Abstract
Background
Trilostane medical treatment of naturally occurring hyperadrenocorticism (NOH) in dogs is common, as is use of the adrenocorticotropic hormone (ACTH) stimulation test (ACTHst) in monitoring response to treatment. There is uncertainty regarding when the ACTHst should be started relative to time of trilostane administration.
Objective
To compare ACTHst results in dogs being treated for NOH with trilostane when the test is begun 2 versus 4 hours after trilostane administration.
Animals
Twenty‐one privately owned dogs with NOH, each treated with trilostane for at least 30 days.
Methods
Each dog had 2 ACTHst completed, 1 started 2 hours and the other 4 hours after trilostane administration. The second test was started no sooner than 46 hours and no later than 74 hours after the first.
Results
For all 21 dogs, the mean post‐ACTH serum cortisol concentration from tests started 2 hours after trilostane administration (5.4 ± 3.7 μg/dL) was significantly lower (P = .03) as compared with results from the tests started 4 hours after administration (6.5 ± 4.5 μg/dL).
Conclusions
Results of ACTHst started at different times yield significantly different results. Dogs with NOH, treated with trilostane, and monitored with ACTHst results should have all of their subsequent ACTHst tests begun at or about the same time after trilostane administration.
Keywords: Hyperadrenocorticism, Trilostane
Abbreviations
- ACTH
adrenocorticotropic hormone
- ACTHst
ACTH stimulation test
- ATH
adrenocortical tumor‐associated hyperadrenocorticism
- LDDst
low dose dexamethasone suppression test
- NOH
naturally occurring hyperadrenocorticism
- PDH
pituitary‐dependent hyperadrenocorticism
- UCCR
urine cortisol to creatinine ratio
Naturally occurring hyperadrenocorticism (NOH; hypercortisolism) is a well‐recognized endocrine disorder in dogs. Approximately 85% of dogs with NOH have an adrenocorticotropic hormone (ACTH)‐secreting pituitary tumor (pituitary‐dependent hyperadrenocorticism; PDH). The autonomous and excessive secretion of ACTH results in secondary adrenocortical hyperplasia and chronic excess in cortisol secretion with associated clinical and biochemical abnormalities. Approximately 15% of dogs with NOH have an autonomous cortisol‐secreting adrenocortical adenoma or adenocarcinoma (ATH) which also results in chronic excesses in circulating cortisol.1
Dogs with either PDH or ATH are commonly treated with trilostane, a drug that interferes with synthesis of glucocorticoids by enzyme blockade.2 Trilostane treatment, at appropriate doses and frequency of administration, may control many of the clinical and biochemical abnormalities associated with canine NOH.3, 4, 5, 6, 7 However, there is uncertainty in the literature regarding what test might serve as an objective, specific, and sensitive means of assessing therapeutic response to trilostane.6, 7, 8 Results from such a test (if it fulfills these criteria) should complement the more subjective information determined from the owner history and physical examination.
For decades, veterinarians have used ACTH stimulation test (ACTHst) results to aid in assessing therapeutic response of dogs with NOH being treated with mitotane (o,p'‐DDD). This test has also been adopted for the assessment of therapeutic response to trilostane. Since trilostane began to be used as a medical treatment for dogs with NOH approximately 15 years ago, no other test has been widely accepted as a more objective, specific, and sensitive means of assessing therapeutic response.6, 7, 8 If ACTHst results are utilized to aid in assessing trilostane dose, frequency of administration, or both, there is a question of when the test should be started relative to time of trilostane administration. In a previous study of dogs with NOH, post‐ACTH serum cortisol concentrations, when the test was started 8–9 hours posttrilostane administration (mean: 8.1 ± 3.4 μg/dL), significantly less inhibition of cortisol synthesis was demonstrated as compared with tests begun 3–4 hours after administration (mean: 2.6 ± 1.3 μg/dL).9 One conclusion from that study was that results of the ACTHst started 3–4 hours after trilostane administration, in most dogs with NOH, were more indicative of trilostane's inhibition of cortisol synthesis than results from tests started 8–9 hours after administration.9
Currently, the literature contains several recommended times to begin ACTHst in trilostane‐treated dogs. Among the suggested times to begin testing after trilostane administration are as follows: 2–3 hours,8 3 hours,9, 10 2–4 hours,11 2–5 hours,12 2–6 hours,13, 14 as long as 24 hours,15 and 4 hours if the drug is given once daily or 8–12 hours if the drug is given twice daily.16 The package insert that accompanies FDA‐approved trilostane recommends beginning ACTHst 4–6 hours after the drug has been administered.1
This study was designed to further our understanding of trilostane's effect on ACTHst results relative to its time of administration in dogs with NOH. Results of tests begun 2 hours after trilostane administration were compared with results of tests begun 4 hours after.
