%). This study was the first to examine the therapeutic index and dose-response relationships of CO therapy in nonhuman primates in an acute inflammation model.47
The potential for protective effects of HO-1/CO have been evaluated in a clinically-relevant model of sterile inflammation induced by mechanical ventilation (MV) which causes ventilator-induced lung injury (VILI) in rats and mice.50–52 Rats ventilated with an injurious (high tidal volume) ventilator setting in combination with LPS injection, exhibited lung injury. The inclusion of CO (250 ppm) during MV reduced the inflammatory cell infiltration, decreased TNF-α and increased IL-10 in BAL fluid.50 CO application conferred tissue protection in a mouse model of VILI, at moderate tidal volume ventilation.51–52 CO reduced MV-induced cytokine and chemokine production and prevented lung injury during ventilation, involving the reduction of BAL protein and cell count, lung neutrophil recruitment, and pulmonary edema.51–52 These effects of CO were associated with the activation of caveolin-151 the activation of PPARγ, and the inhibition of the pro-inflammatory factor Egr-1.52 These studies, taken together, suggest that CO may protect the lung in animal models of VILI. More studies are required to determine the underlying mechanisms of the therapeutic effectiveness of CO in rodent VILI models, and whether these observations may be translatable to humans.
Regulation of the Inflammasome by HO-1/CO
Inflammasomes are cytosolic protein complexes that promote the cleavage of caspase-1, which leads to the maturation and secretion of pro-inflammatory cytokines including interleukin-1β (IL-1β) and IL-18. Among the known inflammasomes, the NOD-, leucine rich region- and pyrin domain-containing-3 (NLRP3)-dependent inflammasome is crucially involved in the pathogenesis of various acute or chronic inflammatory diseases.187–188
Current research suggests a potential regulatory role for HO-1/CO in the inflammasome system. Recent studies demonstrate that heme pre-conditioning to induce HO-1 can reduce IL-1β maturation and downregulate inflammasome activation in a model of sepsis-associated lung injury.189 Furthermore, induction of HO-1 by heme conditioning was associated with protection from acute liver injury induced by D-galactosamine and LPS, and with downregulation of the associated NLRP3 inflammasome-dependent activation of caspase-1.190
Our recent studies demonstrate that application of CO gas can inhibit caspase-1 activation and secretion of IL-1β and IL-18 in bone marrow-derived macrophages in response to LPS and ATP treatment, a NLRP3 inflammasome activation model.191 CO also inhibited IL-18 secretion in response to LPS and nigericin, an alternate NLRP3 inflammasome activator. LPS and ATP stimulation induced the formation of complexes between NLRP3 and ASC, or NLRP3 and caspase-1. CO treatment inhibited these molecular interactions induced by LPS and ATP. In addition, CO inhibited mitochondrial depolarization induced by LPS and ATP in macrophages.191 These results suggest that CO negatively regulates NLRP3 inflammasome activation in part by preventing mitochondrial dysfunction.191 Application of the CO donor compound CORM-2 downregulated caspase-1 activation and IL-1β maturation and secretion in the context of ER-stress induced inflammation.192 In contrast to these observations, another report shows that CO can promote ATP secretion from bacteria which in turn can upregulate NLRP3 inflammasome activation by stimulating the purinergic receptor (P2X7R) in bacteria-treated macrophages.193 The reasons for these contrasting findings remain unclear at present, but may be related to differences between live bacterial sepsis and inflammation induced by cell free LPS. Although further studies will be needed to clarify the precise mechanisms, HO-1 and CO show potential as novel regulators of the NLRP3 inflammasome and secretion of IL-1β and IL-18.
HO-1/CO in Sepsis and Infectious Disease
A protective anti-inflammatory function of HO-1 has been demonstrated in the context of of microbial sepsis.54,194–196 Using the cecal ligation and puncture (CLP) method to induce polymicrobial sepsis, HO-1-deficient mice (Hmox1−/−) displayed higher mortality rates compared with wild type mice. The Hmox1−/−mice also had increased levels of free circulating heme and reduced levels of the heme sequestering protein hemopexin, rendering them more susceptible sepsis-induced mortality.195 Conversely, targeted over-expression of HO-1 to smooth muscle cells and myofibroblasts, and bowel protected against sepsis-induced mortality associated with Enterococcus faecalis infection, and enhanced bacterial clearance by increasing phagocytosis and the endogenous antimicrobial response.194 These studies suggest that endogenous heme is a pro-pathogenic mediator which is associated with propagation of tissue injury and inflammation in sepsis. The pro-inflammatory effects of heme have been demonstrated in cultured cells, and involve activation of TLR4-mediated signaling pathways leading to increased production of ROS in macrophages and activation of endothelial cells.197–198 In contrast, pharmacological application of heme has been used as a preconditioning agent, which can confer protection by virtue of its ability to induce HO-1 in tissues in advance of injury, resulting in cytoprotection. For example, hemin pre-conditioning of healthy animals, which induces HO-1, can protect mice from subsequent lethal endotoxemia and sepsis induced by LPS or CLP, respectively.54
Several studies have demonstrated that circulating levels of HMGB1, a protein that can regulate chemotaxis and accumulation of pro-inflammatory cytokines, contributes to LPS-induced mortality in Hmox1−/− mice.54,196 Furthermore, pharmacological preconditioning with heme, to induce HO-1, significantly reduced plasma levels of HMGB1 in mice challenged with LPS or CLP, which was associated with the reduction of serum TNF-α, and IL-1β levels.54,196 Transfection of HO-1 or induction of HO-1-derived CO resulted in a significant reduction in the translocation and release of HMGB1 in CLP-induced sepsis in vivo. In conclusion, HO-1-derived CO attenuated HMGB1 release during sepsis, which may represent a therapeutic intervention against sepsis.54
Emerging studies suggest that CO can enhance bacterial clearance, though the mechanisms remain incompletely understood.193–194,199 The pharmacological application of CO releasing pro-drugs enhanced bacterial phagocytosis in vivo and rescued Hmox1−/− mice from sepsis-induced mortality.194 Furthermore, CO releasing pro-drugs improved intestinal bacterial clearance in an S. typhimurium infection model.199 In an E. coli infection model, endogenous HO-derived CO was associated with enhanced macrophage phagocytosis and this was shown to require NLRP3 and caspase-1 dependent immune responses.193 In accord with these studies, we have recently shown that CO inhalation (250 ppm) either as pre-treatment or post treatment improved mouse survival in the CLP model. The salutary effects of CO in this model were related to the induction of autophagy and phagocytosis, the reduction of inflammation, and enhanced bacterial clearance from organs and blood. The pro-survival effects of CO in CLP were dependent on the autophagy program, as they were reduced in mice heterozygous for genetic deletion in the autophagy regulator molecule Beclin 1.53
The therapeutic potential of HO-1/CO has also been investigated in infectious disease models. The enhanced gene expression of HO-1 in rat lungs by intratracheal adenoviral mediated gene transfer limited murine acute lung injury following influenza virus infection.200 Current studies have evaluated the therapeutic potential of CO using an S. pneumoniae model of pneumonia in baboons.55 Plasma obtained from S. pneumoniae infected baboons displayed significantly reduced levels of lipid mediator/specialized pro-resolving mediators (SPM), including eicosapentaenoic acid-derived E-series resolvins (RvE) and lipoxins, suggesting that pneumonia can deregulate pro-resolution programs in baboons. In these animals, CO inhalation increased the levels of plasma RvE and lipoxins relative to control levels. These results suggested that altered SPM profiles during pneumonia can be partially restored with inhaled low-dose CO.55
HO-1/CO in Vascular Injury and Disease
Protective roles for HO-1/CO in vascular injury and disease have been reported.29, 58 In injured pig arteries, HO-1 gene transfer increased vasodilation and inhibited vascular smooth muscle proliferation.29 Hmox1−/− mice displayed increased intimal hyperplasia after aortic injury relative to corresponding wild type mice.29 HO-1 expression in vitro reduced vascular smooth muscle cell (SMC) proliferation through upregulation of p21Cip1.29 Inhaled CO prevented arteriosclerotic lesions associated with aorta transplantation in rodents, and reduced intimal hyperplasia associated with vascular injury. Exposure to a low level of CO (250 ppm) for 1 hour before injury inhibited intimal hyperplasia associated with balloon injury.58 The protective effect of CO was associated with inhibition of graft leukocyte infiltration/activation as well as with inhibition of SMC proliferation through upregulation of p21Cip1.58
HO-1 gene transfer was also shown to reduce hypoxia/reoxygenation-induced stasis in mice with sickle cell disease (SCD). The increase of HO-1 expression in this model promoted anti-inflammatory effects associated with the activation (phosphorylation) of p38 MAPK and Akt, decreased the nuclear accumulation of p65 NF-κB in liver tissue, as well as reduced serum levels of soluble vascular cell adhesion molecule-1 (sVCAM-1).201 Pharmacological application of heme to increase HO-1 expression in the liver, inhibited vascular stasis and leukocyte adhesion, and inhibited NF-κB activation. CO inhalation also reduced stasis and conferred similar anti-inflammatory effects in this model.59,60 Additionally, CO inhalation reduced hepatic necrosis, reduced myeloid and lymphoid markers in the bone marrow, and reduced blood cell counts in SCD mice.60
HO-1/CO in Organ Ischemia/Reperfusion Injury
Tissue protective effects of HO-1 or CO have been demonstrated in rodent models of organ I/R injury. For example, overexpression of HO-1 protected against myocardial injury in a mouse cardiac I/R injury model.202 Mice genetically deficient in HO-1 (Hmox-1−/−) were sensitized to the lethal effects of lung I/R injury. Inhaled CO compensated for the HO-1 deficiency in hmox-1−/− mice, and improved survival during pulmonary I/R.30 The protection provided by CO involved activation of fibrinolysis in a guanylate cyclase-dependent mechanism, involving downstream inhibition of plasminogen activator inhibitor-1.30 CO inhibited fibrin deposition and improve circulation in ischemic lungs, by inhibiting the pro-inflammatory transcription factor Egr-1.203 Additional studies in the pulmonary I/R model demonstrate protective effects of CO involving downregulation of apoptosis pathways.56,204
In organ transplantation, I/R injury subsequent to transplantation may represent a major causative factor in graft failure. Transgenic systemic overexpression of HO-1 was shown to improve cardiac graft tolerance and reduce inflammation associated with I/R injury in a cardiac transplantation model.205 CO has been intensively studied as an anti-inflammatory therapeutic in experimental organ transplantation. CO has a demonstrated potential for reducing transplant associated I/R injury and also reducing the probability of graft rejection when applied at low concentration, when added to organ preservation fluid or when applied to donors and/or recipients in gaseous form at low concentration. The application of CO can confer protection during transplantation of multiple organs (see Table 3). In a model of orthotopic lung transplantation in rats, exogenous application of CO (500 ppm), significantly protected the graft, and reduced hemorrhage, fibrosis, and thrombosis after transplantation.61 Furthermore, CO inhibited lung cell apoptosis and downregulated lung and systemic pro-inflammatory cytokine production.61 In a vascular transplantation model, when transplant recipients of aortic grafts were exposed to CO (250 ppm), these animals displayed reduced intimal hyperplasia, and reduced leukocyte, macrophage, and T cell infiltration in the graft.58 The modulation of responses, and the inhibition of apoptosis and inflammation associated with I/R injury, likely represent the main mechanisms by which CO promotes the survival and function of transplanted organs, though effects on circulation and cell proliferation may also contribute.58
Metabolic Disease and Inflammation
HO-1 and CO have been implicated in the modulation of metabolic disease, including high fat diet (HFD)-induced obesity, insulin resistance, hyperglycemia, and diabetes. These topics has been reviewed elsewhere.206–207 Chemical induction of HO-1 using hemin protected against obesity-induced adipose inflammation through M2 macrophage phenotype switching in mice fed a high fed diet (HFD).208 In models of metabolic disease, inhalation of CO gas reduced hepatic steatosis in mice subjected to 30% fructose or methionine-deficient and choline-deficient-diets.209 CO exposure (administered as CORM-2) was shown to confer cardioprotection and restore mitochondrial function in a HFD-induced model of metabolic syndrome.210 Furthermore, the application of CORM-A1, a CO releasing molecule, prevented HFD-induced hypoglycemia, insulin resistance and weight gain in mice.211 The application of BR, an HO end-product, also improved insulin sensitivity in HFD-induced obese mice.212
In contrast to these observations, the adipose-specific transgenic expression of HO-1 in mice failed to modulate insulin resistance in HFD mice.213 Despite reported anti-inflammatory effects associated with HO-1 in models of endotoxemia, ALI, and I/R injury, recent research has indicated a potential pro-inflammatory effect of HO-1 in the context of inflammation associated with metabolic disease.214 Hepatocyte and macrophage conditional HO-1 deletion in mice conferred protection against diet-induced insulin resistance and inflammation, dramatically reducing secondary disease such as steatosis (fatty liver) and liver toxicity.214 Heterozygous Hmox1 knockout mice were also shown to be protected against HFD-induced insulin resistance by reducing macrophage migration.215 These observations, suggestive of pro-pathogenic roles for HO-1, emphasize that the functions of HO-1 in chronic inflammation are not yet fully understood.
Immunomodulation by HO-1/CO
HO-1 can exert significant immunomodulatory potential, though the mechanisms remain incompletely understood. HO-1 expression may promote macrophage class switching from activated M1 macrophages to alternatively activated anti-inflammatory M2 macrophages.216 M2 macrophage subtypes have been identified which display high levels of HO-1 expression.216 HO-1 has also been shown to regulate interferon-β (IFN-β) production in myeloid cells.217 Myeloid cell-specific immunomodulatory functions of HO-1 were observed in myeloid-specific HO-1 knockout mice. Mice with myeloid cell-specific Hmox1 deletion exhibited a defect of the IFN-β pathway. These mice were characterized by aberrant immune responses in experimentally-induced infections, were resistant to Listeria monocytogenes infection, but displayed an aggravated autoimmune response and accelerated pathology in a model of experimental autoimmune encephalomyelitis.217
A number of studies have previously implicated HO-1 in T-cell mediated immunosuppression. Upregulation of HO-1 and associated CO production was proposed to influence the function of CD4+CD25+ regulatory T cells (Treg) in immunosuppression and peripheral tolerance.218 HO-1 dependent tolerance to cardiac transplantation depended on CD4+CD25+ Treg functions.219 HO-1 induction with hemin was also associated with the suppression of allergic airway inflammation through upregulation of CD4+CD25+ Tregs.220–222 The transcription factor Foxp3 which functions in development and function of CD4+CD25+ Tregs was shown to induce HO-1, which in turn was proposed to mediate Foxp3-dependent immunosuppressive functions.224 More recent studies have challenged these conclusions and present evidence that the CD4+CD25+ Treg function does not directly depend on HO-1 expression in these cells.223
Emerging evidence suggests that the impact of HO-1 in immunomodulation may be related to the effects of its expression on activated T cells, dendritic cells (DCs) and antigen-presenting cells (APCs). The absence of HO-1 in APCs abolished the suppressive activity of Treg cells on effector T cells.224 Stimulation of HO-1 induction ex vivo restored GARP+CD4+CD25+ Tregs populations in isolated T lymphocyte samples from patients with acute coronary syndrome, and promoted the expression of effector molecules (i.e., LAP and GARP) on activated T cells.225 HO-1 may be important for the functioning of DCs in antigen-presentation and adaptive immune responses. Targeted up-regulation of HO-1 can influence the maturation and cell-specific functions of DCs in humans and mice.226–227 Induction of HO-1 or treatment with exogenous CO inhibited LPS-induced maturation of DCs and protected against in vivo and in vitro antigen-specific inflammation.228 Current work also shows that HO-1 induction or application of CO blocks antigen presentation by DCs at the step of endosome-lysosome fusion.230 In a Type-1 diabetes model, ex vivo treatment of DCs with gaseous CO was shown to augment dendritic-cell based therapy. Application of CO-conditioned DCs was shown to effectively impair the accumulation and pathogenic activity of autoreactive CD8+ T cells in the pancreas.230 These examples illustrate the complex and incompletely understood role for HO-1 in adaptive immune response.
THERAPEUTIC STATEGIES INVOLVING HO-1 MODULATION
Heme oxygenase inhibitor compounds
HO activity can be inhibited in cells or tissues by several natural and synthetic compounds. The application of HO activity inhibitors was initially proposed as a clinical therapy for neonatal jaundice, where HO promotes the excess formation of BR which may lead to neurological injury in neonates.231
HO activity inhibitors may also potentially have therapeutic value in diseases such as neurological disorders where excess HO activity has been implicated in the pathology.232 First generation competitive inhibitors of HO are represented by synthetic metalloporphyrins (Figure 4). These include heavy metal (i.e., Zn2+, Sn2+, and Cr2+) chelates of protoporphyrin (PP), mesoporphyrin (MP), or deuteroporphyrin (DP).231, 233 Of these SnMP was described as the most potent HO inhibitor.234 Modified derivatives for improved oral absorption have been described, exemplified by Zinc deuteroporphyrin bis-glycol (ZnDP-BG).234–235 Despite the potency of these compounds, their principle drawback is their lack of isoform selectivity (e.g., HO-1 vs. HO-2).236 Furthermore, pleiotropic off-target effects of metalloporphyrins have been reported in some experimental settings, such as inhibition of iNOS or guanylate cyclase, phototoxicity, or ability to induce Hmox1 gene transcription.235–236 Several clinical trials were performed which demonstrated reduction of total BR after SnMP application in neonates. Phototoxicity resulting in erythemia, and increased toxicity with combination phototherapy were the principle reported concerns.237
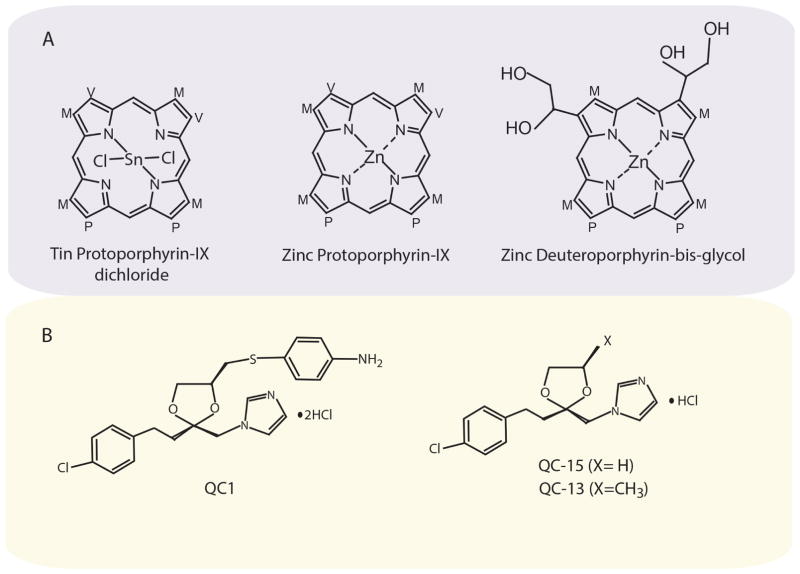
Figure 4.
Structures of HO inhibitor compounds. (Top) Representative metalloporphyrin compounds that act as competitive inhibitors of HO activity. Tin protoporphyrin IX (SnPPIX), Zinc Protoporphyrin (ZnPPIX), and Zinc Deuteroporphyrin Bis Glycol (ZnDPBG). (Bottom) Representative imidazole-dioxolanes that act as non-competitive inhibitors of HO activity. QC-1: (azalanstat) (2S,4S)-2-[2-(4-chlorophenyl)ethyl]-2-[(1H-imidazol-1-yl)methyl]-4-[((4-aminophenyl)thio)methyl]-1,3-dioxolane. QC-13: (2R,4R)-2-[2-(4-chlorophenyl)ethyl]-2-[(1H-imidazol-1-yl)methyl]-4-methyl-1,3-dioxolane hydrochloride QC-15: (2R,4R)-2-[2-(4-chlorophenyl)ethyl]-2-[(1H-imidazol-1-yl)methyl]-1,3-dioxolane hydrochloride.238–244
The imidazole-dioxolanes represent a second generation of HO activity inhibitors, the first of which is represented by azalanstat (QC-1) (Figure 4).238–244 Additional derivatives of this molecule (e.g., QC-13, QC-15 and others) have been shown to inhibit HO activity, with QC-15 reported as highly selective for HO-1.238–240, 243 To date, limited in vivo experiments have been performed using these novel inhibitors. In mice, the intra-renal medullary interstitial infusion or peritoneal injection of QC-13 resulted in the inhibition of HO activity in the kidney.245 Renal delivery of QC-13 aggravated angiotensin-II induced hypertension in this model as effectively as the metalloporphyrin SnMP.246 Recent reports also describe therapeutic effects of azole-based HO-1 inhibitors (e.g., QB-28) in mouse models of neurodegenerative disease.232
Natural and Synthetic HO-1-inducing Compounds
A subclass of HO inducing agents includes electrophilic antioxidant compounds, many of which are plant-derived polyphenols found in the human diet. These compounds activate the Nrf2 system resulting in the enhanced expression of several Nrf2 target genes coding for detoxification-associated proteins (e.g., HO-1, glutathione S-transferase A2 and NADPH: quinone oxidoreductase).247–248 Among the natural compounds that can induce HO-1 in model systems include the plant-derived compounds curcumin,12–14 caffeic acid phenethyl ester,13–14 resveratrol,11 quercetin,15–17 epigallocatechin gallate,18–19 carnosol,248 sulforaphane248 and others. The effectiveness of these dietary compounds at inducing HO-1 using in vitro model systems has led to a hypothesis that these or related compounds may be used as pharmaceuticals for human conditions such as cardiovascular disease.249 A recent study has evaluated the effectiveness of high dose oral curcumin administration in humans.250 This study concluded that oral curcumin was ineffective at inducing HO-1 in human peripheral blood mononuclear cells after administration, due to poor absorption.250 Further studies are needed to identify safe and effective oral compounds that can induce HO-1 in humans.
In addition to dietary compounds, several pharmaceutical compounds have been identified that can activate Nrf2/Keap1 axis, including dimethyl fumarate (DMF) and related compounds.248 Fumarates have been used in the treatment of psoriasis, while DMF has shown therapeutic effects in Phase III clinical trials for multiple sclerosis. An oral formulation of DMF (e.g., BG-12, Tecfidera) has been approved for clinical use in the treatment of multiple sclerosis in the USA, Canada, Europe and Australia.251–252 The mechanism of action of DMF, or its downstream metabolite monomethyl fumarate, involves the direct modification of a critical cysteine thiol group of Keap1 (Cys151) resulting in Nrf2 stabilization.253–254 DMF can act as a potent inducer of HO-1 in microglia cells and can confer anti-inflammatory effects in this cell type.248 Application of DMF can confer neuroprotection, reduce macrophage inflammation, and increase IL-10 production in mouse models of chronic experimental autoimmune encephalomyelitis.253, 255 DMF also reduced intimal hyperplasia in a model of vascular injury, in an Nrf2-dependent mechanism involving upregulation of p21Cip1.256 The effects of Nrf2-inducing compounds such as DMF are not necessarily specific for HO-1, as Nrf2 regulates multiple effector enzymes. Further research may lead to the development of additional Nrf2-activating compounds for safe and effective activation of the antioxidant response in the context of human disease.
HO-1 Gene therapy
Gene therapy approaches to regulate HO-1 in tissues were initially proposed based on preclinical data associating HO-1 with cytoprotection.257 Adenovirus and retroviral-based vectors have been evaluated for HO-1 gene transfer in vivo. For example, HO-1 expression by adenovirus-mediated gene transfer inhibits lung cell injury in response to hyperoxia in vivo after intratracheal administration.258 Microinjection of a human HO-1 transgene into rabbit eyes resulted in the site-specific expression of human HO-1 protein.259 Delivery of human HO-1 using a replication-deficient retroviral vector to spontaneously hypertensive rats reversed the hypertensive phenotype.260 Adenoviral-dependent expression of HO-1 also protected against myocardial injury in a mouse cardiac I/R injury model.202 Gene transfer of HO-1 using the Sleeping Beauty transposase vector was shown to effectively increase hepatic HO-1 expression in SCD mice, and conferred anti-inflammatory effects in this model.201
Recent studies have explored the possibility of targeted downregulation of HO-1 using the retroviral expression of miRNAs. Lung endothelial specific lentiviral expression of miR was used successfully to knockdown HO-1 in the lung, and this resulted in susceptibility to oxidant stress, enhanced pulmonary apoptosis and reduced autophagy.261 These examples support the feasibility of modulating HO-1 expression in a tissue-targeted fashion. Further progress in HO-1 gene therapy depends on the continued development of cell or organ-specific vectors for gene delivery.257 Furthermore, the safety and efficacy of retroviral vectors for human application also remains an important concern.257
THERAPEUTIC STATEGIES INVOLVING APPLICATION OF HO-1 END PRODUCTS
Inhalation CO: Mechanism of action
In addition to genetic or pharmacological strategies aimed at altering HO-1 expression or activity, the direct application of the HO activity end-products (e.g., CO) remains a promising strategy to harness the therapeutic potential of HO-1. Much initial research has focused on the direct application of CO gas by inhalation (in vivo) or ambient application (in vitro). Such experiments have typically utilized relatively low concentrations of CO (e.g., 250 ppm in air) for cell culture, with up to 500 ppm used in vivo.45–49 CO is an odorless and colorless low molecular weight gas. The principle consideration of inhaled CO, albeit at supraphysiological concentrations, is the inherent toxicity associated with this gas, which is a common contaminant of indoor and outdoor air. At high ambient concentrations, CO can act as a chemical asphyxiant. CO has a high affinity for hemoglobin (approximately 250 times that of oxygen), which results in the formation of CO-Hb in the circulation. Partial occupation of CO at the O2 binding sites of hemoglobin inhibits the release of O2 from the remaining heme groups. Accumulation of CO-Hb results in hypoxemia, and impaired oxygen delivery to tissues, which can result in morbidity or death.262 CO also has a high affinity for other heme iron chelates, and thus may target other biological hemoproteins, including guanylate cyclase, cytochrome c oxidase and cytochrome p-450.262 The impairment of mitochondrial respiration by CO may contribute to cellular toxicity, though hypoxemia remains the principle toxic mechanism of action in vivo.
At low concentrations, below the threshold of toxicity, CO may exert physiological effects (e.g., vasoregulation)263–265 and cellular effects, the latter which include modulation of cellular signaling pathways that regulate apoptosis, proliferation, and inflammation, as demonstrated in various cell culture and animal models.28,44,266 Similar to NO, CO serves as an agonist of soluble guanylate cyclase (sGC), via heme liganding, which results in the formation of 3′,5′-cyclic guanosine monophosphate (cGMP).267–268 The sGC/cGMP axis has been implicated in vasoregulation, antiproliferative effects, and neurotransmitter effects of CO.263–265, 269–271 The agonist effect of CO on sGC, however is much less potent than NO.268 In addition to cGMP formation, CO may also activate potassium channels, such as the large conductance voltage- and Ca2+ activated K+ channel (i.e., BKCa), and other ion channels such as the voltage gated L-type Ca2+ channels, which may contribute to vasodilatory and other signaling effects.272–274
CO can modulate the activation of the mitogen activated protein kinases (MAPK), including p38 MAPK, JNK and ERK1/2 activites, though the proximal target(s) remain unclear.45,49,56,180,204,275 MAPKs represent crucial mediators of inflammatory and stress responses. The anti-inflammatory effect of CO as demonstrated in LPS-stimulated macrophages, required activation of p38 MAPK pathway,45 and may also may require the downregulation of Toll-like receptor-4 (TLR4) trafficking and activation,276–277 and inhibition of NADPH oxidase-dependent signaling.276 Additional signal transduction pathway molecules have been implicated in CO dependent anti-inflamamtory effects, including heat shock factor-1 (HSF-1),181 the PPARγ/Egr-1 pathway278 and caveolin-1, which inhibits TLR4 signaling.277
CO has been shown to regulate apoptotic signaling pathways in cultured cells.56,204,275 When applied at low concentration, CO inhibited cell death caused by pro-apoptotic agents (e.g., TNFα) in endothelial cells, which required the p38 MAPK pathway, and modulation of NF-κB signaling.275,279 Additional targets for CO-dependent regulation of cellular apoptosis in various stress models include the STAT3 and PI3K/Akt pathways,280 inhibition of NADPH: oxidase dependent ROS formation,281 and modulation of caspase-8.204, 281–282 CO can exert anti-proliferative effects in vitro, with respect to the proliferation of vascular SMC.269–270 In SMC, both sGC/cGMP and p38 MAPK signaling pathways have been implicated in the anti-proliferative effects of CO these cells.58,269–270 Signaling mechanisms involved in CO-dependent regulation of cell proliferation and/or migration, include the regulation of the lipid-raft associated protein caveolin-1,283 and the modulation of NADPH oxidase.284–285
Additional hemoprotein targets of CO that may be relevant to modulation of cellular signaling, include modulation inducible NOS (iNOS),182, 286 inhibition of cytochrome p-450 degradation,287 and inhibition of mitochondrial cytochrome c oxidase associated with increased mitochondrial ROS formation.288–290 The latter pathway has been linked to autophagy signaling in epithelial cells,290 and to activation of hypoxia-inducible factor (HIF-1) mediated gene regulation.289 Finally, several studies have shown that CO can activate the Nrf2 system,291–292 and thus act as a feedback regulator of HO-1 expression. The activation of the Nrf2 pathway was recently implicated in CO-dependent protection against neural ischemia.293 Endogenous CO, generated from HO-1, can activate Nrf2 nuclear translocation through upregulation of PI3K/Akt and downregulation of glycogen synthase kinase-3β (GSK-3β). This pathway has been implicated in the cardiac upregulation of the nuclear regulatory factor-1 (NRF-1), a cardinal regulator of mitochondrial biogenesis.292 Taken together, these intriguing modulatory effects of CO on the signal transduction pathways that culminate in the regulation of inflammation, apoptosis, cell proliferation, and vascular function all may contribute to the proposed therapeutic effects of this gas.
Pharmacological CO
As an alternative to the inhalation of CO gas for therapeutic delivery, chemical CO-donor compounds termed carbon monoxide releasing compounds (CORMs) have been developed as experimental therapeutics over the last decade.44,294–295 An advantage of CORMs is that they deliver CO to tissues with reduced CO-Hb accumulation as compared with inhalation CO.41,294 CORMs can deliver small amounts of CO in a controlled manner and may represent an experimental therapy for sepsis and inflammatory disorders.44,294
The CORMs that have been developed for experimental applications include the original prototype compound Mn2CO10 (CORM-1) and the ruthenium-based compounds tricarbonyldichlororuthenium-(II)-dimer (CORM-2) and tricarbonylchoro(glycinato)-ruthenium (II) (CORM-3).44, 294–297 CORM-1 and CORM-2 have a hydrophobic backbone, whereas CORM-3 is water-soluble and rapidly releases CO in physiological fluids. A water-soluble boron-containing CORM (CORM-A1) has been developed, which slowly releases CO in a pH and temperature-dependent fashion.298 New generation CORMs based on iron carbonyl structures have been described. The hydrophilic iron carbonyl in this line displayed improved cytotoxicity and slower CO release than the hydrophobic analogues.299 Novel compounds also include light-activated CORMs such as CORM-S1, a compound based on iron and cysteamine.300 New photoactivatable CORMs (Photo-CORMs) based on manganese tricarbonyl structure have recently been introduced. Among these, Mn(CO)3(tpa-κ(3)N)](+) is stable in the dark, releases CO in solution upon photoactivation at 365 nm, and has demonstrated anti-bacterial activity.301–303 Recent innovation in this area also includes the development of nanoparticle delivery systems for CORMs,303–304 as well as bifunctional hybrid molecules with both Nrf2-activating and CORM functions.305
CORMs can exert vasomodulatory effects with CORM-3 shown to produce a rapid vasodilatory response.297 Similar to inhalation CO, protective effects have been demonstrated in preclinical injury and disease models with pharmacological application of CORMs. Among the therapeutic effects of CORMs include the inhibition of pro-inflammatory cytokine production in LPS-stimulated macrophages,306 and inhibition of the inflammatory response in LPS-stimulated endothelial cells.307 In vivo, CORMs have been shown to attenuate systemic inflammation and pro-adhesive vascular cell properties, as well as prolong survival, and reduce liver injury during sepsis and inflammatory injury.308–310 The protective effects of CORMs in sepsis were related to stimulation of mitochondrial biogenesis.311 Furthermore, the beneficial effects of CORMs in sepsis, like gaseous CO, may involve stimulation of bacterial clearance.194,199,312 CO, when generated by application of CORMs, can also exert direct bactericidal activity.313–317 CORM3 exerted antibacterial activity against P. aeruginosa and reduced bacterial counts in a model of live bacterial infection.314–315 CORM3 was also shown to have antibacterial activity against E. coli,313,318 and S. typhimurium after bacterial uptake of the drug.316 Both CORM2 and CORM3 exerted antibacterial activity against antibiotic-resistant strains of H. pylori.319 Recent advances using PhotoCORMs demonstrate photoactivatable toxicity of these compounds toward E. coli bacteria.301 CORMs may also find application in reducing gastrointestinal inflammation in various models including post-operative ileus and necrotizing enterocolitis.312 Finally, applications for CORMs in transplantation models have also been described. For example, in a cardiac transplantation model, inclusion of CORM-3 in the preservation fluid was shown to improve cardiac function following transplantation.320
These examples of salutary effects of CORMs are suggestive of possible therapeutic applications of CORMs in human disease, though more research regarding safety and efficacy of these compounds for human application is needed.
BV/BR therapy
BV and BR are known to have potent chemical antioxidant properties, which have been well documented using in vitro model systems,321–323 as well as cytoprotective effects in cultured cells.324–327 Elevated serum BR conferred oxidative stress resistance in hyperbilirubinemic animals.328 In human subjects, circulating BR has been correlated to reduced risk of cardiovascular disease.329–332 Exogenous applications of BV or BR, can confer anti-inflammatory and protective effects in models of organ injury and transplantation.63,67,81,84–86 For example, exogenous application of BR preserved myocardial function during cardiac I/R injury.326 BR or BV conferred tissue protection from I/R injury in various organ transplantation models, including liver,84 kidney,85 and heart.86 Perfusion with BV increased the survival in rats undergoing orthotopic liver transplantation by preserving liver function,84 by decreasing neutrophil influx, reducing pro-inflammatory cytokine expression, and reducing iNOS activation.84 In an isolated perfused kidney model, perfusion with BR protected against warm I/R-induced tissue injury and preserved renal function.85 BV improved the survivability of rat cardiac and pancreatic allografts, by reducing leukocyte infiltration and inhibiting T-cell proliferation.81,86 Application of both BV and CO provided a synergistic tissue protection against transplant-associated cold I/R injury of heart and kidney grafts.67 Hyperbilirubinemic rats were resistant to bleomycin-induced lung injury (fibrosis) and associated mortality, and displayed reduced lung hydroxyproline content, and reduced polymorphonuclear lymphocyte and leukocyte counts, as well as reduced levels of TGF-β in the BAL.333 Administration of BV to rats protected against systemic inflammation and lung injury, and extended survival after exposure to a lethal dose of LPS, associated with a reduction of pro-inflammatory cytokines in the serum (i.e., IL-6) and upregulation of serum IL-10 levels, as well as reduced lung permeability.183 Recent studies have proposed a novel pathway for anti-inflammatory effects of BV in macrophages, which depend on eNOS upregulation, and eNOS/NO-dependent downregulation of TLR4 expression.334 Exogenous BV administration inhibited neointimal hyperplasia associated with vascular balloon injury in rats,335–336 while hyperbillirubinemic animals also displayed increased resistance to vascular injury.336 An antiproliferative effect of BV/BR was demonstrated in vascular SMC culture, whereby exogenous BV/BR arrested cells in G1 phase associated with inhibition of p38 MAPK and retinoblastoma protein phosphorylation in vitro.336 Taken together, these studies suggest that BV/BR may have therapeutic applications in disease.
Heme and Iron modulation
Free circulating hemoglobin can represent a toxic insult to the vascular endothelium. Heme, the prosthetic group of hemoglobin and other hemoproteins, in free form is regarded as a potentially toxic molecule based on its nature as an iron chelate, which can catalyze free radical generating reactions, leading to LDH oxidation and NO depletion, causing injury to endothelium, and promoting inflammatory pathways.43, 337–338 Thus, the removal of cell-free hemoglobin or free heme with scavenging proteins such as haptoglobin or hemopexin, respectively, has been proposed as a therapeutic strategy in inflammatory diseases.339–340 HO-1 has a well recognized role in iron metabolism by redistributing heme iron. Hmox−/− mice are known to display aberrant tissue iron deposition.26 HO-1-derived iron drives the synthesis of ferritin, which in turn sequesters the liberated iron in an inert state, and this may represent a cytoprotective pathway that limits the potential of iron to catalyze harmful reactions.341–343 Iron chelators, although not specific for HO-derived iron, may be of therapeutic value in iron-dependent pathologies.344–345 In diseases where HO-1 derived iron has been directly implicated in the pathology (e.g., Alzheimer’s disease), such a neurodegenerative diseases, inhibition of HO activity may provide therapeutic benefit.232,346
IMPLICATIONS FOR CO THERAPY IN HUMAN DISEASE
The widespread and general success of CO as an anti-inflammatory agent in preclinical rodent models, as well as additional progress in primate and porcine models of inflammatory disease have fueled the continued aspiration that CO will eventually provide useful clinical applications as a gaseous molecular medicine for human disease. Ongoing Phase I/II clinical trials may soon yield additional information. In a randomized, double-blinded, placebo-controlled, two-way cross-over trial, experimental endotoxemia was induced in healthy volunteers by injection of 2 ng/kg LPS. The potential anti-inflammatory effects of CO inhalation were investigated under these conditions by inhalation of 500 ppm CO (which increased CO-Hb to 7%) versus synthetic air as a placebo for 1 h. Under these conditions, CO inhalation had no effect on the inflammatory response as measured by systemic cytokine production. In this study, no adverse effects of CO inhalation were observed.347 Another human clinical trial demonstrated the feasibility of administering inhaled CO to humans with chronic obstructive pulmonary disease (COPD).348 In this study, ex-smoking patients with stable COPD were subjected to CO inhalation (100–125 ppm for 2 hours/day for 4 days), which increased CO-Hb levels to 4.5%. Inhalation of CO by patients with stable COPD displayed a trend in reduction of sputum eosinophils and improvement of methacholine responsiveness, without adverse effects.348 In summary, the protective phenotype of CO in with respect to reducing inflammation in mice has been partially recapitulated in higher animals. Further experiments are required to confirm the safety and efficacy of CO inhalation as a treatment for inflammatory lung diseases in humans. Ongoing clinical trials in sepsis, acute lung injury, and fibrosis may yield additional information in the foreseeable future.
CONCLUDING REMARKS
HO-1 continues to fascinate the research community as a molecule with a multiplicity of implications in the pathogenesis and therapeutics of human disease. Although many pre-clinical research studies have pointed to anti-inflammatory effects of HO-1 in tissue injury, recent studies now also propose a pro-pathogenic effect of HO-1 in the propagation of chronic inflammation.214 Thus, the function of HO-1 in inflammation remains complex and incompletely understood. The putative role of HO-1 in the modulation of the adaptive immune response represents an area of active investigation.
The modulation of HO-1 by genetic overexpression or counter-regulation remains a candidate for gene therapy applications, yet this approach is limited by the overall constraints of human gene therapy approaches, as well as potential for overdosing and off-target effects. Similarly, the application of synthetic chemical inhibitors of HO activity may find therapeutic application, though further development of isoform-selective and specific inhibitors is ongoing. The modulation of HO-1 by dietary antioxidants and related compounds has long been proposed as a therapeutic avenue based on model studies and has met with limited success in humans using the model compound curcumin, though the pharmaceutical DMF shows promise.