Methods
Animals
The study included dogs evaluated at the Veterinary Medical Teaching Hospital, University of California at Davis and at the Animal Specialty and Emergency Center, Los Angeles, from February 2012 through December 2012. For inclusion in this study informed consent must have been obtained from each owner. Also, each dog must have had a history and physical examination consistent with NOH.12 Each dog must have had polyuria as an owner concern and each must have had at least 4 of the following 6 clinicopathologic findings: serum activities of alkaline phosphatase or alanine aminotransferase above the reference range, or both above the reference range, serum cholesterol concentration above the reference range, serum urea nitrogen concentration (SUN) below or in the lower half of the reference range. The urine specific gravity had to have been <1.020, or there could have been microbial growth on culture of urine.12 No dog could have had an SUN above the reference range or glucosuria. Before initiation of trilostane treatment, each dog must have had an abdominal ultrasonographic examination performed. For each dog, results of at least 2 of the following 3 screening tests (eg, ACTHst, low‐dose dexamethasone suppression test [LDDst], or urine cortisol to creatinine ratio [UCCR]) must have been consistent with NOH.12 Dogs need not have had all 3 screening tests.
A diagnosis of PDH was made if a dog had at least 2 of the following 3 diagnostic test results: an LDDst result indicative of PDH (transient suppression of serum cortisol concentration), ultrasonographic evidence of 2 relatively equal‐sized adrenal glands with no evidence of an adrenal gland tumor, or plasma concentration of endogenous ACTH >45 pg/mL.6, 17, 18 A diagnosis of ATH was made if all of the following were present: a LDDst result consistent with ATH, ultrasonographic evidence of an adrenal mass, and, after trilostane control of NOH, histologic confirmation of an adrenocortical tumor (adenoma or carcinoma).6, 17, 18, 19
Treatment and Assessment
Each dog must have been treated with FDA‐approved, commercially available trilostane (Vetoryl)1 for at least 30 days before enrollment.3, 10, 12, 15 Resolution of NOH‐related clinical signs and biochemical changes were not inclusion requirements to increase the likelihood that a spectrum of results, similar to those encountered in any practice, would be included.
Each dog underwent 2 ACTHst, one initiated 2 hours and the other 4 hours after trilostane administration. The second test was started no sooner than 46 hours and no later than 74 hours after the first. Dogs with PDH, separately from dogs suspected and later confirmed to have had ATH, were to be numbered as they were entered into the study, beginning with 1. Odd‐numbered dogs had the 2‐hour test first and even‐numbered dogs had the 4‐hour test first. Neither dose nor frequency of trilostane administration was changed after the first test in any dog. For the ACTHst, blood samples (2 mL each) were collected before and 1 hour after each dog was given 0.25 mg of reconstituted lyophilized ACTH (Cortrosyn) as an IM injection.2
Hormone Assay
Blood samples obtained for determination of plasma endogenous ACTH concentration were collected, stored, and assayed as previously described.1 Serum cortisol concentrations were measured by use of a commercial cortisol radioimmunoassay3 that has been validated for the use in dogs.10 The analytic sensitivity of this radioimmunoassay was 0.3 μ/dL. All serum samples were batched and assayed together after all of the dogs had been tested.