Finally, the pharmacological application of HO activity end-products including systemic administration of BV/BR or inhalation of CO gas has now been tested in multiple pre-clinical studies. It should be noted that the exogenous application of these substances as pharmaceuticals, even at low concentrations, may result in non-physiological effects that may not necessarily have the identical effect as the endogenous production of these substances in their natural contexts.349
As an alternative to inhaled CO, pharmacological application of CO using CORMs or PhotoCORMs may provide a promising therapeutic strategy.44 Other novel CO-based therapies may include the use of CO-saturated hemoglobins as well as the combination of CO preconditioning with cell based therapies.230, 350
The success of inhalation CO in preclinical animal studies using rodents and higher mammals has led to continued efforts toward the clinical application of inhaled CO. To date, human studies recapitulating the therapeutic effects of CO observed in animals are ongoing. Several studies have been completed which lend support for the safety and feasibility of low-dose CO application in humans. Further progress in clinical CO awaits the completion and analysis of several ongoing clinical trials in organ transplantation, pulmonary fibrosis, and ARDS.
Acknowledgments
This work was supported by NIH grants P01 HL108801, R01 HL079904, and R01 HL060234 (AMKC); and R01 HL060234 (SWR). All authors have read the journal’s policy on disclosure of potential conflicts of interest and have declared that no competing interests exist. None of the authors have financial or personal relationship with organizations that could potentially be perceived as influencing the described research. The work is solely that of the authors and no editorial support was used in the preparation of this manuscript. All authors have read the journal’s authorship agreement and the manuscript has been reviewed by and approved by all named authors.
Abbreviations
- ALI
Acute lung injury
- APCs
Antigen presenting cells
- ARE
Antioxidant responsive element
- Bach 1
BTB and CNC homologue 1
- BAL
Bronchoalveolar lavage
- BR
bilirubin-IXα
- BV
biliverdin-IXα
- BVR
Biliverdin reductase, CO, carbon monoxide
- CO-Hb
Carboxyhemoglobin
- COPD
Chronic obstructive pulmonary disease
- CORM
CO releasing molecule
- CPR
Cytochrome p-450 reductase, DCs, Dendritic cells
- DMF
Dimethylfumarate
- EGR-1
Early growth response-1
- ERK1/2
Extracellular regulated protein kinase 1/2
- FoxP3
Forkhead Box P3
- HO
Heme oxygenase
- HFD
High fat diet
- HO-1
Heme oxygenase-1
- HO-2
Heme oxygenase-2
- HMGB1
high-mobility group box-1
- HSF-1
Heat shock factor-1
- IFN-β
Interferon-β
- IL
Interleukin
- iNOS
Inducible nitric oxide synthase
- I/R
Ischemia/reperfusion
- JNK
c-Jun NH2-terminal kinase
- Keap1
Kelch-like ECH-associated protein
- LPS
Lipopolysaccharide
- MAPK
Mitogen activated protein kinase
- miR
MicroRNA
- MV
Mechanical ventilation
- NADH
Nicotinamide adenine dinucleotide, reduced form
- NADPH
Nicotinamide adenine dinucleotide phosphate, reduced form
- NLRP3
NOD-, leucine rich region- and pyrin domain-containing-3
- Nrf2
Nuclear factor erythroid 2-related factor-2
- p38 MAPK
p38 Mitogen activated protein kinase
- PI3K/Akt
Phosphatidylinositol-3-kinase/Akt
- PhotoCORMs
Photoactivatable carbon monoxide releasing molecules
- ROS
Reactive oxygen species
- SCD
Sickle cell disease
- SMC
smooth muscle cells (SMC)
- SNP
small nucleotide polymorphism
- SnPPIX
Tin Protoporphyrin-IX
- StRE
Stress-responsive element
- SPM
Specialized pro-resolving mediators
- TLR4
Toll-like receptor-4
- Treg
Regulatory T lymphocyte
- VILI
Ventilator-induced lung injury
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ryter SW, Choi AM. Heme oxygenase-1/carbon monoxide: from metabolism to molecular therapy. Am J Respir Cell Mol Biol. 2009;41:251–260. doi: 10.1165/rcmb.2009-0170TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci USA. 1968;61:748–755. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tenhunen R, Marver HS, Schmid R. Microsomal heme oxygenase. Characterization of the enzyme. J Biol Chem. 1969;244:6388–6394. [PubMed] [Google Scholar]
- 4.Maines MD. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 1988;2:2557–2568. [PubMed] [Google Scholar]
- 5.Alam J, Shibahara S, Smith A. Transcriptional activation of the heme oxygenase gene by heme and cadmium in mouse hepatoma cells. J Biol Chem. 1989;264:6371–6375. [PubMed] [Google Scholar]
- 6.Keyse SM, Tyrrell RM. Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc Natl Acad Sci USA. 1989;86:99–103. doi: 10.1073/pnas.86.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keyse SM, Tyrrell RM. Both near ultraviolet radiation and the oxidizing agent hydrogen peroxide induce a 32-kDa stress protein in normal human skin fibroblasts. J Biol Chem. 1987;262:14821–14825. [PubMed] [Google Scholar]
- 8.Saunders EL, Maines MD, Meredith MJ, Freeman ML. Enhancement of heme oxygenase-1 synthesis by glutathione depletion in Chinese hamster ovary cells. Arch Biochem Biophys. 1991;288:368–373. doi: 10.1016/0003-9861(91)90208-z. [DOI] [PubMed] [Google Scholar]
- 9.Hartsfield CL, Alam J, Cook JL, Choi AM. Regulation of heme oxygenase-1 gene expression in vascular smooth muscle cells by nitric oxide. Am J Physiol. 1997;273:L980–L988. doi: 10.1152/ajplung.1997.273.5.L980. [DOI] [PubMed] [Google Scholar]
- 10.Camhi SL, Alam J, Wiegand GW, Chin BY, Choi AM. Transcriptional activation of the HO-1 gene by lipopolysaccharide is mediated by 5′ distal enhancers: role of reactive oxygen intermediates and AP-1. Am J Respir Cell Mol Biol. 1998;18:226–234. doi: 10.1165/ajrcmb.18.2.2910. [DOI] [PubMed] [Google Scholar]
- 11.Chen CY, Jang JH, Li MH, Surh YJ. Resveratrol upregulates heme oxygenase-1 expression via activation of NF-E2-related factor 2 in PC12 cells. Biochem Biophys Res Commun. 2005;331:993–1000. doi: 10.1016/j.bbrc.2005.03.237. [DOI] [PubMed] [Google Scholar]
- 12.Motterlini R, Foresti R, Bassi R, Green CJ. Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radic Biol Med. 2000;28:1303–1312. doi: 10.1016/s0891-5849(00)00294-x. [DOI] [PubMed] [Google Scholar]
- 13.Balogun E, Hoque M, Gong P, Killeen E, Green CJ, Foresti R, Alam J, Motterlini R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem J. 2003;371:887–895. doi: 10.1042/BJ20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scapagnini G, Foresti R, Calabrese V, Giuffrida Stella AM, Green CJ, Motterlini R. Caffeic acid phenethyl ester and curcumin: a novel class of heme oxygenase-1 inducers. Mol Pharmacol. 2002;61:554–561. doi: 10.1124/mol.61.3.554. [DOI] [PubMed] [Google Scholar]
- 15.Lin HC, Cheng TH, Chen YC, Juan SH. Mechanism of heme oxygenase-1 gene induction by quercetin in rat aortic smooth muscle cells. Pharmacology. 2004;71:107–112. doi: 10.1159/000076947. [DOI] [PubMed] [Google Scholar]
- 16.Yao P, Nussler A, Liu L, Hao L, Song F, Schirmeier A, Nussler N. Quercetin protects human hepatocytes from ethanol-derived oxidative stress by inducing heme oxygenase-1 via the MAPK/Nrf2 pathways. J Hepatol. 2007;47:253–261. doi: 10.1016/j.jhep.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Rayamajhi N, Kim SK, Go H, Joe Y, Callaway Z, Kang JG, Ryter SW, Chung HT. Quercetin induces mitochondrial biogenesis through activation of HO-1 in HepG2 cells. Oxid Med Cell Longev. 2013;2013:154279. doi: 10.1155/2013/154279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pullikotil P, Chen H, Muniyappa R, Greenberg CC, Yang S, Reiter CE, Lee JW, Chung JH, Quon MJ. Epigallocatechin gallate induces expression of heme oxygenase-1 in endothelial cells via p38 MAPK and Nrf-2 that suppresses proinflammatory actions of TNF-α. J Nutr Biochem. 2012;23:1134–1145. doi: 10.1016/j.jnutbio.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andreadi CK, Howells LM, Atherfold PA, Manson MM. Involvement of Nrf2, p38, B-Raf, and nuclear factor-kappaB, but not phosphatidylinositol 3-kinase, in induction of hemeoxygenase-1 by dietary polyphenols. Mol Pharmacol. 2006;69:1033–1040. doi: 10.1124/mol.105.018374. [DOI] [PubMed] [Google Scholar]
- 20.Shibahara S, Müller RM, Taguchi H. Transcriptional control of rat heme oxygenase by heat shock. J Biol Chem. 1987;262:12889–12892. [PubMed] [Google Scholar]
- 21.Lee PJ, Jiang BH, Chin BY, Iyer NV, Alam J, Semenza GL, Choi AM. Hypoxia-inducible factor-1 mediates transcriptional activation of the heme oxygenase-1 gene in response to hypoxia. J Biol Chem. 1997;272:5375–5381. [PubMed] [Google Scholar]
- 22.Lee PJ, Alam J, Sylvester SL, Inamdar N, Otterbein L, Choi AM. Regulation of heme oxygenase-1 expression in vivo and in vitro in hyperoxic lung injury. Am J Respir Cell Mol Biol. 1996;14:556–568. doi: 10.1165/ajrcmb.14.6.8652184. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, Bedard EL, Potter R, Zhong R, Alam J, Choi AM, Lee PJ. Mitogen-activated protein kinases regulate HO-1 gene transcription after ischemia-reperfusion lung injury. Am J Physiol Lung Cell Mol Physiol. 2002;283:L815–L829. doi: 10.1152/ajplung.00485.2001. [DOI] [PubMed] [Google Scholar]
- 24.Ryter SW, Choi AM. Heme oxygenase-1/carbon monoxide: novel therapeutic strategies in critical care medicine. Curr Drug Targets. 2010;11:1485–1494. doi: 10.2174/1389450111009011485. [DOI] [PubMed] [Google Scholar]
- 25.Yachie A, Niida Y, Wada T, Igarashi N, Kaneda H, Toma T, Ohta K, Kasahara Y, Koizumi S. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J Clin Invest. 1999;103:129–135. doi: 10.1172/JCI4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poss KD, Tonegawa S. Heme oxygenase 1 is required for mammalian iron reutilization. Proc Natl Acad Sci USA. 1997;94:10919–10924. doi: 10.1073/pnas.94.20.10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poss KD, Tonegawa S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc Natl Acad Sci USA. 1997;94:10925–10930. doi: 10.1073/pnas.94.20.10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 29.Duckers HJ, Boehm M, True AL, Yet SF, San H, Park JL, Webb RC, Lee ME, Nabel GJ, Nabel EG. Heme oxygenase-1 protects against vascular constriction and proliferation. Nat Med. 2001;7:693–698. doi: 10.1038/89068. [DOI] [PubMed] [Google Scholar]
- 30.Fujita T, Toda K, Karimova A, Yan SF, Naka Y, Yet SF, Pinsky DJ. Paradoxical rescue from ischemic lung injury by inhaled carbon monoxide driven by derepression of fibrinolysis. Nat Med. 2001;7:598–604. doi: 10.1038/87929. [DOI] [PubMed] [Google Scholar]
- 31.Yet SF, Perrella MA, Layne MD, Hsieh CM, Maemura K, Kobzik L, Wiesel P, Christou H, Kourembanas S, Lee ME. Hypoxia induces severe right ventricular dilatation and infarction in heme oxygenase-1 null mice. J Clin Invest. 1999;103:R23–R29. doi: 10.1172/JCI6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yet SF, Tian R, Layne MD, Wang ZY, Maemura K, Solovyeva M, Ith B, Melo LG, Zhang L, Ingwall JS, Dzau VJ, Lee ME, Perrella MA. Cardiac-specific expression of heme oxygenase-1 protects against ischemia and reperfusion injury in transgenic mice. Circ Res. 2001;89:168–173. doi: 10.1161/hh1401.093314. [DOI] [PubMed] [Google Scholar]
- 33.Vulapalli SR, Chen Z, Chua BH, Wang T, Liang CS. Cardioselective overexpression of HO-1 prevents I/R-induced cardiac dysfunction and apoptosis. Am J Physiol Heart Circ Physiol. 2002;283:H688–H694. doi: 10.1152/ajpheart.00133.2002. [DOI] [PubMed] [Google Scholar]
- 34.Hull TD, Bolisetty S, DeAlmeida AC, Litovsky SH, Prabhu SD, Agarwal A, George JF. Heme oxygenase-1 expression protects the heart from acute injury caused by inducible Cre recombinase. Lab Invest. 2013;93:868–879. doi: 10.1038/labinvest.2013.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Minamino T, Christou H, Hsieh CM, Liu Y, Dhawan V, Abraham NG, Perrella MA, Mitsialis SA, Kourembanas S. Targeted expression of heme oxygenase-1 prevents the pulmonary inflammatory and vascular responses to hypoxia. Proc Natl Acad Sci USA. 2001;98:8798–8803. doi: 10.1073/pnas.161272598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zampetaki A, Minamino T, Mitsialis SA, Kourembanas S. Effect of heme oxygenase-1 overexpression in two models of lung inflammation. Exp Biol Med (Maywood) 2003;228:442–446. doi: 10.1177/15353702-0322805-02. [DOI] [PubMed] [Google Scholar]
- 37.Grochot-Przeczek A, Lach R, Mis J, Skrzypek K, Gozdecka M, Sroczynska P, Dubiel M, Rutkowski A, Kozakowska M, Zagorska A, Walczynski J, Was H, Kotlinowski J, Drukala J, Kurowski K, Kieda C, Herault Y, Dulak J, Jozkowicz A. Heme oxygenase-1 accelerates cutaneous wound healing in mice. PLoS ONE. 2009;4:e5803. doi: 10.1371/journal.pone.0005803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song W, Zukor H, Lin SH, Liberman A, Tavitian A, Mui J, Vali H, Fillebeen C, Pantopoulos K, Wu TD, Guerquin-Kern JL, Schipper HM. Unregulated brain iron deposition in transgenic mice over-expressing HMOX1 in the astrocytic compartment. J Neurochem. 2012;123:325–336. doi: 10.1111/j.1471-4159.2012.07914.x. [DOI] [PubMed] [Google Scholar]
- 39.Zukor H, Song W, Liberman A, Mui J, Vali H, Fillebeen C, Pantopoulos K, Wu TD, Guerquin-Kern JL, Schipper HM. HO-1-mediated macroautophagy: a mechanism for unregulated iron deposition in aging and degenerating neural tissues. J Neurochem. 2009;109:776–791. doi: 10.1111/j.1471-4159.2009.06007.x. [DOI] [PubMed] [Google Scholar]
- 40.Song W, Su H, Song S, Paudel HK, Schipper HM. Over-expression of hemeoxygenase-1 promotes oxidative mitochondrial damage in rat astroglia. J Cell Physiol. 2006;206:655–663. doi: 10.1002/jcp.20509. [DOI] [PubMed] [Google Scholar]
- 41.Suttner DM, Dennery PA. Reversal of HO-1 related cytoprotection with increased expression is due to reactive iron. FASEB J. 1999;13:1800–1809. doi: 10.1096/fasebj.13.13.1800. [DOI] [PubMed] [Google Scholar]
- 42.Kvam E, Hejmadi V, Ryter S, Pourzand C, Tyrrell RM. Heme oxygenase activity causes transient hypersensitivity to oxidative ultraviolet A radiation that depends on release of iron from heme. Free Radic Biol Med. 2000;28:1191–1196. doi: 10.1016/s0891-5849(00)00205-7. [DOI] [PubMed] [Google Scholar]
- 43.Ryter SW, Tyrrell RM. The heme synthesis and degradation pathways: role in oxidant sensitivity. Heme oxygenase has both pro- and antioxidant properties. Free Radic Biol Med. 2000;28:289–309. doi: 10.1016/s0891-5849(99)00223-3. [DOI] [PubMed] [Google Scholar]
- 44.Motterlini R, Otterbein LE. The therapeutic potential of carbon monoxide. Nat Rev Drug Discov. 2010;9:728–743. doi: 10.1038/nrd3228. [DOI] [PubMed] [Google Scholar]
- 45.Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, Davis RJ, Flavell RA, Choi AM. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 46.Mazzola S, Forni M, Albertini M, Bacci ML, Zannoni A, Gentilini F, Lavitrano M, Bach FH, Otterbein LE, Clement MG. Carbon monoxide pretreatment prevents respiratory derangement and ameliorates hyperacute endotoxic shock in pigs. FASEB J. 2005;19:2045–2047. doi: 10.1096/fj.05-3782fje. [DOI] [PubMed] [Google Scholar]
- 47.Mitchell LA, Channell MM, Royer CM, Ryter SW, Choi AM, McDonald JD. Evaluation of inhaled carbon monoxide as an anti-inflammatory therapy in a nonhuman primate model of lung inflammation. Am J Physiol Lung Cell Mol Physiol. 2010;299:L891–L897. doi: 10.1152/ajplung.00366.2009. [DOI] [PubMed] [Google Scholar]
- 48.Otterbein LE, Mantell LL, Choi AM. Carbon monoxide provides protection against hyperoxic lung injury. Am J Physiol. 1999;276:L688–94. doi: 10.1152/ajplung.1999.276.4.L688. [DOI] [PubMed] [Google Scholar]
- 49.Otterbein LE, Otterbein SL, Ifedigbo E, Liu F, Morse DE, Fearns C, Ulevitch RJ, Knickelbein R, Flavell RA, Choi AM. MKK3 mitogen-activated protein kinase pathway mediates carbon monoxide-induced protection against oxidant-induced lung injury. Am J Pathol. 2003;163:2555–2563. doi: 10.1016/S0002-9440(10)63610-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dolinay T, Szilasi M, Liu M, Choi AM. Inhaled carbon monoxide confers antiinflammatory effects against ventilator-induced lung injury. Am J Respir Crit Care Med. 2004;170:613–620. doi: 10.1164/rccm.200401-023OC. [DOI] [PubMed] [Google Scholar]
- 51.Hoetzel A, Schmidt R, Vallbracht S, Goebel U, Dolinay T, Kim HP, Ifedigbo E, Ryter SW, Choi AM. Carbon monoxide prevents ventilator-induced lung injury via caveolin-1. Crit Care Med. 2009;37:1708–1715. doi: 10.1097/CCM.0b013e31819efa31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoetzel A, Dolinay T, Vallbracht S, Zhang Y, Kim HP, Ifedigbo E, Alber S, Kaynar AM, Schmidt R, Ryter SW, Choi AM. Carbon monoxide protects against ventilator-induced lung injury via PPAR-gamma and inhibition of Egr-1. Am J Respir Crit Care Med. 2008;177:1223–1232. doi: 10.1164/rccm.200708-1265OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee S, Lee SJ, Coronata AA, Fredenburgh LE, Chung SW, Perrella MA, Nakahira K, Ryter SW, Choi AM. Carbon monoxide confers protection in sepsis by enhancing beclin 1-dependent autophagy and phagocytosis. Antioxid Redox Signal. 2014;20:432–442. doi: 10.1089/ars.2013.5368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsoyi K, Lee TY, Lee YS, Kim HJ, Seo HG, Lee JH, Chang KC. Heme-oxygenase-1 induction and carbon monoxide-releasing molecule inhibit lipopolysaccharide (LPS)-induced high-mobility group box 1 release in vitro and improve survival of mice in LPS- and cecal ligation and puncture-induced sepsis model in vivo. Mol Pharmacol. 2009;76:173–82. doi: 10.1124/mol.109.055137. [DOI] [PubMed] [Google Scholar]
- 55.Dalli J, Kraft BD, Colas RA, Shinohara M, Fredenburgh LE, Hess DR, Chiang N, Welty-Wolf KE, Choi AM, Piantadosi CA, Serhan CN. Proresolving lipid mediator profiles in baboon pneumonia are regulated by inhaled carbon monoxide. Am J Respir Cell Mol Biol. 2015 Jan 8; doi: 10.1165/rcmb.2014-0299OC. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang X, Shan P, Otterbein LE, Alam J, Flavell RA, Davis RJ, Choi AM, Lee PJ. Carbon monoxide inhibition of apoptosis during ischemia-reperfusion lung injury is dependent on the p38 mitogen-activated protein kinase pathway and involves caspase 3. J Biol Chem. 2003;278:1248–1258. doi: 10.1074/jbc.M208419200. [DOI] [PubMed] [Google Scholar]
- 57.Amersi F, Shen XD, Anselmo D, Melinek J, Iyer S, Southard DJ, Katori M, Volk HD, Busuttil RW, Buelow R, Kupiec-Weglinski JW. Ex vivo exposure to carbon monoxide prevents hepatic ischemia/reperfusion injury through p38 MAP kinase pathway. Hepatology. 2002;35:815–823. doi: 10.1053/jhep.2002.32467. [DOI] [PubMed] [Google Scholar]
- 58.Otterbein LE, Zuckerbraun BS, Haga M, Liu F, Song R, Usheva A, Stachulak C, Bodyak N, Smith RN, Csizmadia E, Tyagi S, Akamatsu Y, Flavell RJ, Billiar TR, Tzeng E, Bach FH, Choi AM, Soares MP. Carbon monoxide suppresses arteriosclerotic lesions associated with chronic graft rejection and with balloon injury. Nat Med. 2003;9:183–190. doi: 10.1038/nm817. [DOI] [PubMed] [Google Scholar]
- 59.Belcher JD, Mahaseth H, Welch TE, Otterbein LE, Hebbel RP, Vercellotti GM. Heme oxygenase-1 is a modulator of inflammation and vaso-occlusion in transgenic sickle mice. J Clin Invest. 2006;116:808–816. doi: 10.1172/JCI26857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beckman JD, Belcher JD, Vineyard JV, Chen C, Nguyen J, Nwaneri MO, O’Sullivan MG, Gulbahce E, Hebbel RP, Vercellotti GM. Inhaled carbon monoxide reduces leukocytosis in a murine model of sickle cell disease. Am J Physiol Heart Circ Physiol. 2009;297:H1243–253. doi: 10.1152/ajpheart.00327.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Song R, Kubo M, Morse D, Zhou Z, Zhang X, Dauber JH, Fabisiak J, Alber SM, Watkins SC, Zuckerbraun BS, Otterbein LE, Ning W, Oury TD, Lee PJ, McCurry KR, Choi AM. Carbon monoxide induces cytoprotection in rat orthotopic lung transplantation via anti-inflammatory and anti-apoptotic effects. Am J Pathol. 2003;163:231–242. doi: 10.1016/S0002-9440(10)63646-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kohmoto J, Nakao A, Kaizu T, Tsung A, Ikeda A, Tomiyama K, Billiar TR, Choi AM, Murase N, McCurry KR. Low-dose carbon monoxide inhalation prevents ischemia/reperfusion injury of transplanted rat lung grafts. Surgery. 2006;140:179–185. doi: 10.1016/j.surg.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 63.Zhou H, Qian H, Liu J, Zhu D, Ding W, Pan P, Jin D, Wang J, Li W. Protection against lung graft injury from brain-dead donors with carbon monoxide, biliverdin, or both. J Heart Lung Transplant. 2011;30:460–466. doi: 10.1016/j.healun.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 64.Sahara H, Shimizu A, Setoyama K, Oku M, Okumi M, Nishimura H, Oriyanhan W, Tasaki M, Scalea J, Wada H, Bando T, Date H, Yamada K. Beneficial effects of perioperative low-dose inhaled carbon monoxide on pulmonary allograft survival in MHC-inbred CLAWN miniature swine. Transplantation. 2010;90:1336–1343. doi: 10.1097/TP.0b013e3181ff8730. [DOI] [PubMed] [Google Scholar]
- 65.Nakao A, Huang CS, Stolz DB, Wang Y, Franks JM, Tochigi N, Billiar TR, Toyoda Y, Tzeng E, McCurry KR. Ex vivo carbon monoxide delivery inhibits intimal hyperplasia in arterialized vein grafts. Cardiovasc Res. 2011;89:457–463. doi: 10.1093/cvr/cvq298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sato K, Balla J, Otterbein L, Smith RN, Brouard S, Lin Y, Csizmadia E, Sevigny J, Robson SC, Vercellotti G, Choi AM, Bach FH, Soares MP. Carbon monoxide generated by heme oxygenase-1 suppresses the rejection of mouse-to-rat cardiac transplants. J Immunol. 2001;166:4185–4194. doi: 10.4049/jimmunol.166.6.4185. [DOI] [PubMed] [Google Scholar]
- 67.Nakao A, Neto JS, Kanno S, Stolz DB, Kimizuka K, Liu F, Bach FH, Billiar TR, Choi AM, Otterbein LE, Murase N. Protection against ischemia/reperfusion injury in cardiac and renal transplantation with carbon monoxide, biliverdin and both. Am J Transplant. 2005;5:282–291. doi: 10.1111/j.1600-6143.2004.00695.x. [DOI] [PubMed] [Google Scholar]
- 68.Nakao A, Toyokawa H, Abe M, Kiyomoto T, Nakahira K, Choi AM, Nalesnik MA, Thomson AW, Murase N. Heart allograft protection with low-dose carbon monoxide inhalation: effects on inflammatory mediators and alloreactive T-cell responses. Transplantation. 2006;81:220–230. doi: 10.1097/01.tp.0000188637.80695.7f. [DOI] [PubMed] [Google Scholar]
- 69.Akamatsu Y, Haga M, Tyagi S, Yamashita K, Graça-Souza AV, Ollinger R, Czismadia E, May GA, Ifedigbo E, Otterbein LE, Bach FH, Soares MP. Heme oxygenase-1-derived carbon monoxide protects hearts from transplant associated ischemia reperfusion injury. FASEB J. 2004;18:771–772. doi: 10.1096/fj.03-0921fje. [DOI] [PubMed] [Google Scholar]
- 70.Nakao A, Kaczorowski DJ, Wang Y, Cardinal JS, Buchholz BM, Sugimoto R, Tobita K, Lee S, Toyoda Y, Billiar TR, McCurry KR. Amelioration of rat cardiac cold ischemia/reperfusion injury with inhaled hydrogen or carbon monoxide, or both. J Heart Lung Transplant. 2010;29:544–553. doi: 10.1016/j.healun.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 71.Ozaki KS, Yoshida J, Ueki S, Pettigrew GL, Ghonem N, Sico RM, Lee LY, Shapiro R, Lakkis FG, Pacheco-Silva A, Murase N. Carbon monoxide inhibits apoptosis during cold storage and protects kidney grafts donated after cardiac death. Transpl Int. 2012;25:107–117. doi: 10.1111/j.1432-2277.2011.01363.x. [DOI] [PubMed] [Google Scholar]
- 72.Nakao A, Faleo G, Nalesnik MA, Seda-Neto J, Kohmoto J, Murase N. Low-dose carbon monoxide inhibits progressive chronic allograft nephropathy and restoresrenal allograft function. Am J Physiol Renal Physiol. 2009;297:F19–F26. doi: 10.1152/ajprenal.90728.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Faleo G, Neto JS, Kohmoto J, Tomiyama K, Shimizu H, Takahashi T, Wang Y, Sugimoto R, Choi AM, Stolz DB, Carrieri G, McCurry KR, Murase N, Nakao A. Carbon monoxide ameliorates renal cold ischemia-reperfusion injury with an upregulation of vascular endothelial growth factor by activation of hypoxia-inducible factor. Transplantation. 2008;85:1833–4180. doi: 10.1097/TP.0b013e31817c6f63. [DOI] [PubMed] [Google Scholar]
- 74.Neto JS, Nakao A, Kimizuka K, Romanosky AJ, Stolz DB, Uchiyama T, Nalesnik MA, Otterbein LE, Murase N. Protection of transplant-induced renal ischemia-reperfusion injury with carbon monoxide. Am J Physiol Renal Physiol. 2004;287:F979–F989. doi: 10.1152/ajprenal.00158.2004. [DOI] [PubMed] [Google Scholar]
- 75.Hanto DW, Maki T, Yoon MH, Csizmadia E, Chin BY, Gallo D, Konduru B, Kuramitsu K, Smith NR, Berssenbrugge A, Attanasio C, Thomas M, Wegiel B, Otterbein LE. Intraoperative administration of inhaled carbon monoxide reduces delayed graft function in kidney allografts in Swine. Am J Transplant. 2010;10:2421–2430. doi: 10.1111/j.1600-6143.2010.03289.x. [DOI] [PubMed] [Google Scholar]
- 76.Yoshida J, Ozaki KS, Nalesnik MA, Ueki S, Castillo-Rama M, Faleo G, Ezzelarab M, Nakao A, Ekser B, Echeverri GJ, Ross MA, Stolz DB, Murase N. Ex vivo application of carbon monoxide in UW solution prevents transplant-induced renal ischemia/reperfusion injury in pigs. Am J Transplant. 2010;10:763–772. doi: 10.1111/j.1600-6143.2010.03040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kaizu T, Nakao A, Tsung A, Toyokawa H, Sahai R, Geller DA, Murase N. Carbon monoxide inhalation ameliorates cold ischemia/reperfusion injury after rat liver transplantation. Surgery. 2005;138:229–235. doi: 10.1016/j.surg.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 78.Ikeda A, Ueki S, Nakao A, Tomiyama K, Ross MA, Stolz DB, Geller DA, Murase N. Liver graft exposure to carbon monoxide during cold storage protects sinusoidal endothelial cells and ameliorates reperfusion injury in rats. Liver Transpl. 2009;15:1458–1468. doi: 10.1002/lt.21918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nakao A, Kimizuka K, Stolz DB, Seda Neto J, Kaizu T, Choi AM, Uchiyama T, Zuckerbraun BS, Bauer AJ, Nalesnik MA, Otterbein LE, Geller DA, Murase N. Protective effect of carbon monoxide inhalation for cold-preserved small intestinal grafts. Surgery. 2003;134:285–292. doi: 10.1067/msy.2003.238. [DOI] [PubMed] [Google Scholar]
- 80.Nakao A, Toyokawa H, Tsung A, Nalesnik MA, Stolz DB, Kohmoto J, Ikeda A, Tomiyama K, Harada T, Takahashi T, Yang R, Fink MP, Morita K, Choi AM, Murase N. Ex vivo application of carbon monoxide in University of Wisconsin solution to prevent intestinal cold ischemia/reperfusion injury. Am J Transplant. 2006;6:2243–2255. doi: 10.1111/j.1600-6143.2006.01465.x. [DOI] [PubMed] [Google Scholar]
- 81.Lee SS, Gao W, Mazzola S, Thomas MN, Csizmadia E, Otterbein LE, Bach FH, Wang H. Heme oxygenase-1, carbon monoxide, and bilirubin induce tolerance in recipients toward islet allografts by modulating T regulatory cells. FASEB J. 2007;21:3450–3457. doi: 10.1096/fj.07-8472com. [DOI] [PubMed] [Google Scholar]
- 82.Wang H, Lee SS, Gao W, Czismadia E, McDaid J, Ollinger R, Soares MP, Yamashita K, Bach FH. Donor treatment with carbon monoxide can yield islet allograft survival and tolerance. Diabetes. 2005;54:1400–1406. doi: 10.2337/diabetes.54.5.1400. [DOI] [PubMed] [Google Scholar]
- 83.Günther L, Berberat PO, Haga M, Brouard S, Smith RN, Soares MP, Bach FH, Tobiasch E. Carbon monoxide protects pancreatic beta-cells from apoptosis and improves islet function/survival after transplantation. Diabetes. 2002;51:994–999. doi: 10.2337/diabetes.51.4.994. [DOI] [PubMed] [Google Scholar]
- 84.Fondevila C, Shen XD, Tsuchiyashi S, Yamashita K, Csizmadia E, Lassman C, Busuttil RW, Kupiec-Weglinski JW, Bach FH. Biliverdin therapy protects rat livers from ischemia and reperfusion injury. Hepatology. 2004;40:1333–1341. doi: 10.1002/hep.20480. [DOI] [PubMed] [Google Scholar]
- 85.Adin CA, Croker BP, Agarwal A. Protective effects of exogenous bilirubin on ischemia–reperfusion injury in the isolated, perfused rat kidney. Am J Physiol Renal Physiol. 2005;288:F778–F784. doi: 10.1152/ajprenal.00215.2004. [DOI] [PubMed] [Google Scholar]
- 86.Yamashita K, McDaid J, Ollinger R, Tsui TY, Berberat PO, Usheva A, Csizmadia E, Smith RN, Soares MP, Bach FH. Biliverdin, a natural product of heme catabolism, induces tolerance to cardiac allografts. FASEB J. 2004;18:765–767. doi: 10.1096/fj.03-0839fje. [DOI] [PubMed] [Google Scholar]
- 87.Morse D, Lin L, Choi AM, Ryter SW. Heme oxygenase-1, a critical arbitrator of cell death pathways in lung injury and disease. Free Radic Biol Med. 2009;47:1–12. doi: 10.1016/j.freeradbiomed.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Simon T, Anegon I, Blancou P. Heme oxygenase and carbon monoxide as an immunotherapeutic approach in transplantation and cancer. Immunotherapy. 2011;3:15–18. doi: 10.2217/imt.11.43. [DOI] [PubMed] [Google Scholar]
- 89.Motterlini R, Foresti R. Heme oxygenase-1 as a target for drug discovery. Antioxid Redox Signal. 2014;20:1810–1826. doi: 10.1089/ars.2013.5658. [DOI] [PubMed] [Google Scholar]
- 90.Yoshida T, Noguchi M, Kikuchi G. The step of carbon monoxide liberation in the sequence of heme degradation catalyzed by the reconstituted microsomal heme oxygenase system. J Biol Chem. 1982;257:9345–9348. [PubMed] [Google Scholar]
- 91.Noguchi M, Yoshida T, Kikuchi G. Identification of the product of heme degradation catalyzed by the heme oxygenase system as biliverdin IX alpha by reversed-phase high-performance liquid chromatography. J Biochem. 1982;91:1479–1483. doi: 10.1093/oxfordjournals.jbchem.a133839. [DOI] [PubMed] [Google Scholar]
- 92.Kikuchi G, Yoshida T, Noguchi M. Heme oxygenase and heme degradation. Biochem Biophys Res Commun. 2005;338:558–567. doi: 10.1016/j.bbrc.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 93.Matsui T, Unno M, Ikeda-Saito M. Heme oxygenase reveals its strategy for catalyzing three successive oxygenation reactions. Acc Chem Res. 2010;43:240–247. doi: 10.1021/ar9001685. [DOI] [PubMed] [Google Scholar]
- 94.Matsui T, Iwasaki M, Sugiyama R, Unno M, Ikeda-Saito M. Dioxygen activation for the self-degradation of heme: reaction mechanism and regulation of heme oxygenase. Inorg Chem. 2010;49:3602–3609. doi: 10.1021/ic901869t. [DOI] [PubMed] [Google Scholar]
- 95.Noguchi M, Yoshida T, Kikuchi G. Specific requirement of NADPH-cytochrome c reductase for the microsomal heme oxygenase reaction yielding biliverdin IX alpha. FEBS Lett. 1979;98:281–284. doi: 10.1016/0014-5793(79)80200-8. [DOI] [PubMed] [Google Scholar]
- 96.Sugishima M, Sato H, Higashimoto Y, Harada J, Wada K, Fukuyama K, Noguchi M. Structural basis for the electron transfer from an open form of NADPH-cytochrome P450 oxidoreductase to heme oxygenase. Proc Natl Acad Sci USA. 2014;111:2524–2529. doi: 10.1073/pnas.1322034111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Higashimoto Y, Sakamoto H, Hayashi S, Sugishima M, Fukuyama K, Palmer G, Noguchi M. Involvement of NADPH in the interaction between heme oxygenase-1 and cytochrome P450 reductase. J Biol Chem. 2005;280:729–737. doi: 10.1074/jbc.M406203200. [DOI] [PubMed] [Google Scholar]
- 98.Yoshida T, Noguchi M, Kikuchi G. Oxygenated form of heme. heme oxygenase complex and requirement for second electron to initiate heme degradation from the oxygenated complex. J Biol Chem. 1980;255:4418–4420. [PubMed] [Google Scholar]
- 99.Yoshida T, Migita CT. Mechanism of heme degradation by heme oxygenase. J Inorg Biochem. 2000;82:33–41. doi: 10.1016/s0162-0134(00)00156-2. [DOI] [PubMed] [Google Scholar]
- 100.Yoshida T, Noguchi M, Kikuchi G, Sano S. Degradation of mesoheme and hydroxymesoheme catalyzed by the heme oxygenase system: involvement of hydroxyheme in the sequence of heme catabolism. J Biochem. 1981;90:125–131. doi: 10.1093/oxfordjournals.jbchem.a133441. [DOI] [PubMed] [Google Scholar]
- 101.Higashimoto Y, Sato H, Sakamoto H, Takahashi K, Palmer G, Noguchi M. The reactions of heme- and verdoheme-heme oxygenase-1 complexes with FMN-depleted NADPH-cytochrome P450 reductase. Electrons required for verdoheme oxidation can be transferred through a pathway not involving FMN. J Biol Chem. 2006;281:31659–31667. doi: 10.1074/jbc.M606163200. [DOI] [PubMed] [Google Scholar]
- 102.Matera KM, Takahashi S, Fujii H, Zhou H, Ishikawa K, Yoshimura T, Rousseau DL, Yoshida T, Ikeda-Saito M. Oxygen and one reducing equivalent are both required for the conversion of alpha-hydroxyhemin to verdoheme in heme oxygenase. J Biol Chem. 1996;271:6618–6624. doi: 10.1074/jbc.271.12.6618. [DOI] [PubMed] [Google Scholar]
- 103.Gohya T, Sato M, Zhang X, Migita CT. Variation of the oxidation state of verdoheme in the heme oxygenase reaction. Biochem Biophys Res Commun. 2008;376:293–298. doi: 10.1016/j.bbrc.2008.08.141. [DOI] [PubMed] [Google Scholar]
- 104.Tenhunen R, Ross ME, Marver HS, Schmid R. Reduced nicotinamide-adenine dinucleotide phosphate dependent biliverdin reductase: partial purification and characterization. Biochemistry. 1970;9:298–303. doi: 10.1021/bi00804a016. [DOI] [PubMed] [Google Scholar]
- 105.Maines MD, Trakshel GM, Kutty RK. Characterization of two constitutive forms of rat liver microsomal heme oxygenase. Only one molecular species of the enzyme is inducible. J Biol Chem. 1986;261:411–419. [PubMed] [Google Scholar]
- 106.Trakshel GM, Kutty RK, Maines MD. Purification and characterization of the major constitutive form of testicular heme oxygenase. J Biol Chem. 1986;261:11131–11137. [PubMed] [Google Scholar]
- 107.Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 108.Cruse I, Maines MD. Evidence suggesting that the two forms of heme oxygenase are products of different genes. J Biol Chem. 1998;263:3348–3353. [PubMed] [Google Scholar]
- 109.Rotenberg MO, Maines MD. Isolation, characterization, and expression in Escherichia coli of a cDNA encoding rat heme oxygenase-2. J Biol Chem. 1990;265:7501–7506. [PubMed] [Google Scholar]
- 110.Rotenberg MO, Maines MD. Characterization of a cDNA-encoding rabbit brain heme oxygenase-2 and identification of a conserved domain among mammalian heme oxygenase isozymes: possible heme-binding site? Arch Biochem Biophys. 1991;290:336–344. doi: 10.1016/0003-9861(91)90549-x. [DOI] [PubMed] [Google Scholar]
- 111.Ishikawa K, Sato M, Yoshida T. Expression of rat heme oxygenase in Esherichia coli as a catalytically active, full length form that binds to bacterial membranes. Eur J Biochem. 1991;202:161–165. doi: 10.1111/j.1432-1033.1991.tb16357.x. [DOI] [PubMed] [Google Scholar]