Statistical Analysis
Paired samples from the same dogs were analyzed using the exact Wilcoxon signed‐rank test. Comparisons between the 2 groups of patients were made using the exact Mann‐Whitney test. Testing was performed using commercial statistical software.4 Results are presented as mean ± SD and median with range, as indicated. Statistical significance was set at P < .05.
Results
Twenty‐one client‐owned dogs met the inclusion criteria. Four dogs had ATH and 17 had PDH. The 21 dogs included 10 males and 11 females; 19 purebred dogs and 2 mixed breed dogs. The following breeds were represented: Labrador Retriever (2 dogs), English Bulldog (2 dogs), Beagle (2 dogs), Australian Cattle dog, Shih Tzu, Coton de Tullier, Chihuahua, Basset Hound, German Shepherd Dog, Jack Russell Terrier, Airedale Terrier, Maltese, Chesapeake Bay Retriever, Rhodesian Ridgeback, Dachshund, and Springer Spaniel (1 each). The mean age of the dogs was 10.7 years (median 10 years) with a range of 7–14 years of age. Mean body weight of the dogs was 20.7 kg (median 17 kg) with a range of 4.1–42.2 kg. Mean trilostane dosage was 0.61 mg/kg PO q12h (median 0.5 mg/kg PO q12h) with a range of 0.1–1.5 mg/kg PO q12h.
Group 1 had 9 dogs with PDH and 2 with ATH. Group 2 had 8 dogs with PDH and 2 with ATH. Group 1 dogs underwent the 2‐hour posttrilostane‐administration ACTHst first and Group 2 dogs underwent the 4‐hour posttrilostane‐administration ACTHst first. There was no significant difference comparing the first and second set of basal cortisol concentrations obtained from the 11 Group 1 dogs (2.2 ± 1.3 and 2.9 ± 2.1 μg/dL, respectively). There was no significant difference comparing the first and second set of basal cortisol concentrations obtained from the 10 Group 2 dogs (3.0 ± 2.0 and 3.2 ± 3.3 μg/dL, respectively). There also was no significant difference in comparing either set of basal serum cortisol concentrations from the Group 1 dogs with either set of basal serum cortisol concentrations from the Group 2 dogs. Furthermore, there were no significant differences between Group 1 and Group 2 dogs for either 2 or 4 hours post‐ACTH serum cortisol concentrations (P = .29). Therefore, results from the ACTHst started 2 hours after trilostane administration from both groups were combined as were all results from ACTHst started 4 hours after trilostane administration.
For all 21 dogs, the post‐ACTH serum cortisol concentrations from tests initiated 2 hours after trilostane administration (5.4 ± 3.7 μg/dL) were significantly lower (P = .03) as compared with results from tests started 4 hours after administration (6.5 ± 4.5 μg/dL). One dog had a post‐ACTH serum cortisol concentration <1.5 μg/dL (1.4 μg/dL) on the 2‐hour test. The result after the 4‐hour test, in that dog, was 2.5 μg/dL. No other post‐ACTH serum cortisol concentration was <1.5 μg/dL. One of the 21 dogs had identical post‐ACTH serum cortisol concentrations (3.7 μg/dL) on both tests. Fifteen of the 21 dogs (71%, 95% confidence interval, 48–89%; including 3 dogs with ATH) had a lower post‐ACTH serum cortisol concentration on ACTHst started 2 hours after trilostane had been given as compared with the result when testing was started 4 hours after (Fig 1). Five of the 21 dogs (24%, 95% confidence interval, 8–47%; including 1 dog with ATH) had a lower post‐ACTH serum cortisol concentration on ACTHst started 4 hours after trilostane had been given as compared with the result when testing started 2 hours after (Fig 2).
Figure 1.

Individual postadrenocorticotropic hormone stimulation serum cortisol concentrations from 15 dogs with naturally occurring hyperadrenocorticism whose results on tests started 2 hours after the administration of trilostane were lower than on tests begun 4 hours after. The 3 data sets with symbols are the results from 3 dogs with adrenocortical tumor‐associated hyperadrenocorticism, all other data sets are from dogs with pituitary‐dependent hyperadrenocorticism.