- 112.McCoubrey WK, Jr, Huang TJ, Maines MD. Heme oxygenase-2 is a hemoprotein and binds heme through heme regulatory motifs that are not involved in heme catalysis. J Biol Chem. 1997;272:12568–12574. doi: 10.1074/jbc.272.19.12568. [DOI] [PubMed] [Google Scholar]
- 113.Converso DP, Taillé C, Carreras MC, Jaitovich A, Poderoso JJ, Boczkowski J. HO-1 is located in liver mitochondria and modulates mitochondrial heme content and metabolism. FASEB J. 2006;20:1236–1238. doi: 10.1096/fj.05-4204fje. [DOI] [PubMed] [Google Scholar]
- 114.Slebos DJ, Ryter SW, van der Toorn M, Liu F, Guo F, Baty CJ, Karlsson JM, Watkins SC, Kim HP, Wang X, Lee JS, Postma DS, Kauffman HF, Choi AM. Mitochondrial localization and function of heme oxygenase-1 in cigarette smoke-induced cell death. Am J Respir Cell Mol Biol. 2007;36:409–417. doi: 10.1165/rcmb.2006-0214OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 115.Kim HP, Wang X, Galbiati F, Ryter SW, Choi AM. Caveolae compartmentalization of heme oxygenase-1 in endothelial cells. FASEB J. 2004;18:1080–1089. doi: 10.1096/fj.03-1391com. [DOI] [PubMed] [Google Scholar]
- 116.Taira J, Sugishima M, Kida Y, Oda E, Noguchi M, Higashimoto Y. Caveolin-1 is a competitive inhibitor of heme oxygenase-1 (HO-1) with heme: identification of a minimum sequence in caveolin-1 for binding to HO-1. Biochemistry. 2011;50:6824–6831. doi: 10.1021/bi200601t. [DOI] [PubMed] [Google Scholar]
- 117.Schacter BA, Nelson EB, Marver HS, Masters BS. Immunochemical evidence for an association of heme oxygenase with the microsomal electron transport system. J Biol Chem. 1972;247:3601–3607. [PubMed] [Google Scholar]
- 118.Dennery PA. Signaling function of heme oxygenase proteins. Antioxid Redox Signal. 2014;20:1743–1753. doi: 10.1089/ars.2013.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Alam J, Cai J, Smith A. Isolation and characterization of the mouse heme oxygenase-1 gene. Distal 5′ sequences are required for induction by heme or heavy metals. J Biol Chem. 1994;269:1001–1009. [PubMed] [Google Scholar]
- 120.Alam J, Camhi S, Choi AM. Identification of a second region upstream of the mouse heme oxygenase-1 gene that functions as a basal level and inducer-dependent transcription enhancer. J Biol Chem. 1995;270:11977–11984. doi: 10.1074/jbc.270.20.11977. [DOI] [PubMed] [Google Scholar]
- 121.Alam J, Den Z. Distal AP-1 binding sites mediate basal level enhancement and TPA induction of the mouse heme oxygenase-1 gene. J Biol Chem. 1992;267:21894–21900. [PubMed] [Google Scholar]
- 122.Alam J, Stewart D, Touchard C, Boinapally S, Choi AM, Cook JL. Nrf2, a Cap’n’Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J Biol Chem. 1999;274:26071–26078. doi: 10.1074/jbc.274.37.26071. [DOI] [PubMed] [Google Scholar]
- 123.Itoh K, Wakabayashi N, Katoh Y, Ishii T, O’Connor T, Yamamoto M. Keap1 regulates both cytoplasmic-nuclear shuttling and degradation of Nrf2 in response to electrophiles. Genes Cells. 2003;8:379–391. doi: 10.1046/j.1365-2443.2003.00640.x. [DOI] [PubMed] [Google Scholar]
- 124.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kobayashi M, Itoh K, Suzuki T, Osanai H, Nishikawa K, Katoh Y, Takagi Y, Yamamoto M. Identification of the interactive interface and phylogenic conservation of the Nrf2-Keap1 system. Genes Cells. 2002;7:807–820. doi: 10.1046/j.1365-2443.2002.00561.x. [DOI] [PubMed] [Google Scholar]
- 126.Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci USA. 2002;99:11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Komatsu M, Kurokawa H, Waguri S, Taguchi K, Kobayashi A, Ichimura Y, Sou YS, Ueno I, Sakamoto A, Tong KI, Kim M, Nishito Y, Iemura S, Natsume T, Ueno T, Kominami E, Motohashi H, Tanaka K, Yamamoto M. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol. 2010;12:213–223. doi: 10.1038/ncb2021. [DOI] [PubMed] [Google Scholar]
- 128.Taguchi K, Fujikawa N, Komatsu M, Ishii T, Unno M, Akaike T, Motohashi H, Yamamoto M. Keap1 degradation by autophagy for the maintenance of redox homeostasis. Proc Natl Acad Sci U S A. 2012;109:13561–13566. doi: 10.1073/pnas.1121572109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sun J, Hoshino H, Takaku K, Nakajima O, Muto A, Suzuki H, Tashiro S, Takahashi S, Shibahara S, Alam J, Taketo MM, Yamamoto M, Igarashi K. Hemoprotein Bach1 regulates enhancer availability of heme oxygenase-1 gene. EMBO J. 2002;21:5216–5224. doi: 10.1093/emboj/cdf516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sun J, Brand M, Zenke Y, Tashiro S, Groudine M, Igarashi K. Heme regulates the dynamic exchange of Bach1 and NF-E2-related factors in the Maf transcription factor network. Proc Natl Acad Sci USA. 2004;101:1461–1466. doi: 10.1073/pnas.0308083100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Miyazaki T, Kirino Y, Takeno M, Samukawa S, Hama M, Tanaka M, Yamaji S, Ueda A, Tomita N, Fujita H, Ishigatsubo Y. Expression of heme oxygenase-1 in human leukemic cells and its regulation by transcriptional repressor Bach1. Cancer Sci. 2010;101:1409–1416. doi: 10.1111/j.1349-7006.2010.01550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dorresteijn MJ, Paine A, Zilian E, Fenten MG, Frenzel E, Janciauskiene S, Figueiredo C, Eiz-Vesper B, Blasczyk R, Dekker D, Pennings B, Scharstuhl A, Smits P, Larmann J, Theilmeier G, van der Hoeven JG, Wagener FA, Pickkers P, Immenschuh S. Cell-type-specific downregulation of heme oxygenase-1 by lipopolysaccharide via Bach1 in primary human mononuclear cells. Free Radic Biol Med. 2015;78:224–232. doi: 10.1016/j.freeradbiomed.2014.10.579. [DOI] [PubMed] [Google Scholar]
- 133.Takeda K, Ishizawa S, Sato M, Yoshida T, Shibahara S. Identification of a cis-acting element that is responsible for cadmium-mediated induction of the human heme oxygenase gene. J Biol Chem. 1994;269:22858–22867. [PubMed] [Google Scholar]
- 134.Yang G, Nguyen X, Ou J, Rekulapelli P, Stevenson DK, Dennery PA. Unique effects of zinc protoporphyrin on HO-1 induction and apoptosis. Blood. 2001;97:1306–1313. doi: 10.1182/blood.v97.5.1306. [DOI] [PubMed] [Google Scholar]
- 135.Sikorski EM, Hock T, Hill-Kapturczak N, Agarwal A. The story so far: Molecular regulation of the heme oxygenase-1 gene in renal injury. Am J Physiol Renal Physiol. 2004;286:F425–F441. doi: 10.1152/ajprenal.00297.2003. [DOI] [PubMed] [Google Scholar]
- 136.Alam J, Igarashi K, Immenschuh S, Shibahara S, Tyrrell RM. Regulation of heme oxygenase-1 gene transcription: recent advances and highlights from the International Conference (Uppsala, 2003) on Heme Oxygenase. Antioxid Redox Signal. 2004;6:924–933. doi: 10.1089/ars.2004.6.924. [DOI] [PubMed] [Google Scholar]
- 137.Alam J, Cook JL. How many transcription factors does it take to turn on the heme oxygenase-1 gene? Am J Respir Cell Mol Biol. 2007;36:166–174. doi: 10.1165/rcmb.2006-0340TR. [DOI] [PubMed] [Google Scholar]
- 138.Exner M, Minar E, Wagner O, Schillinger M. The role of heme oxygenase-1 promoter polymorphisms in human disease. Free Radic Biol Med. 2004;37:1097–1104. doi: 10.1016/j.freeradbiomed.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 139.Bao W, Song F, Li X, Rong S, Yang W, Wang D, Xu J, Fu J, Zhao Y, Liu L. Association between heme oxygenase-1 gene promoter polymorphisms and type 2 diabetes mellitus: a HuGE review and meta-analysis. Am J Epidemiol. 2010;172:631–636. doi: 10.1093/aje/kwq162. [DOI] [PubMed] [Google Scholar]
- 140.Pechlaner R, Willeit P, Summerer M, Santer P, Egger G, Kronenberg F, Demetz E, Weiss G, Tsimikas S, Witztum JL, Willeit K, Iglseder B, Paulweber B, Kedenko L, Haun M, Meisinger C, Gieger C, Müller-Nurasyid M, Peters A, Willeit J, Kiechl S. Heme oxygenase-1 gene promoter microsatellite polymorphism is associated with progressive atherosclerosis and incident cardiovascular disease. Arterioscler Thromb Vasc Biol. 2015;35:229–236. doi: 10.1161/ATVBAHA.114.304729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chen YH, Kuo KL, Hung SC, Hsu CC, Chen YH, Tarng DC. Length polymorphism in heme oxygenase-1 and risk of CKD among patients with coronary artery disease. J Am Soc Nephrol. 2014;25:2669–2677. doi: 10.1681/ASN.2013111205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gulla A, Evans BJ, Navenot JM, Pundzius J, Barauskas G, Gulbinas A, Dambrauskas Z, Arafat H, Wang ZX. Heme oxygenase-1 gene promoter polymorphism is associated with the development of necrotizing acute pancreatitis. Pancreas. 2014;43:1271–1276. doi: 10.1097/MPA.0000000000000171. [DOI] [PubMed] [Google Scholar]
- 143.Kaartokallio T, Klemetti MM, Timonen A, Uotila J, Heinonen S, Kajantie E, Kere J, Kivinen K, Pouta A, Lakkisto P, Laivuori H. Microsatellite polymorphism in the heme oxygenase-1 promoter is associated with nonsevere and late-onset preeclampsia. Hypertension. 2014;64:172–177. doi: 10.1161/HYPERTENSIONAHA.114.03337. [DOI] [PubMed] [Google Scholar]
- 144.Yamada N, Yamaya M, Okinaga S, Nakayama K, Sekizawa K, Shibahara S, Sasaki H. Microsatellite polymorphism in the heme oxygenase-1 gene promoter is associated with susceptibility to emphysema. Am J Hum Genet. 2000;66:187–195. doi: 10.1086/302729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sheu CC, Zhai R, Wang Z, Gong MN, Tejera P, Chen F, Su L, Thompson BT, Christiani DC. Heme oxygenase-1 microsatellite polymorphism and haplotypes are associated with the development of acute respiratory distress syndrome. Intensive Care Med. 2009;35:1343–1351. doi: 10.1007/s00134-009-1504-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Exner M, Schillinger M, Minar E, Mlekusch W, Schlerka G, Haumer M, Mannhalter C, Wagner O. Heme oxygenase-1 gene promoter microsatellite polymorphism is associated with restenosis after percutaneous transluminal angioplasty. J Endovasc Ther. 2001;8:433–440. doi: 10.1177/152660280100800501. [DOI] [PubMed] [Google Scholar]
- 147.Chen YH, Chau LY, Lin MW, Chen LC, Yo MH, Chen JW, Lin S. Heme oxygenase-1 gene promotor microsatellite polymorphism is associated with angiographic restenosis after coronary stenting. Eur Heart J. 2004;25:39–47. doi: 10.1016/j.ehj.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 148.Schillinger M, Exner M, Minar E, Mlekusch W, Müllner M, Mannhalter C, Bach FH, Wagner O. Heme oxygenase-1 genotype and restenosis after balloon angioplasty: a novel vascular protective factor. J Am Coll Cardiol. 2004;43:950–957. doi: 10.1016/j.jacc.2003.09.058. [DOI] [PubMed] [Google Scholar]
- 149.Rueda B, Oliver J, Robledo G, López-Nevot MA, Balsa A, Pascual-Salcedo D, González-Gay MA, González-Escribano MF, Martín J. HO-1 promoter polymorphism associated with rheumatoid arthritis. Arthritis Rheum. 2007;56:3953–3958. doi: 10.1002/art.23048. [DOI] [PubMed] [Google Scholar]
- 150.Exner M, Böhmig GA, Schillinger M, Regele H, Watschinger B, Hörl WH, Raith M, Mannhalter C, Wagner OF. Donor heme oxygenase-1 genotype is associated with renal allograft function. Transplantation. 2004;77:538–542. doi: 10.1097/01.tp.0000113467.36269.f8. [DOI] [PubMed] [Google Scholar]
- 151.Tiwari PK, Sethi A, Basu S, Raman R, Kumar A. Heme oxygenase-1 gene variants and hyperbilirubinemia risk in North Indian newborns. Eur J Pediatr. 2013;172:1627–1632. doi: 10.1007/s00431-013-2091-7. [DOI] [PubMed] [Google Scholar]
- 152.Kramer M, Sponholz C, Slaba M, Wissuwa B, Claus RA, Menzel U, Huse K, Platzer M, Bauer M. Alternative 5′ untranslated regions are involved in expression regulation of human heme oxygenase-1. PLoS One. 2013;8:e77224. doi: 10.1371/journal.pone.0077224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cao L, Zhang Z, Cai B, Bai W, Zhang Y, Sun W, Xie X, Sun W, Cai Q, Li Z, Liu D, Xiong Y, Ma M, Liu X, Xu G. Association of heme oxygenase-1 gene rs2071746 polymorphism with vascular outcomes in patients with atherosclerotic stroke. J Neurol Sci. 2014;344:154–157. doi: 10.1016/j.jns.2014.06.046. [DOI] [PubMed] [Google Scholar]
- 154.Ayuso P, Martínez C, Pastor P, Lorenzo-Betancor O, Luengo A, Jiménez-Jiménez FJ, Alonso-Navarro H, Agúndez JA, García-Martín E. An association study between Heme oxygenase-1 genetic variants and Parkinson’s disease. Front Cell Neurosci. 2014;8:298. doi: 10.3389/fncel.2014.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ullrich R, Exner M, Schillinger M, Zuckermann A, Raith M, Dunkler D, Horvat R, Grimm M, Wagner O. Microsatellite polymorphism in the heme oxygenase-1 gene promoter and cardiac allograft vasculopathy. J Heart Lung Transplant. 2005;24:1600–1605. doi: 10.1016/j.healun.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 156.Courtney AE, McNamee PT, Middleton D, Heggarty S, Patterson CC, Maxwell AP. Association of functional heme oxygenase-1 gene promoter polymorphism with renal transplantation outcomes. Am J Transplant. 2007;7:908–913. doi: 10.1111/j.1600-6143.2006.01726.x. [DOI] [PubMed] [Google Scholar]
- 157.Han SW, Song W, Kim HS, Shin KS, Kang H, Cho HC, Ki CS, Park MJ. HMOX1 gene promoter polymorphism is not associated with coronary artery disease in Koreans. Ann Lab Med. 2014;34:337–344. doi: 10.3343/alm.2014.34.5.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lemaire A, Trépo E, Ouziel R, Gustot T, Moreno C, Degré D, Minsart C, Quertinmont E, Vercruysse V, De Wilde V, le Moine O, Devière J, Abramowicz M, le Moine A, Lemmers A. Microsatellite polymorphism in the heme oxygenase-1 gene promoter is not associated with alcoholic liver disease severity. Hepatology. 2014;59:352–253. doi: 10.1002/hep.26534. [DOI] [PubMed] [Google Scholar]
- 159.Tanaka G, Aminuddin F, Akhabir L, He JQ, Shumansky K, Connett JE, Anthonisen NR, Abboud RT, Paré PD, Sandford AJ. Effect of heme oxygenase-1 polymorphisms on lung function and gene expression. BMC Med Genet. 2011;12:117. doi: 10.1186/1471-2350-12-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cheng X, Ku CH, Siow RC. Regulation of the Nrf2 antioxidant pathway by microRNAs: New players in micromanaging redox homeostasis. Free Radic Biol Med. 2013;64:4–11. doi: 10.1016/j.freeradbiomed.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 161.Narasimhan M, Patel D, Vedpathak D, Rathinam M, Henderson G, Mahimainathan L. Identification of novel microRNAs in post-transcriptional control of Nrf2 expression and redox homeostasis in neuronal, SH-SY5Y cells. PLoS One. 2012;7:e51111. doi: 10.1371/journal.pone.0051111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Stachurska A, Ciesla M, Kozakowska M, Wolffram S, Boesch-Saadatmandi C, Rimbach G, Jozkowicz A, Dulak J, Loboda A. Cross-talk between microRNAs, nuclear factor E2-related factor 2, and heme oxygenase-1 in ochratoxin A-induced toxic effects in renal proximal tubular epithelial cells. Mol Nutr Food Res. 2013;57:504–515. doi: 10.1002/mnfr.201200456. [DOI] [PubMed] [Google Scholar]
- 163.Do MT, Kim HG, Choi JH, Jeong HG. Metformin induces microRNA-34a to downregulate the Sirt1/Pgc-1α/Nrf2 pathway, leading to increased susceptibility of wild-type p53 cancer cells to oxidative stress and therapeutic agents. Free Radic Biol Med. 2014;74:21–34. doi: 10.1016/j.freeradbiomed.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 164.Xiao S, Wang X, Ni H, Li N, Zhang A, Liu H, Pu F, Xu L, Gao J, Zhao Q, Mu Y, Wang C, Sun Y, Du T, Xu X, Zhang G, Hiscox JA, Goodfellow IG, Zhou EM. MiR-24-3p promotes porcine reproductive and respiratory syndrome virus replication through suppression of heme oxygenase-1 expression. J Virol. 2015 Feb 4; doi: 10.1128/JVI.02810-14. pii:JVI.02810–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lorenzen JM, Kaucsar T, Schauerte C, Schmitt R, Rong S, Hübner A, Scherf K, Fiedler J, Martino F, Kumarswamy R, Kölling M, Sörensen I, Hinz H, Heineke J, van Rooij E, Haller H, Thum T. MicroRNA-24 antagonism prevents renal ischemia reperfusion injury. J Am Soc Nephrol. 2014;25:2717–2729. doi: 10.1681/ASN.2013121329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Fiedler J, Stöhr A, Gupta SK, Hartmann D, Holzmann A, Just A, Hansen A, Hilfiker-Kleiner D, Eschenhagen T, Thum T. Functional microRNA library screening identifies the hypoxamir miR-24 as a potent regulator of smooth muscle cell proliferation and vascularization. Antioxid Redox Signal. 2014;21:1167–76. doi: 10.1089/ars.2013.5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Li XY, Zhang K, Jiang ZY, Cai LH. MiR-204/miR-211 downregulation contributes to candidemia-induced kidney injuries via derepression of Hmx1 expression. Life Sci. 2014;102:139–144. doi: 10.1016/j.lfs.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 168.Zhang J, Vandevenne P, Hamdi H, Van Puyvelde M, Zucchi A, Bettonville M, Weatherly K, Braun MY. Micro-RNA-155-mediated control of heme oxygenase 1 (HO-1) is required for restoring adaptively tolerant CD4(+) T-cell function in rodents. Eur J Immunol. 2015 Jan 12; doi: 10.1002/eji.201445066. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 169.Skrzypek K, Tertil M, Golda S, Ciesla M, Weglarczyk K, Collet G, Guichard A, Kozakowska M, Boczkowski J, Was H, Gil T, Kuzdzal J, Muchova L, Vitek L, Loboda A, Jozkowicz A, Kieda C, Dulak J. Interplay between heme oxygenase-1 and miR-378 affects non-small cell lung carcinoma growth, vascularization, and metastasis. Antioxid Redox Signal. 2013;19:644–660. doi: 10.1089/ars.2013.5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Beckman JD, Chen C, Nguyen J, Thayanithy V, Subramanian S, Steer CJ, Vercellotti GM. Regulation of heme oxygenase-1 protein expression by miR-377 in combination with miR-217. J Biol Chem. 2011;286:3194–3202. doi: 10.1074/jbc.M110.148726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pulkkinen KH, Ylä-Herttuala S, Levonen AL. Heme oxygenase 1 is induced by miR-155 via reduced BACH1 translation in endothelial cells. Free Radic Biol Med. 2011;51:2124–2131. doi: 10.1016/j.freeradbiomed.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 172.Hou W, Tian Q, Zheng J, Bonkovsky HL. MicroRNA-196 represses Bach1 protein and hepatitis C virus gene expression in human hepatoma cells expressing hepatitis C viral proteins. Hepatology. 2010;51:1494–1504. doi: 10.1002/hep.23401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hou W, Tian Q, Steuerwald NM, Schrum LW, Bonkovsky HL. The let-7 microRNA enhances heme oxygenase-1 by suppressing Bach1 and attenuates oxidant injury in human hepatocytes. Biochim Biophys Acta. 2012;1819:1113–1122. doi: 10.1016/j.bbagrm.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kim JH, Lee KS, Lee DK, Kim J, Kwak SN, Ha KS, Choe J, Won MH, Cho BR, Jeoung D, Lee H, Kwon YG, Kim YM. Hypoxia-responsive MicroRNA-101 promotes angiogenesis via heme oxygenase-1/Vascular endothelial growth factor axis by targeting Cullin 3. Antioxid Redox Signal. 2014;21:2469–2482. doi: 10.1089/ars.2014.5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Eades G, Yang M, Yao Y, Zhang Y, Zhou Q. miR-200a regulates Nrf2 activation by targeting Keap1 mRNA in breast cancer cells. J Biol Chem. 2011;286:40725–40733. doi: 10.1074/jbc.M111.275495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kozakowska M, Szade K, Dulak J, Jozkowicz A. Role of heme oxygenase-1 in postnatal differentiation of stem cells: a possible cross-talk with microRNAs. Antioxid Redox Signal. 2014;20:1827–1850. doi: 10.1089/ars.2013.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kozakowska M, Ciesla M, Stefanska A, Skrzypek K, Was H, Jazwa A, Grochot-Przeczek A, Kotlinowski J, Szymula A, Bartelik A, Mazan M, Yagensky O, Florczyk U, Lemke K, Zebzda A, Dyduch G, Nowak W, Szade K, Stepniewski J, Majka M, Derlacz R, Loboda A, Dulak J, Jozkowicz A. Heme oxygenase-1 inhibits myoblast differentiation by targeting myomirs. Antioxid Redox Signal. 2012;16:113–127. doi: 10.1089/ars.2011.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Willis D, Moore AR, Frederick R, Willoughby DA. Heme oxygenase: a novel target for the modulation of the inflammatory response. Nat Med. 1996;2:87–90. doi: 10.1038/nm0196-87. [DOI] [PubMed] [Google Scholar]
- 179.Inoue S, Suzuki M, Nagashima Y, Suzuki S, Hashiba T, Tsuburai T, Ikehara K, Matsuse T, Ishigatsubo Y. Transfer of heme oxygenase 1 cDNA by a replication-deficient adenovirus enhances interleukin 10 production from alveolar macrophages that attenuates lipopolysaccharide-induced acute lung injury in mice. Hum Gene Ther. 2001;12:967–979. doi: 10.1089/104303401750195926. [DOI] [PubMed] [Google Scholar]
- 180.Morse D, Pischke SE, Zhou Z, Davis RJ, Flavell RA, Loop T, Otterbein SL, Otterbein LE, Choi AM. Suppression of inflammatory cytokine production by carbon monoxide involves the JNK pathway and AP-1. J Biol Chem. 2003;278:36993–36998. doi: 10.1074/jbc.M302942200. [DOI] [PubMed] [Google Scholar]
- 181.Kim HP, Wang X, Zhang J, Suh GY, Benjamin I, Ryter SW, Choi AM. Heat shock factor-1 mediates the cytoprotective effect of carbon monoxide. J Immunol. 2005;175:2622–2629. doi: 10.4049/jimmunol.175.4.2622. [DOI] [PubMed] [Google Scholar]
- 182.Sarady JK, Zuckerbraun BS, Bilban M, Wagner O, Usheva A, Liu F, Ifedigbo E, Zamora R, Choi AM, Otterbein LE. Carbon monoxide protection against endotoxic shock involves reciprocal effects on iNOS in the lung and liver. FASEB J. 2004;18:854–856. doi: 10.1096/fj.03-0643fje. [DOI] [PubMed] [Google Scholar]
- 183.Sarady-Andrews JK, Liu F, Gallo D, Nakao A, Overhaus M, Ollinger R, Choi AM, Otterbein LE. Biliverdin administration protects against endotoxin-induced acute lung injury in rats. Am J Physiol Lung Cell Mol Physiol. 2005;289:L1131–L1137. doi: 10.1152/ajplung.00458.2004. [DOI] [PubMed] [Google Scholar]
- 184.Lee TS, Chau LY. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med. 2002;8:240–246. doi: 10.1038/nm0302-240. [DOI] [PubMed] [Google Scholar]
- 185.Nakahira K, Kim HP, Geng XH, Nakao A, Wang X, Murase N, Drain PF, Wang X, Sasidhar M, Nabel EG, Takahashi T, Lukacs NW, Ryter SW, Morita K, Choi AM. Carbon monoxide differentially inhibits TLR signaling pathways by regulating ROS-induced trafficking of TLRs to lipid rafts. J Exp Med. 2006;203:2377–2389. doi: 10.1084/jem.20060845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Jamal Uddin M, Joe Y, Kim SK, Oh Jeong S, Ryter SW, Pae HO, Chung HT. IRG1 induced by heme oxygenase-1/carbon monoxide inhibits LPS-mediated sepsis and pro-inflammatory cytokine production. Cell Mol Immunol. 2015 Feb 2; doi: 10.1038/cmi.2015.02. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 188.Davis BK, Wen H, Ting JP. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annual Rev Imunol. 2011;29:707–735. doi: 10.1146/annurev-immunol-031210-101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Luo YP, Jiang L, Kang K, Fei DS, Meng XL, Nan CC, Pan SH, Zhao MR, Zhao MY. Hemin inhibits NLRP3 inflammasome activation in sepsis-induced acute lung injury, involving heme oxygenase-1. Int Immunopharmacol. 2014;20:24–32. doi: 10.1016/j.intimp.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 190.Kim SJ, Lee SM. NLRP3 inflammasome activation in D-galactosamine and lipopolysaccharide-induced acute liver failure: role of heme oxygenase-1. Free Radic Biol Med. 2013;65:997–1004. doi: 10.1016/j.freeradbiomed.2013.08.178. [DOI] [PubMed] [Google Scholar]
- 191.Jung SS, Moon JS, Xu J, Ifedigbo E, Ryter SW, Choi AM, Nakahira K. Carbon monoxide negatively regulates NLRP3 inflammasome activation in macrophages. Am J Physiol Lung Cell Mol Physiol. 2015;308:L1058–L1067. doi: 10.1152/ajplung.00400.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kim S, Joe Y, Jeong SO, Zheng M, Back SH, Park SW, Ryter SW, Chung HT. Endoplasmic reticulum stress is sufficient for the induction of IL-1β production via activation of the NF-κB and inflammasome pathways. Innate Immun. 2014;20:799–815. doi: 10.1177/1753425913508593. [DOI] [PubMed] [Google Scholar]
- 193.Wegiel B, Larsen R, Gallo D, Chin BY, Harris C, Mannam P, Kaczmarek E, Lee PJ, Zuckerbraun BS, Flavell R, Soares MP, Otterbein LE. Macrophages sense and kill bacteria through carbon monoxide-dependent inflammasome activation. J Clin Invest. 2014;124:4926–4940. doi: 10.1172/JCI72853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Chung SW, Liu X, Macias AA, Baron RM, Perrella MA. Heme oxygenase-1-derived carbon monoxide enhances the host defense response to microbial sepsis in mice. J Clin Invest. 2008;118:239–247. doi: 10.1172/JCI32730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Larsen R, Gozzelino R, Jeney V, Tokaji L, Bozza FA, Japiassú AM, Bonaparte D, Cavalcante MM, Chora A, Ferreira A, Marguti I, Cardoso S, Sepúlveda N, Smith A, Soares MP. A central role for free heme in the pathogenesis of severe sepsis. Sci Transl Med. 2010;2:51ra71. doi: 10.1126/scitranslmed.3001118. [DOI] [PubMed] [Google Scholar]
- 196.Takamiya R, Hung CC, Hall SR, Fukunaga K, Nagaishi T, Maeno T, Owen C, Macias AA, Fredenburgh LE, Ishizaka A, Blumberg RS, Baron RM, Perrella MA. High-mobility group box 1 contributes to lethality of endotoxemia in heme oxygenase-1-deficient mice. Am J Respir Cell Mol Biol. 2009;41:129–135. doi: 10.1165/rcmb.2008-0331OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Figueiredo RT, Fernandez PL, Mourao-Sa DS, et al. Characterization of heme as activator of Toll-like receptor 4. J Biol Chem. 2007;282:20221–20229. doi: 10.1074/jbc.M610737200. [DOI] [PubMed] [Google Scholar]
- 198.Belcher JD, Chen C, Nguyen J, et al. Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease. Blood. 2014;123:377–390. doi: 10.1182/blood-2013-04-495887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Onyiah JC, Sheikh SZ, Maharshak N, Steinbach EC, Russo SM, Kobayashi T, Mackey LC, Hansen JJ, Moeser AJ, Rawls JF, Borst LB, Otterbein LE, Plevy SE. Carbon monoxide and heme oxygenase-1 prevent intestinal inflammation in mice by promoting bacterial clearance. Gastroenterology. 2013;144:789–798. doi: 10.1053/j.gastro.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hashiba T, Suzuki M, Nagashima Y, Suzuki S, Inoue S, Tsuburai T, Matsuse T, Ishigatubo Y. Adenovirus-mediated transfer of heme oxygenase-1 cDNA attenuates severe lung injury induced by the influenza virus in mice. Gene Ther. 2001;8:1499–1507. doi: 10.1038/sj.gt.3301540. [DOI] [PubMed] [Google Scholar]
- 201.Belcher JD, Vineyard JV, Bruzzone CM, Chen C, Beckman JD, Nguyen J, Steer CJ, Vercellotti GM. Heme oxygenase-1 gene delivery by Sleeping Beauty inhibits vascular stasis in a murine model of sickle cell disease. J Mol Med (Berl) 2010;88:665–675. doi: 10.1007/s00109-010-0613-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Liu X, Pachori AS, Ward CA, Davis JP, Gnecchi M, Kong D, Zhang L, Murduck J, Yet SF, Perrella MA, Pratt RE, Dzau VJ, Melo LG. Heme oxygenase-1 (HO-1) inhibits postmyocardial infarct remodeling and restores ventricular function. FASEB J. 2006;20:207–216. doi: 10.1096/fj.05-4435com. [DOI] [PubMed] [Google Scholar]
- 203.Mishra S, Fujita T, Lama VN, Nam D, Liao H, Okada M, Minamoto K, Yoshikawa Y, Harada H, Pinsky DJ. Carbon monoxide rescues ischemic lungs by interrupting MAPK-driven expression of early growth response 1 gene and its downstream target genes. Proc Natl Acad Sci USA. 2006;13:5191–5196. doi: 10.1073/pnas.0600241103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zhang X, Shan P, Alam J, Davis RJ, Flavell RA, Lee PJ. Carbon monoxide modulates Fas/Fas ligand, caspases, and Bcl-2 family proteins via the p38alpha mitogen-activated protein kinase pathway during ischemia-reperfusion lung injury. J Biol Chem. 2003;278:22061–22070. doi: 10.1074/jbc.M301858200. [DOI] [PubMed] [Google Scholar]
- 205.Araujo JA, Meng L, Tward AD, Hancock WW, Zhai Y, Lee A, Ishikawa K, Iyer S, Buelow R, Busuttil RW, Shih DM, Lusis AJ, Kupiec-Weglinski JW. Systemic rather than local heme oxygenase-1 overexpression improves cardiac allograft outcomes in a new transgenic mouse. J Immunol. 2003;171:1572–1580. doi: 10.4049/jimmunol.171.3.1572. [DOI] [PubMed] [Google Scholar]
- 206.Barbagallo I, Nicolosi A, Calabrese G, David S, Cimino S, Madonia M, Cappello F. The role of the heme oxygenase system in the metabolic syndrome. Curr Pharm Des. 2014;20:4970–4974. doi: 10.2174/1381612819666131206103824. [DOI] [PubMed] [Google Scholar]
- 207.Hosick PA, Stec DE. Heme oxygenase, a novel target for the treatment of hypertension and obesity? Am J Physiol Regul Integr Comp Physiol. 2012;302:R207–R214. doi: 10.1152/ajpregu.00517.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Tu TH, Joe Y, Choi HS, Chung HT, Yu R. Induction of heme oxygenase-1 with hemin reduces obesity-induced adipose tissue inflammation via adipose macrophage phenotype switching. Mediators Inflamm. 2014;2014:290708. doi: 10.1155/2014/290708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Joe Y, Zheng M, Kim SK, Kim S, Uddin JM, Min TS, Ryu do G, Chung HT. The role of carbon monoxide in metabolic disease. Ann N Y Acad Sci. 2011;1229:156–161. doi: 10.1111/j.1749-6632.2011.06121.x. [DOI] [PubMed] [Google Scholar]
- 210.Lancel S, Montaigne D, Marechal X, Marciniak C, Hassoun SM, Decoster B, Ballot C, Blazejewski C, Corseaux D, Lescure B, Motterlini R, Neviere R. Carbon monoxide improves cardiac function and mitochondrial population quality in a mouse model of metabolic syndrome. PLoS One. 2012;7:e41836. doi: 10.1371/journal.pone.0041836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hosick PA, AlAmodi AA, Storm MV, Gousset MU, Pruett BE, Gray W, 3rd, Stout J, Stec DE. Chronic carbon monoxide treatment attenuates development of obesity and remodels adipocytes in mice fed a high-fat diet. Int J Obes (Lond) 2014;38:132–139. doi: 10.1038/ijo.2013.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Dong H, Huang H, Yun X, Kim DS, Yue Y, Wu H, Sutter A, Chavin KD, Otterbein LE, Adams DB, Kim YB, Wang H. Bilirubin increases insulin sensitivity in leptin-receptor deficient and diet-induced obese mice through suppression of ER stress and chronic inflammation. Endocrinology. 2014;155:818–828. doi: 10.1210/en.2013-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Huang JY, Chiang MT, Chau LY. Adipose overexpression of heme oxygenase-1 does not protect against high fat diet-induced insulin resistance in mice. PLoS One. 2013;8:e55369. doi: 10.1371/journal.pone.0055369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Jais A, Einwallner E, Sharif O, Gossens K, Lu TT, Soyal SM, Medgyesi D, Neureiter D, Paier-Pourani J, Dalgaard K, Duvigneau JC, Lindroos-Christensen J, Zapf TC, Amann S, Saluzzo S, Jantscher F, Stiedl P, Todoric J, Martins R, Oberkofler H, Müller S, Hauser-Kronberger C, Kenner L, Casanova E, Sutterlüty-Fall H, Bilban M, Miller K, Kozlov AV, Krempler F, Knapp S, Lumeng CN, Patsch W, Wagner O, Pospisilik JA, Esterbauer H. Heme oxygenase-1 drives metaflammation and insulin resistance in mouse and man. Cell. 2014;158:25–40. doi: 10.1016/j.cell.2014.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Huang JY, Chiang MT, Yet SF, Chau LY. Myeloid heme oxygenase-1 haploinsufficiency reduces high fat diet-induced insulin resistance by affecting adipose macrophage infiltration in mice. PLoS One. 2012;7:e38626. doi: 10.1371/journal.pone.0038626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Naito Y, Takagi T, Higashimura Y. Heme oxygenase-1 and anti-inflammatory M2 macrophages. Arch Biochem Biophys. 2014;564C:83–88. doi: 10.1016/j.abb.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 217.Tzima S, Victoratos P, Kranidioti K, Alexiou M, Kollias G. Myeloid heme oxygenase-1 regulates innate immunity and autoimmunity by modulating IFN-beta production. J Exp Med. 2009;206:1167–1179. doi: 10.1084/jem.20081582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Brusko TM, Wasserfall CH, Agarwal A, Kapturczak MH, Atkinson MA. An integral role for heme oxygenase-1 and carbon monoxide in maintaining peripheral tolerance by CD4+CD25+ regulatory T cells. J Immunol. 2005;174:5181–5186. doi: 10.4049/jimmunol.174.9.5181. [DOI] [PubMed] [Google Scholar]
- 219.Yamashita K, Ollinger R, McDaid J, Sakahama H, Wang H, Tyagi S, Csizmadia E, Smith NR, Soares MP, Bach FH. Heme oxygenase-1 is essential for and promotes tolerance to transplanted organs. FASEB J. 2006;20:776–778. doi: 10.1096/fj.05-4791fje. [DOI] [PubMed] [Google Scholar]
- 220.Xia ZW, Xu LQ, Zhong WW, Wei JJ, Li NL, Shao J, Li YZ, Yu SC, Zhang ZL. Heme oxygenase-1 attenuates ovalbumin-induced airway inflammation by up-regulation of foxp3 T-regulatory cells, interleukin-10, and membrane-bound transforming growth factor-1. Am J Pathol. 2007;171:1904–1914. doi: 10.2353/ajpath.2007.070096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Xia ZW, Zhong WW, Xu LQ, Sun JL, Shen QX, Wang JG, Shao J, Li YZ, Yu SC. Heme oxygenase-1-mediated CD4+CD25high regulatory T cells suppress allergic airwayinflammation. J Immunol. 2006;177:5936–5945. doi: 10.4049/jimmunol.177.9.5936. [DOI] [PubMed] [Google Scholar]
- 222.Choi BM, Pae HO, Jeong YR, Kim YM, Chung HT. Critical role of heme oxygenase-1min Foxp3-mediated immune suppression. Biochem Biophys Res Commun. 2005;327:1066–1071. doi: 10.1016/j.bbrc.2004.12.106. [DOI] [PubMed] [Google Scholar]
- 223.Biburger M, Theiner G, Schädle M, Schuler G, Tiegs G. Pivotal Advance: Hemeoxygenase 1 expression by human CD4+ T cells is not sufficient for their development of immunoregulatory capacity. J Leukoc Biol. 2010;87:193–202. doi: 10.1189/jlb.0508280. [DOI] [PubMed] [Google Scholar]
- 224.George JF, Braun A, Brusko TM, Joseph R, Bolisetty S, Wasserfall CH, Atkinson MA, Agarwal A, Kapturczak MH. Suppression by CD4+CD25+ regulatory T cells is dependent on expression of heme oxygenase-1 in antigen-presenting cells. Am J Pathol. 2008;173:154–160. doi: 10.2353/ajpath.2008.070963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Liu Y, Zhao X, Zhong Y, Meng K, Yu K, Shi H, Wu B, Tony H, Zhu J, Zhu R, Peng Y, Mao Y, Cheng P, Mao X, Zeng Q. Heme oxygenase-1 restores impaired GARPCD4(+)CD25(+) regulatory T cells from patients with acute coronary syndrome by upregulating LAP and GARP expression on activated T lymphocytes. Cell Physiol Biochem. 2015;35:553–570. doi: 10.1159/000369719. [DOI] [PubMed] [Google Scholar]
- 226.Rémy S, Blancou P, Tesson L, Tardif V, Brion R, Royer PJ, Motterlini R, Foresti R, Painchaut M, Pogu S, Gregoire M, Bach JM, Anegon I, Chauveau C. Carbon monoxide inhibits TLR-induced dendritic cell immunogenicity. J Immunol. 2009;182:1877–1884. doi: 10.4049/jimmunol.0802436. [DOI] [PubMed] [Google Scholar]