Figure 2.
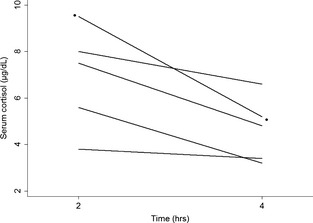
Individual postadrenocorticotropic hormone stimulation serum cortisol concentrations from 5 dogs with naturally occurring hyperadrenocorticism whose results on tests started 4 hours after the administration of trilostane were lower than results on tests begun 2 hours after. The data set with symbols is the results from the dog with adrenocortical tumor‐associated hyperadrenocorticism, all other data sets are from dogs with pituitary‐dependent hyperadrenocorticism.
Discussion
The results of an ACTHst can be of value in monitoring and adjusting trilostane dosage in dogs treated for NOH.8 It is hoped that trilostane‐treated dogs would respond favorably and survive for an extended period of time once treatment has been initiated. The manufacturer of trilostane recommends monitoring dogs with an ACTHst 10–14 days after beginning medication and again “at 30 days, 90 days, and every 3 months thereafter.”1 It is also recommended that an ACTHst be conducted 10–14 days after every trilostane dose adjustment. Results of this study, however, indicate that the 4–6 hour posttrilostane starting time recommended for ACTHst1 would likely yield higher, and potentially misleading results as compared with tests started 2 hours after trilostane administration. Furthermore, although not evaluated, it is likely that ACTHst begun 4 hours after trilostane administration would yield lower results as compared with those begun 6 hours after.
The reason for repeatedly obtaining ACTHst results from trilostane‐treated NOH dogs is that response to treatment is not consistently predictable from assessment of history and physical examination. As previously discussed, the ACTHst is accepted as the most informative biochemical test for monitoring this treatment. Trilostane dose and frequency of administration may need to be periodically altered to achieve the desired response, and results from the ACTHst are used to provide an objective assessment of response to complement owner opinion and physical examination findings. If ACTHst results are not reliable, not consistent, or misleading, inappropriate adjustments in dose or frequency of administration may be implemented. For example, post‐ACTHst serum cortisol concentration <1–1.5 μg/dL may be documented and trilostane dose decreased before clinical over dosage occurs. Such a result may be missed, however, if the test is begun after trilostane effect has begun to dissipate postadministration. Similarly, post‐ACTHst serum cortisol concentrations >5.5–6.5 μg/dL may prompt an increase in the trilostane dose in an attempt to improve response or before signs of under dosage are seen and become intolerable. However, a post‐ACTH serum cortisol concentration >5.5–6.5 μg/dL may be documented in a dog that may have had lower serum cortisol concentrations had the test been started earlier in the day. If it is arbitrarily assumed that a difference in 2‐ versus 4‐hour post‐ACTHst serum cortisol concentrations >20% would not be clinically acceptable, 14 of the 21 dogs (66%) had unacceptable differences in results. If it is arbitrarily assumed that 1 post‐ACTH serum cortisol concentration <5.5 μg/dL and 1 >5.5 μg/dL would not be clinically acceptable, 6 of the 21 dogs (28%) had unacceptably different results. Additional studies are needed to indicate whether it might be of benefit to have new guidelines for interpreting ACTHst results relative to time after trilostane administration.
Veterinarians are instructed, in the literature, to begin ACTHst testing 2–24 hours after trilostane is administered to dogs with NOH.8, 9, 10, 11, 12, 13, 14, 15, 16 As demonstrated in an earlier study, ACTHst begun 3–4 hours after trilostane administration yielded significantly lower results when compared with tests begun 8–9 hours after.9 Results from this study demonstrate significantly lower post‐ACTH serum cortisol concentrations when results of testing begun 2 hours after trilostane administration were compared with results from tests begun 4 hours after. Furthermore, although the number of dogs with ATH was relatively small, there was no evidence of difference in response or need for different timing recommendations for dogs with ATH versus those with PDH.
When monitoring dogs treated for NOH with trilostane, veterinarians usually assume that each set of ACTHst results can be compared directly with previous results to determine trends in response. Also, when monitoring such dogs, it is assumed that dose requirements occasionally may need to be altered to meet patient needs. To meet the criteria suggested in the introduction, this objective assessment should be as consistently reliable as possible if used to complement owner opinion and physical examination findings. Results of this study demonstrate that post‐ACTHst serum cortisol concentrations in a dog with NOH being treated with trilostane are significantly influenced by varying the starting time of the test as little as 2 hours after the drug was given. Therefore, the ACTHst in each trilostane‐treated dog should be started at or about the same time postadministration to have the strongest chance for repeatable results. The insert provided with FDA‐approved trilostane states that “maximal plasma concentrations of trilostane occur within 1.5 hours” of administration in healthy dogs.1 From results of this study, it would appear likely that this time from administration to maximal plasma concentrations is similar in dogs with NOH and that starting ACTHst approximately 2 hours after administration results in lower cortisol concentrations than at 4 hours or later.9
Veterinarians treating dogs for NOH should avoid the confusion of trying to determine if a change in ACTHst results indicates need for dose adjustment or is the change simply reflective of the time at which the test was started. Regardless of the time postadministration chosen for testing, that approximate time should be used for all subsequent testing. Lower serum cortisol concentrations should be anticipated if testing is begun approximately 2 hours posttrilostane administration as compared with starting the test later.
Conclusion
Results of ACTHst started at 2 versus 4 hours after administration of trilostane yielded significantly different results. In a previous study, ACTHst results in dogs with NOH treated with trilostane were significantly lower if the test was started approximately 3 hours after trilostane administration as compared with results if the test was started approximately 9 hours after trilostane administration. It is recommended that dogs with NOH being treated with trilostane and monitored with ACTHst results have each test begun at or about the same time after administration of trilostane.
Acknowledgments
This work was supported in part by donations from the late Ruth Johnston.
Conflict of Interest: The authors disclose no conflict of interest.
Previously presented at the House Officer Seminar Day, School of Veterinary Medicine, University of California, March 2013.
Veterinary Medical Teaching Hospital, University of California at Davis, CA and Animal Specialty and Emergency Center, Los Angeles, CA
Footnotes
Vetoryl [package insert]. Dechra Veterinary Products, Overland Park, KS 2008
Cortrosyn, Amphastar Pharmaceuticals Inc, Rancho Cucamonga, CA
Coat‐a‐count cortisol assay, Diagnostic Products Corp, Los Angeles, CA
StatXact‐9, Cytel Software Corporation, Cambridge, MA
References
- 1. Feldman EC. Distinguishing dogs with functional adrenocortical tumors from dogs with pituitary‐dependent hyperadrenocorticism. J Am Vet Med Assoc 1982;183:195–200. [PubMed] [Google Scholar]
- 2. Potts GO, Creange JE, Hardong HR, Schane HP. Trilostane, an orally active inhibitor of steroid biosynthesis. Steroids 1978;32:257–267. [DOI] [PubMed] [Google Scholar]
- 3. Ramsey IK. Trilostane in dogs. Vet Clin Small Anim 2010;40:269–283. [DOI] [PubMed] [Google Scholar]