- 227.Park DJ, Agarwal A, George JF. Heme oxygenase-1 expression in murine dendritic cell subpopulations: effect on CD8+ dendritic cell differentiation in vivo. Am J Pathol. 2010;176:2831–2839. doi: 10.2353/ajpath.2010.090845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Chauveau C, Rémy S, Royer PJ, Hill M, Tanguy-Royer S, Hubert FX, Tesson L, Brion R, Beriou G, Gregoire M, Josien R, Cuturi MC, Anegon I. Heme oxygenase-1 expression inhibits dendritic cell maturation and proinflammatory function but conserves IL-10 expression. Blood. 2005;106:1694–1702. doi: 10.1182/blood-2005-02-0494. [DOI] [PubMed] [Google Scholar]
- 229.Tardif V, Riquelme SA, Remy S, Carreño LJ, Cortés CM, Simon T, Hill M, Louvet C, Riedel CA, Blancou P, Bach JM, Chauveau C, Bueno SM, Anegon I, Kalergis AM. Carbon monoxide decreases endosome-lysosome fusion and inhibits soluble antigen presentation by dendritic cells to T cells. Eur J Immunol. 2013;43:2832–2844. doi: 10.1002/eji.201343600. [DOI] [PubMed] [Google Scholar]
- 230.Simon T, Pogu S, Tardif V, Rigaud K, Rémy S, Piaggio E, Bach JM, Anegon I, Blancou P. Carbon monoxide-treated dendritic cells decrease β1-integrin induction on CD8+ T cells and protect from type 1 diabetes. Eur J Immunol. 2013;43:209–218. doi: 10.1002/eji.201242684. [DOI] [PubMed] [Google Scholar]
- 231.Stevenson DK, Wong RJ. Metalloporphyrins in the management of neonatal hyperbilirubinemia. Semin Fetal Neonatal Med. 2010;15:164–8. doi: 10.1016/j.siny.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Gupta A, Lacoste B, Pistel PJ, Ingram DK, Hamel E, Alaoui-Jamali MA, Szarek WA, Vlahakis JZ, Jie S, Song W, Schipper HM. Neurotherapeutic effects of novel HO-1 inhibitors in vitro and in a transgenic mouse model of Alzheimer’s disease. J Neurochem. 2014;131:778–790. doi: 10.1111/jnc.12927. [DOI] [PubMed] [Google Scholar]
- 233.Vreman HJ, Ekstrand BC, Stevenson DK. Selection of metalloporphyrin heme oxygenase inhibitors based on potency and photoreactivity. Pediatr Res. 1993;33:195–200. doi: 10.1203/00006450-199302000-00021. [DOI] [PubMed] [Google Scholar]
- 234.Wong RJ, Vreman HJ, Schulz S, Kalish FS, Pierce NW, Stevenson DK. In vitro inhibition of heme oxygenase isoenzymes by metalloporphyrins. J Perinatol. 2011;31 (Suppl 1):S35–S41. doi: 10.1038/jp.2010.173. [DOI] [PubMed] [Google Scholar]
- 235.He CX, Campbell CM, Zhao H, Kalish FS, Schulz S, Vreman HJ, Wong RJ, Stevenson DK. Effects of zinc deuteroporphyrin bis glycol on newborn mice after heme loading. Pediatr Res. 2011;70:467–472. doi: 10.1203/PDR.0b013e31822e1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Grundemar L, Ny L. Pitfalls using metalloporphyrins in carbon monoxide research. Trends Pharmacol Sci. 1997;18:193–195. doi: 10.1016/s0165-6147(97)01065-1. [DOI] [PubMed] [Google Scholar]
- 237.Schulz S, Wong RJ, Vreman HJ, Stevenson DK. Metalloporphyrins - an update. Front Pharmacol. 2012;3:68. doi: 10.3389/fphar.2012.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Rahman MN, Vukomanovic D, Vlahakis JZ, Szarek WA, Nakatsu K, Jia Z. Structural insights into human heme oxygenase-1 inhibition by potent and selective azole-based compounds. J R Soc Interface. 2012;10:20120697. doi: 10.1098/rsif.2012.0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Vlahakis JZ, Kinobe RT, Bowers RJ, Brien JF, Nakatsu K, Szarek WA. Imidazole-dioxolane compounds as isozyme-selective heme oxygenase inhibitors. J Med Chem. 2006;49:4437–4441. doi: 10.1021/jm0511435. [DOI] [PubMed] [Google Scholar]
- 240.Vlahakis JZ, Kinobe RT, Bowers RJ, Brien JF, Nakatsu K, Szarek WA. Synthesis and evaluation of azalanstat analogues as heme oxygenase inhibitors. Bioorg Med Chem Lett. 2005;15:1457–1461. doi: 10.1016/j.bmcl.2004.12.075. [DOI] [PubMed] [Google Scholar]
- 241.Sugishima M, Higashimoto Y, Oishi T, Takahashi H, Sakamoto H, Noguchi M, Fukuyama K. X-ray crystallographic and biochemical characterization of the inhibitory action of an imidazole-dioxolane compound on heme oxygenase. Biochemistry. 2007;46:1860–1867. doi: 10.1021/bi062264p. [DOI] [PubMed] [Google Scholar]
- 242.Kinobe RT, Ji Y, Vlahakis JZ, Motterlini R, Brien JF, Szarek WA, Nakatsu K. Effectiveness of novel imidazole-dioxolane heme oxygenase inhibitors in renal proximal tubule epithelial cells. J Pharmacol Exp Ther. 2007;323:763–770. doi: 10.1124/jpet.107.119800. [DOI] [PubMed] [Google Scholar]
- 243.Kinobe RT, Vlahakis JZ, Vreman HJ, Stevenson DK, Brien JF, Szarek WA, Nakatsu K. Selectivity of imidazole-dioxolane compounds for in vitro inhibition of microsomal haem oxygenase isoforms. Br J Pharmacol. 2006;147:307–315. doi: 10.1038/sj.bjp.0706555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Kinobe RT, Dercho RA, Nakatsu K. Inhibitors of the heme oxygenase – carbon monoxide system: on the doorstep of the clinic? Can J Physiol Pharmacol. 2008;86:577–99. doi: 10.1139/y08-066. [DOI] [PubMed] [Google Scholar]
- 245.Csongradi E, Vera T, Rimoldi JM, Gadepalli RS, Stec DE. In vivo inhibition of renal heme oxygenase with an imidazole-dioxolane inhibitor. Pharmacol Res. 2010;61:525–530. doi: 10.1016/j.phrs.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Csongradi E, Storm MV, Stec DE. Renal Inhibition of Heme Oxygenase-1 Increases Blood Pressure in Angiotensin II-Dependent Hypertension. Int J Hypertens. 2012;2012:497213. doi: 10.1155/2012/497213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Kundu JK, Surh YJ. Nrf2-Keap1 signaling as a potential target for chemoprevention of inflammation-associated carcinogenesis. Pharm Res. 2010;27:999–1013. doi: 10.1007/s11095-010-0096-8. [DOI] [PubMed] [Google Scholar]
- 248.Foresti R, Bains SK, Pitchumony TS, de Castro Brás LE, Drago F, Dubois-Randé JL, Bucolo C, Motterlini R. Small molecule activators of the Nrf2-HO-1 antioxidant axis modulate heme metabolism and inflammation in BV2 microglia cells. Pharmacol Res. 2013;76:132–148. doi: 10.1016/j.phrs.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 249.Barbagallo I, Galvano F, Frigiola A, Cappello F, Riccioni G, Murabito P, D’Orazio N, Torella M, Gazzolo D, Li Volti G. Potential therapeutic effects of natural heme oxygenase-1 inducers in cardiovascular diseases. Antioxid Redox Signal. 2013;18:507–521. doi: 10.1089/ars.2011.4360. [DOI] [PubMed] [Google Scholar]
- 250.Klickovic U, Doberer D, Gouya G, Aschauer S, Weisshaar S, Storka A, Bilban M, Wolzt M. Human pharmacokinetics of high dose oral curcumin and its effect on heme oxygenase-1 expression in healthy male subjects. Biomed Res Int. 2014;2014:458592. doi: 10.1155/2014/458592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Dubey D, Kieseier BC, Hartung HP, Hemmer B, Warnke C, Menge T, Miller-Little WA, Stuve O. Dimethyl fumarate in relapsing-remitting multiple sclerosis: rationale, mechanisms of action, pharmacokinetics, efficacy and safety. Expert Rev Neurother. 2015;15:339–346. doi: 10.1586/14737175.2015.1025755. [DOI] [PubMed] [Google Scholar]
- 252.Nicholas JA, Boster AL, Imitola J, O’Connell C, Racke MK. Design of oral agents for the management of multiple sclerosis: benefit and risk assessment for dimethyl fumarate. Drug Des Devel Ther. 2014;8:897–908. doi: 10.2147/DDDT.S50962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Linker RA, Lee DH, Ryan S, van Dam AM, Conrad R, Bista P, Zeng W, Hronowsky X, Buko A, Chollate S, Ellrichmann G, Brück W, Dawson K, Goelz S, Wiese S, Scannevin RH, Lukashev M, Gold R. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain. 2011;134:678–692. doi: 10.1093/brain/awq386. [DOI] [PubMed] [Google Scholar]
- 254.Takaya K, Suzuki T, Motohashi H, Onodera K, Satomi S, Kensler TW, Yamamoto M. Validation of the multiple sensor mechanism of the Keap1-Nrf2 system. Free Radic Biol Med. 2012;53:817–827. doi: 10.1016/j.freeradbiomed.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Schilling S, Goelz S, Linker R, Luehder F, Gold R. Fumaric acid esters are effective in chronic experimental autoimmune encephalomyelitis and suppress macrophage infiltration. Clin Exp Immunol. 2006;145:101–107. doi: 10.1111/j.1365-2249.2006.03094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Oh CJ, Park S, Kim JY, Kim HJ, Jeoung NH, Choi YK, Go Y, Park KG, Lee IK. Dimethylfumarate attenuates restenosis after acute vascular injury by cell-specific and Nrf2-dependent mechanisms. Redox Biol. 2014;2:855–864. doi: 10.1016/j.redox.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Abraham NG, Asija A, Drummond G, Peterson S. Heme oxygenase-1 gene therapy: recent advances and therapeutic applications. Curr Gene Ther. 2007;7:89–108. doi: 10.2174/156652307780363134. [DOI] [PubMed] [Google Scholar]
- 258.Otterbein LE, Kolls JK, Mantell LL, Cook JL, Alam J, Choi AM. Exogenous administration of heme oxygenase-1 by gene transfer provides protection against hyperoxic lung injury. J Clin Invest. 1999;103:1047–1054. doi: 10.1172/JCI5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Abraham NG, da Silva JL, Lavrovsky Y, Stoltz RA, Kappas A, Dunn MW, Schwartzman ML. Adenovirus-mediated heme oxygenase-1 gene transfer into rabbit ocular tissues. Invest Ophthalmol Vis Sci. 1995;36:2202–2210. [PubMed] [Google Scholar]
- 260.Sabaawy HE, Zhang F, Nguyen X, ElHosseiny A, Nasjletti A, Schwartzman M, Dennery P, Kappas A, Abraham NG. Human heme oxygenase-1 gene transfer lowers blood pressure and promotes growth in spontaneously hypertensive rats. Hypertension. 2001;38:210–215. doi: 10.1161/01.hyp.38.2.210. [DOI] [PubMed] [Google Scholar]
- 261.Zhang Y, Jiang G, Sauler M, Lee PJ. Lung endothelial HO-1 targeting in vivo using lentiviral miRNA regulates apoptosis and autophagy during oxidant injury. FASEB J. 2013;27:4041–4058. doi: 10.1096/fj.13-231225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Ryter SW, Otterbein LE. Carbon monoxide in biology and medicine. Bioessays. 2004;26:270–280. doi: 10.1002/bies.20005. [DOI] [PubMed] [Google Scholar]
- 263.Durante W, Johnson FK, Johnson RA. Role of carbon monoxide in cardiovascular function. J Cell Mol Med. 2006;10:672–686. doi: 10.1111/j.1582-4934.2006.tb00427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Suematsu M, Goda N, Sano T, Kashiwagi S, Egawa T, Shinoda Y, Ishimura Y. Carbon monoxide: an endogenous modulator of sinusoidal tone in the perfused ratliver. J Clin Invest. 1995;96:2431–2437. doi: 10.1172/JCI118300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Suematsu M, Kashiwagi S, Sano T, Goda N, Shinoda Y, Ishimura Y. Carbon monoxide as an endogenous modulator of hepatic vascular perfusion. Biochem Biophys Res Commun. 1994;205:1333–1337. doi: 10.1006/bbrc.1994.2811. [DOI] [PubMed] [Google Scholar]
- 266.Kim HP, Ryter SW, Choi AM. CO as a cellular signaling molecule. Annu Rev Pharmacol Toxicol. 2006;46:411–449. doi: 10.1146/annurev.pharmtox.46.120604.141053. [DOI] [PubMed] [Google Scholar]
- 267.Stone JR, Marletta MA. Soluble guanylate cyclase from bovine lung: activation with nitric oxide and carbon monoxide and spectral characterization of the ferrous and ferric states. Biochemistry. 1994;33:5636–5640. doi: 10.1021/bi00184a036. [DOI] [PubMed] [Google Scholar]
- 268.Furchgott RF, Jothianandan D. Endothelium-dependent and -independent vasodilation involving cyclic GMP: relaxation induced by nitric oxide, carbon monoxide and light. Blood Vessels. 1991;28:52–61. doi: 10.1159/000158843. [DOI] [PubMed] [Google Scholar]
- 269.Morita T, Mitsialis SA, Koike H, Liu Y, Kourembanas S. Carbon monoxide controls the proliferation of hypoxic vascular smooth muscle cells. J Biol Chem. 1997;272:32804–32809. doi: 10.1074/jbc.272.52.32804. [DOI] [PubMed] [Google Scholar]
- 270.Morita T, Perrella MA, Lee ME, Kourembanas S. Smooth muscle cell-derived carbon monoxide is a regulator of vascular cGMP. Proc Natl Acad Sci USA. 1995;92:1475–1479. doi: 10.1073/pnas.92.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Verma A, Hirsch DJ, Glatt CE, Ronnett GV, Snyder SH. Carbon monoxide: a putative neural messenger. Science. 1993;259:381–384. doi: 10.1126/science.7678352. [DOI] [PubMed] [Google Scholar]
- 272.Wang R. Resurgence of carbon monoxide: an endogenous gaseous vasorelaxing factor. Can J Physiol Pharmacol. 1998;76:1–15. doi: 10.1139/cjpp-76-1-1. [DOI] [PubMed] [Google Scholar]
- 273.Wilkinson WJ, Kemp PJ. Carbon monoxide: an emerging regulator of ion channels. J Physiol. 2011;589:3055–3062. doi: 10.1113/jphysiol.2011.206706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Peers C. Ion channels as target effectors for carbon monoxide. Exp Physiol. 2011;96:836–839. doi: 10.1113/expphysiol.2011.059063. [DOI] [PubMed] [Google Scholar]
- 275.Brouard S, Otterbein LE, Anrather J, Tobiasch E, Bach FH, Choi AM, Soares MP. Carbon monoxide generated by heme oxygenase-1 suppresses endothelial cell apoptosis. J Exp Med. 2000;192:1015–1026. doi: 10.1084/jem.192.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Nakahira K, Kim HP, Geng XH, Nakao A, Wang X, Murase N, Drain PF, Wang X, Sasidhar M, Nabel EG, Takahashi T, Lukacs NW, Ryter SW, Morita K, Choi AM. Carbon monoxide differentially inhibits TLR signaling pathways by regulating ROS-induced trafficking of TLRs to lipid rafts. J Exp Med. 2006;203:2377–2389. doi: 10.1084/jem.20060845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Wang XM, Kim HP, Nakahira K, Ryter SW, Choi AM. The heme oxygenase-1/carbon monoxide pathway suppresses TLR4 signaling by regulating the interaction of TLR4 with caveolin-1. J Immunol. 2009;182:3809–3818. doi: 10.4049/jimmunol.0712437. [DOI] [PubMed] [Google Scholar]
- 278.Bilban M, Bach FH, Otterbein SL, Ifedigbo E, d’Avila JC, Esterbauer H, Chin BY, Usheva A, Robson SC, Wagner O, Otterbein LE. Carbon monoxide orchestrates a protective response through PPARgamma. Immunity. 2006;24:601–610. doi: 10.1016/j.immuni.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 279.Brouard S, Berberat PO, Tobiasch E, Seldon MP, Bach FH, Soares MP. Heme oxygenase-1-derived carbon monoxide requires the activation of transcription factor NF-kappa B to protect endothelial cells from tumor necrosis factor-alpha-mediated apoptosis. J Biol Chem. 2002;277:17950–17961. doi: 10.1074/jbc.M108317200. [DOI] [PubMed] [Google Scholar]
- 280.Zhang X, Shan P, Alam J, Fu XY, Lee PJ. Carbon monoxide differentially modulates STAT1 and STAT3 and inhibits apoptosis via a phosphatidylinositol 3-kinase/Akt and p38 kinase-dependent STAT3 pathway during anoxia-reoxygenation injury. J Biol Chem. 2005;280:8714–8721. doi: 10.1074/jbc.M408092200. [DOI] [PubMed] [Google Scholar]
- 281.Wang X, Wang Y, Kim HP, Nakahira K, Ryter SW, Choi AM. Carbon monoxide protects against hyperoxia-induced endothelial cell apoptosis by inhibiting reactive oxygen species formation. J Biol Chem. 2007;282:1718–1726. doi: 10.1074/jbc.M607610200. [DOI] [PubMed] [Google Scholar]
- 282.Wang X, Wang Y, Lee SJ, Kim HP, Choi AM, Ryter SW. Carbon monoxide inhibits Fas activating antibody-induced apoptosis in endothelial cells. Med Gas Res. 2011;1:8. doi: 10.1186/2045-9912-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 283.Kim HP, Wang X, Nakao A, Kim SI, Murase N, Choi ME, Ryter SW, Choi AMK. Caveolin-1 expression by means of p38β mitogen activated protein kinase mediates the antiproliferative effect of carbon monoxide. Proc Natl Acad Sci. 2005;102:11319–11324. doi: 10.1073/pnas.0501345102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Taille C, El-Benna J, Lanone S, Boczkowski J, Motterlini R. Mitochondrial respiratory chain and NAD(P)H oxidase are targets for the antiproliferative effect of carbon monoxide in human airway smooth muscle. J Biol Chem. 2005;280:25350–25360. doi: 10.1074/jbc.M503512200. [DOI] [PubMed] [Google Scholar]
- 285.Rodriguez AI, Gangopadhyay A, Kelley EE, Pagano PJ, Zuckerbraun BS, Bauer PM. HO-1 and CO decrease platelet-derived growth factor-induced vascular smooth muscle cell migration via inhibition of Nox1. Arterioscler Thromb Vasc Biol. 2010;30:98–104. doi: 10.1161/ATVBAHA.109.197822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Meyer G, André L, Kleindienst A, Singh F, Tanguy S, Richard S, Obert P, Boucher F, Jover B, Cazorla O, Reboul C. Carbon monoxide increases inducible NOS expression that mediates CO-induced myocardial damage during ischemia-reperfusion. Am J Physiol Heart Circ Physiol. 2015;308:H759–H767. doi: 10.1152/ajpheart.00702.2014. [DOI] [PubMed] [Google Scholar]
- 287.Nakao A, Faleo G, Shimizu H, Nakahira K, Kohmoto J, Sugimoto R, Choi AM, McCurry KR, Takahashi T, Murase N. Ex vivo carbon monoxide prevents cytochrome P450 degradation and ischemia/reperfusion injury of kidney grafts. Kidney Int. 2008;74:1009–10016. doi: 10.1038/ki.2008.342. [DOI] [PubMed] [Google Scholar]
- 288.Zuckerbraun BS, Chin BY, Bilban M, d’Avila JC, Rao J, Billiar TR, Otterbein LE. Carbon monoxide signals via inhibition of cytochrome c oxidase and generation of mitochondrial reactive oxygen species. FASEB J. 2007;21:1099–1106. doi: 10.1096/fj.06-6644com. [DOI] [PubMed] [Google Scholar]