- 4. Arteaga A, Dhand NK, McCann T, et al. Monitoring the response of canine hyperadrenocorticism to Trilostane treatment by assessment of acute phase protein concentrations. J Small Anim Practice 2010;51:204–209. [DOI] [PubMed] [Google Scholar]
- 5. Bell R, Neiger R, McGrotty Y, Ramsey IK. Study of the effects of once daily doses of trilostane on cortisol concentrations and responsiveness to adrenocorticotropic hormone in hyperadrenocorticoid dogs. Vet Rec 2006;159:277–281. [DOI] [PubMed] [Google Scholar]
- 6. Galac S, Butijels JJCWM, Kooistra HS. Urinary corticoid:creatinine ratios in dogs with pituitary‐dependent hypercortisolism during trilostane therapy. J Vet Intern Med 2009;23:1214–1219. [DOI] [PubMed] [Google Scholar]
- 7. Sieber‐Ruckstuhl NS, Boretti FS, Wenger M, Maser‐Gluth C, Reusch CE. Cortisol, aldosterone, cortisol precursor, androgen and endogenous ACTH concentration in dogs with pituitary‐dependent hyperadrenocorticism treated with trilostane. Domes Anim Endocrinol 2006;31:63–75. [DOI] [PubMed] [Google Scholar]
- 8. Burkhardt WA, Boretti FS, Reusch CE, Sieber‐Ruckstuhl NS. Evaluation of baseline cortisol, endogenous ACTH, and cortisol/ACTH ratio to monitor trilostane treatment in dogs with pituitary‐dependent hypercortisolism. J Vet Intern Med 2013;27:919–923. [DOI] [PubMed] [Google Scholar]
- 9. Vaughan MA, Feldman EC, Hoar BR, Nelson RW. Evaluation of twice‐daily, low‐dose trilostane treatment administered orally in dogs with naturally occurring hyperadrenocorticism. J Am Vet Med Assoc 2008;232:1321–1328. [DOI] [PubMed] [Google Scholar]
- 10. Feldman EC. Evaluation of twice‐daily lower‐dose trilostane treatment administered orally in dogs with naturally occurring hyperadrenocorticism. J Am Vet Med Assoc 2011;238:1441–1451. [DOI] [PubMed] [Google Scholar]
- 11. Galac S, Buijtels JJCWM, Mol JA, Kooistra HS. Effects of trilostane on the pituitary‐adrenocorticoal and renin‐aldosterone axis in dogs with pituitary‐dependent hypercortisolism. Vet J 2010;183:75–80. [DOI] [PubMed] [Google Scholar]
- 12. Ramsey I, Neiger R. Canine hyperadrenocorticism In: Bonagura JD, Twedt DC, eds. Current Veterinary Therapy. St. Louis, MO: Saunders Elsevier; 2009:224–228. [Google Scholar]
- 13. Ruckstuhl NS, Nett CS, Reusch CE. Results of clinical examination, laboratory tests, and ultrasonography in dogs with pituitary‐dependent hyperadrenocorticism treated with trilostane. Am J Vet Res 2002;63:506–512. [DOI] [PubMed] [Google Scholar]
- 14. Augusto M, Burden A, Neiger R, Ramsey I. A comparison of once versus twice daily administration of trilostane in dogs. Tierarztl Prax 2012; 40(k): 415–424. [PubMed] [Google Scholar]
- 15. Braddock JA, Church DB, Roberston ID, et al. Trilostane treatment in dogs with pituitary‐dependent hyperadrenocorticism. Aust Vet J 2003;81:600–607. [DOI] [PubMed] [Google Scholar]
- 16. Alenza DP, Arenas C, Lopez ML, Melian C. Long‐term efficacy of trilostane administered twice daily in dogs with pituitary‐dependent hyperadrenocorticism. J Am Anim Hosp Assoc 2006;42:269–276. [DOI] [PubMed] [Google Scholar]
- 17. Feldman EC, Nelson RW, Feldman MS. Use of low‐ and high‐dose dexamethasone tests for distinguishing pituitary‐dependent from adrenal tumor hyperadrenocorticism in dogs. J Am Vet Med Assoc 1996;209:772–775. [PubMed] [Google Scholar]
- 18. Reusch CE, Feldman EC. Canine hyperadrenocorticism due to adrenocortical neoplasia. Pretreatment evaluation of 41 dogs. J Vet Intern Med 1991;5:3–10. [DOI] [PubMed] [Google Scholar]
- 19. Helm JR, McLauchlan G, Boden LA, et al. A comparison of factors that influence survival in dogs with adrenal‐dependent hyperadrenocorticism treated with mitotane or trilostane. J Vet Intern Med 2011;25:251–260. [DOI] [PubMed] [Google Scholar]