- 289.Chin BY, Jiang G, Wegiel B, Wang HJ, Macdonald T, Zhang XC, Gallo D, Cszimadia E, Bach FH, Lee PJ, Otterbein LE. Hypoxia-inducible factor 1alpha stabilization by carbon monoxide results in cytoprotective preconditioning. Proc Natl Acad Sci U S A. 2007;104:5109–5114. doi: 10.1073/pnas.0609611104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Lee SJ, Ryter SW, Xu JF, Nakahira K, Kim HP, Choi AM, Kim YS. Carbon monoxide activates autophagy via mitochondrial reactive oxygen species formation. Am J Respir Cell Mol Biol. 2011;45:867–873. doi: 10.1165/rcmb.2010-0352OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Li MH, Jang JH, Na HK, Cha YN, Surh YJ. Carbon monoxide produced by heme oxygenase-1 in response to nitrosative stress induces expression of glutamate-cysteine ligase in PC12 cells via activation of phosphatidylinositol 3-kinase and Nrf2 signaling. J Biol Chem. 2007;282:28577–28586. doi: 10.1074/jbc.M701916200. [DOI] [PubMed] [Google Scholar]
- 292.Piantadosi CA, Carraway MS, Babiker A, Suliman HB. Heme oxygenase-1 regulates cardiac mitochondrial biogenesis via Nrf2-mediated transcriptional control of nuclear respiratory factor-1. Circ Res. 2008;103:1232–1240. doi: 10.1161/01.RES.0000338597.71702.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Wang B, Cao W, Biswal S, Dore S. Carbon monoxide-activated Nrf2 pathway leads to protection against permanent focal cerebral ischemia. Stroke. 2011;42:2605–2610. doi: 10.1161/STROKEAHA.110.607101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Motterlini R, Haas B, Foresti R. Emerging concepts on the anti-inflammatory actions of carbon monoxide-releasing molecules (CO-RMs) Med Gas Res. 2012;2:2–28. doi: 10.1186/2045-9912-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Motterlini R, Mann BE, Foresti R. Therapeutic applications of carbon monoxide-releasing molecules (CO-RMs) Expert Opin Invest Drugs. 2005;14:1305–1318. doi: 10.1517/13543784.14.11.1305. [DOI] [PubMed] [Google Scholar]
- 296.Foresti R, Bani-Hani MG, Motterlini R. Use of carbon monoxide as a therapeutic agent: promises and challenges. Intensive Care Med. 2008;34:649–658. doi: 10.1007/s00134-008-1011-1. [DOI] [PubMed] [Google Scholar]
- 297.Foresti R, Hammad J, Clark JE, Johnson TR, Mann BE, Friebe A, Green CJ, Motterlini R. Vasoactive properties of CORM-3, a novel water-soluble carbon monoxide-releasing molecule. Brit J Pharmacol. 2004;142:453–460. doi: 10.1038/sj.bjp.0705825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Motterlini R, Sawle P, Hammad J, Bains S, Alberto R, Foresti R, Green CJ. CORM-A1: a new pharmacologically active carbon monoxide-releasing molecule. FASEB J. 2005;19:284–286. doi: 10.1096/fj.04-2169fje. [DOI] [PubMed] [Google Scholar]
- 299.Motterlini R, Sawle P, Hammad J, Mann BE, Johnson TR, Green CJ, Foresti R. Vasorelaxing effects and inhibition of nitric oxide in macrophages by new iron-containing carbon monoxide-releasing molecules (CO-RMs) Pharmacol Res. 2013;68:108–117. doi: 10.1016/j.phrs.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 300.Kretschmer R, Gessner G, Görls H, Heinemann SH, Westerhausen M. Dicarbonyl-bis(cysteamine)iron(II): a light induced carbon monoxide releasing molecule based on iron (CORM-S1) J Inorg Biochem. 2011;105:6–9. doi: 10.1016/j.jinorgbio.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 301.Nagel C, McLean S, Poole RK, Braunschweig H, Kramer T, Schatzschneider U. Introducing [Mn(CO)3(tpa-κ(3)N)](+) as a novel photoactivatable CO-releasing molecule with well-defined iCORM intermediates - synthesis, spectroscopy, and antibacterial activity. Dalton Trans. 2014;43:9986–9997. doi: 10.1039/c3dt51848e. [DOI] [PubMed] [Google Scholar]
- 302.Pai S, Hafftlang M, Atongo G, Nagel C, Niesel J, Botov S, Schmalz HG, Yard B, Schatzschneider U. New modular manganese(I) tricarbonyl complexes as PhotoCORMs: in vitro detection of photoinduced carbon monoxide release using COP-1 as a fluorogenic switch-on probe. Dalton Trans. 2014;43:8664–8678. doi: 10.1039/c4dt00254g. [DOI] [PubMed] [Google Scholar]
- 303.Schatzschneider U. Novel lead structures and activation mechanisms for CO-releasing molecules (CORMs) Br J Pharmacol. 2015;172:1638–1650. doi: 10.1111/bph.12688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Dördelmann G, Pfeiffer H, Birkner A, Schatzschneider U. Silicium dioxide nanoparticles as carriers for photoactivatable CO-releasing molecules (PhotoCORMs) Inorg Chem. 2011;50:4362–4367. doi: 10.1021/ic1024197. [DOI] [PubMed] [Google Scholar]
- 305.Wilson JL, Fayad Kobeissi S, Oudir S, Haas B, Michel B, Dubois Randé JL, Ollivier A, Martens T, Rivard M, Motterlini R, Foresti R. Design and synthesis of new hybrid molecules that activate the transcription factor Nrf2 and simultaneously release carbon monoxide. Chemistry. 2014;20:14698–146704. doi: 10.1002/chem.201403901. [DOI] [PubMed] [Google Scholar]
- 306.Sawle P, Foresti R, Mann BE, Johnson JR, Green CJ, Motterlini R. Carbon monoxide-releasing molecules (CO-RMs) attenuate the inflammatory response elicited by lipopolysaccharide in RAW264. 7 murine macrophages. Br J Pharmacol. 2005;145:800–810. doi: 10.1038/sj.bjp.0706241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Sun B, Zou X, Chen Y, Zhang P, Shi G. Preconditioning of carbon monoxide releasing molecule-derived CO attenuates LPS-induced activation of HUVEC. Int J Biol Sci. 2008;4:270–278. doi: 10.7150/ijbs.4.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Sun B, Sun H, Liu C, Shen J, Chen Z, Chen X. Role of CO-releasing molecules liberated CO in attenuating leukocytes sequestration and inflammatory responses in the lung of thermally injured mice. J Surg Res. 2007;139:128–135. doi: 10.1016/j.jss.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 309.Cepinskas G, Katada K, Bihari A, Potter RF. Carbon monoxide liberated from carbon monoxide-releasing molecule CORM-2 attenuates inflammation in the liver of septic mice. Am J Physiol Gastrointest Liver Physiol. 2008;294:G184–G191. doi: 10.1152/ajpgi.00348.2007. [DOI] [PubMed] [Google Scholar]
- 310.Mizuguchi S, Stephen J, Bihari R, Markovic N, Suehiro S, Capretta A, Potter RF, Cepinskas G. CORM-3-derived CO modulates polymorphonuclear leukocyte migration across the vascular endothelium by reducing levels of cell surface-bound elastase. Am J Physiol Heart Circ Physiol. 2009;297:H920–H929. doi: 10.1152/ajpheart.00305.2009. [DOI] [PubMed] [Google Scholar]
- 311.Lancel S, Hassoun SM, Favory R, Decoster B, Motterlini R, Neviere R. Carbon monoxide rescues mice from lethal sepsis by supporting mitochondrial energetic metabolism and activating mitochondrial biogenesis. J Pharmacol Exp Ther. 2009;329:641–648. doi: 10.1124/jpet.108.148049. [DOI] [PubMed] [Google Scholar]
- 312.Babu D, Motterlini R, Lefebvre RA. CO and CO-releasing molecules (CO-RMs) in acute gastrointestinal inflammation. Br J Pharmacol. 2015;172:1557–1573. doi: 10.1111/bph.12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Wilson JL, Jesse HE, Hughes B, Lund V, Naylor K, Davidge KS, Cook GM, Mann BE, Poole RK. Ru(CO)3Cl(Glycinate) (CORM-3): a carbon monoxide-releasing molecule with broad-spectrum antimicrobial and photosensitive activities against respiration and cation transport in Escherichia coli. Antioxid Redox Signal. 2013;19:497–509. doi: 10.1089/ars.2012.4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Desmard M, Foresti R, Morin D, Dagouassat M, Berdeaux A, Denamur E, Crook SH, Mann BE, Scapens D, Montravers P, Boczkowski J, Motterlini R. Differential antibacterial activity against Pseudomonas aeruginosa by carbon monoxide-releasing molecules. Antioxid Redox Signal. 2012;16:153–163. doi: 10.1089/ars.2011.3959. [DOI] [PubMed] [Google Scholar]
- 315.Desmard M, Davidge KS, Bouvet O, Morin D, Roux D, Foresti R, Ricard JD, Denamur E, Poole RK, Montravers P, Motterlini R, Boczkowski J. A carbon monoxide-releasing molecule (CORM-3) exerts bactericidal activity against Pseudomonas aeruginosa and improves survival in an animal model of bacteraemia. FASEB J. 2009;23:1023–1031. doi: 10.1096/fj.08-122804. [DOI] [PubMed] [Google Scholar]
- 316.Rana N, McLean S, Mann BE, Poole RK. Interaction of the carbon monoxide-releasing molecule Ru(CO)3Cl(glycinate) (CORM-3) with Salmonella enterica serovar Typhimurium: in situ measurements of carbon monoxide binding by integrating cavity dual-beam spectrophotometry. Microbiology. 2014;160:2771–2779. doi: 10.1099/mic.0.081042-0. [DOI] [PubMed] [Google Scholar]
- 317.Chin BY, Otterbein LE. Carbon monoxide is a poison.. to microbes! CO as a bactericidal molecule. Curr Opin Pharmacol. 2009;9:490–500. doi: 10.1016/j.coph.2009.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Davidge KS, Sanguinetti G, Yee CH, Cox AG, McLeod CW, Monk CE, Mann BE, Motterlini R, Poole RK. Carbon monoxide-releasing antibacterial molecules target respiration and global transcriptional regulators. J Biol Chem. 2009;284:4516–4524. doi: 10.1074/jbc.M808210200. [DOI] [PubMed] [Google Scholar]
- 319.Tavares AF, Parente MR, Justino MC, Oleastro M, Nobre LS, Saraiva LM. The bactericidal activity of carbon monoxide-releasing molecules against Helicobacter pylori. PLoS One. 2013;8:e83157. doi: 10.1371/journal.pone.0083157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Musameh MD, Green CJ, Mann BE, Fuller BJ, Motterlini R. Improved myocardial function after cold storage with preservation solution supplemented with a carbon monoxide-releasing molecule (CORM-3) J Heart Lung Transplant. 2007;26:1192–1198. doi: 10.1016/j.healun.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 321.Stocker R. Antioxidant activities of bile pigments. Antioxid Redox Signal. 2004;6:841–849. doi: 10.1089/ars.2004.6.841. [DOI] [PubMed] [Google Scholar]
- 322.Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 323.Neuzil J, Stocker R. Free and albumin-bound bilirubin are efficient co-antioxidants for alpha-tocopherol, inhibiting plasma and low density lipoprotein lipid peroxidation. J Biol Chem. 1994;269:16712–16719. [PubMed] [Google Scholar]
- 324.Baranano DE, Rao M, Ferris CD, Snyder SH. Biliverdin reductase: a major physiologic cytoprotectant. Proc Natl Acad Sci USA. 2002;99:16093–16098. doi: 10.1073/pnas.252626999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Wu TW, Wu J, Li RK, Mickle D, Carey D. Albumin-bound bilirubins protect human ventricular myocyctes against oxyradical damage. Biochem Cell Biol. 1991;69:683–688. doi: 10.1139/o91-102. [DOI] [PubMed] [Google Scholar]
- 326.Clark JE, Foresti R, Sarathchandra P, Kaur H, Green CJ, Motterlini R. Heme oxygenase-1-derived bilirubin ameliorates postischemic myocardial dysfunction. Am J Physiol. 2000;278:H643–H651. doi: 10.1152/ajpheart.2000.278.2.H643. [DOI] [PubMed] [Google Scholar]
- 327.Dore S, Takahashi M, Ferris CD, Zakhary R, Hester LD, Guastella D, Snyder SH. Bilirubin, formed by activation of heme oxygenase-2, protects neurons against oxidative stress injury. Proc Natl Acad Sci USA. 1999;96:2445–2450. doi: 10.1073/pnas.96.5.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Dennery PA, McDonagh AF, Spitz DR, Rodgers PA, Stevenson DK. Hyperbilirubinemia results in reduced oxidative injury in neonatal Gunn rats exposed to hyperoxia. Free Radic Biol Med. 1995;19:395–404. doi: 10.1016/0891-5849(95)00032-s. [DOI] [PubMed] [Google Scholar]
- 329.Ryter SW. Bile pigments in pulmonary and vascular disease. Front Pharmacol. 2012;3:39. doi: 10.3389/fphar.2012.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Schwertner HA, Jackson WG, Tolan G. Association of low serum concentration of bilirubin with increased risk of coronary artery disease. Clin Chem. 1994;40:18–23. [PubMed] [Google Scholar]
- 331.Breimer LH, Wannamethee G, Ebrahim S, Shaper AG. Serum bilirubin and risk of ischemic heart disease in middle-aged British men. Clin Chem. 1995;41:1504–1508. [PubMed] [Google Scholar]
- 332.Erdogan D, Gullu H, Yildirim E, Tok D, Kirabas I, Ciftci O, Baycan ST, Muderrisoglu H. Low serum bilirubin levels are independently and inversely related to impaired flow-mediated vasodilation and increased carotid intima-media thickness in both men and women. Atherosclerosis. 2005;184:431–437. doi: 10.1016/j.atherosclerosis.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 333.Wang HD, Yamaya M, Okinaga S, Jia YX, Kamanaka M, Takahashi H, Guo LY, Ohrui T, Sasaki H. Bilirubin ameliorates bleomycin-induced pulmonary fibrosis in rats. Am J Respir Crit Care Med. 2002;165:406–411. doi: 10.1164/ajrccm.165.3.2003149. [DOI] [PubMed] [Google Scholar]
- 334.Wegiel B, Gallo D, Csizmadia E, Roger T, Kaczmarek E, Harris C, Zuckerbraun BS, Otterbein LE. Biliverdin inhibits Toll-like receptor-4 (TLR4) expression through nitric oxide-dependent nuclear translocation of biliverdin reductase. Proc Natl Acad Sci USA. 2011;108:18849–18854. doi: 10.1073/pnas.1108571108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Nakao A, Murase N, Ho C, Toyokawa H, Billiar TR, Kanno S. Biliverdin administration prevents the formation of intimal hyperplasia induced by vascular injury. Circulation. 2005;112:587–591. doi: 10.1161/CIRCULATIONAHA.104.509778. [DOI] [PubMed] [Google Scholar]
- 336.Ollinger R, Bilban M, Erat A, Froio A, McDaid J, Tyagi S, Csizmadia E, Graça-Souza AV, Liloia A, Soares MP, Otterbein LE, Usheva A, Yamashita K, Bach FH. Bilirubin: a natural inhibitor of vascular smooth muscle cell proliferation. Circulation. 2005;112:1030–1039. doi: 10.1161/CIRCULATIONAHA.104.528802. [DOI] [PubMed] [Google Scholar]
- 337.Jeney V, Balla J, Yachie A, Varga Z, Vercellotti GM, Eaton JW, Balla G. Pro-oxidant and cytotoxic effects of circulating heme. Blood. 2002;100:879–887. doi: 10.1182/blood.v100.3.879. [DOI] [PubMed] [Google Scholar]
- 338.Belcher JD, Beckman JD, Balla G, Balla J, Vercellotti G. Heme degradation and vascular injury. Antioxid Redox Signal. 2010;12:233–248. doi: 10.1089/ars.2009.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Vinchi F, De Franceschi L, Ghigo A, Townes T, Cimino J, Silengo L, Hirsch E, Altruda F, Tolosano E. Hemopexin therapy improves cardiovascular function by preventing heme-induced endothelial toxicity in mouse models of hemolytic diseases. Circulation. 2013;127:1317–1329. doi: 10.1161/CIRCULATIONAHA.112.130179. [DOI] [PubMed] [Google Scholar]
- 340.Schaer DJ, Buehler PW, Alayash AI, Belcher JD, Vercellotti GM. Hemolysis and free hemoglobin revisited: exploring hemoglobin and hemin scavengers as a novel class of therapeutic proteins. Blood. 2013;121:1276–1284. doi: 10.1182/blood-2012-11-451229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Vile GF, Tyrrell RM. Oxidative stress resulting from ultraviolet A irradiation of human skin fibroblasts leads to a heme oxygenase-dependent increase in ferritin. J Biol Chem. 1993;268:14678–14681. [PubMed] [Google Scholar]
- 342.Vile GF, Basu-Modak S, Waltner C, Tyrrell RM. Heme oxygenase 1 mediates an adaptive response to oxidative stress in human skin fibroblasts. Proc Natl Acad Sci U S A. 1994;91:2607–2610. doi: 10.1073/pnas.91.7.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Gozzelino R, Jeney V, Soares MP. Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol. 2010;50:323–354. doi: 10.1146/annurev.pharmtox.010909.105600. [DOI] [PubMed] [Google Scholar]
- 344.Gumienna-Kontecka E, Pyrkosz-Bulska M, Szebesczyk A, Ostrowska M. Iron chelating strategies in systemic metal overload, neurodegeneration and cancer. Curr Med Chem. 2014;21:3741–3767. doi: 10.2174/0929867321666140706143402. [DOI] [PubMed] [Google Scholar]
- 345.Ward RJ, Dexter DT, Crichton RR. Neurodegenerative diseases and therapeutic strategies using iron chelators. J Trace Elem Med Biol. 2015;31:267–273. doi: 10.1016/j.jtemb.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 346.Schipper HM, Gupta A, Szarek WA. Suppression of glial HO-1 activity as a potential neurotherapeutic intervention in AD. Curr Alzheimer Res. 2009;6:424–430. doi: 10.2174/156720509789207985. [DOI] [PubMed] [Google Scholar]
- 347.Mayr FB, Spiel A, Leitner J, Marsik C, Germann P, Ullrich R, Wagner O, Jilma B. Effects of carbon monoxide inhalation during experimental endotoxemia in humans. Am J Respir Crit Care Med. 2006;171:354–360. doi: 10.1164/rccm.200404-446OC. [DOI] [PubMed] [Google Scholar]
- 348.Bathoorn E, Slebos DJ, Postma DS, Koeter GH, van Oosterhout AJ, van der Toorn M, Boezen HM, Kerstjens HA. Anti-inflammatory effects of inhaled carbon monoxide in patients with COPD: a pilot study. Eur Resp J. 2007;30:1131–1137. doi: 10.1183/09031936.00163206. [DOI] [PubMed] [Google Scholar]
- 349.Levitt DG, Levitt MD. Carbon monoxide: a critical quantitative analysis and review of the extent and limitations of its second messenger function. Clin Pharmacol. 2015;7:37–56. doi: 10.2147/CPAA.S79626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Belcher JD, Young M, Chen C, Nguyen J, Burhop K, Tran P, Vercellotti GM. MP4CO, a pegylated hemoglobin saturated with carbon monoxide, is a modulator of HO-1, inflammation, and vaso-occlusion in transgenic sickle mice. Blood. 2013;122:2757–2764. doi: 10.1182/blood-2013-02-486282. [DOI] [PMC free article] [PubMed] [Google Scholar]