Abstract
Background
The prognostic value of early magnetic resonance imaging (MRI) in dogs after traumatic brain injury (TBI) remains unclear.
Objectives
Determine whether MRI findings are associated with prognosis after TBI in dogs.
Animals
Fifty client‐owned dogs.
Methods
Retrospective study of dogs with TBI that underwent 1.5T MRI within 14 days after head trauma. MRI evaluators were blinded to the clinical presentation, and all images were scored based on an MRI grading system (Grade I [normal brain parenchyma] to Grade VI [bilateral lesions affecting the brainstem with or without any lesions of lesser grade]). Skull fractures, percentage of intraparenchymal lesions, degree of midline shift, and type of brain herniation were evaluated. MGCS was assessed at presentation. The presence of seizures was recorded. Outcome was assessed at 48 h (alive or dead) and at 3, 6, 12, and 24 months after TBI.
Results
Sixty‐six percent of the dogs had abnormal MRI findings. MRI grade was negatively correlated (P < .001) with MGCS. A significant negative correlation of MRI grade, degree of midline shift, and percentage of intraparenchymal lesions with follow‐up scores was identified. The MGCS was lower in dogs with brain herniation (P = .0191). Follow‐up scores were significantly lower in dogs that had brain herniation or skull fractures. The possibility of having seizures was associated with higher percentage of intraparenchymal lesions (P = 0.0054) and 10% developed PTE.
Conclusions and Clinical Importance
Significant associations exist between MRI findings and prognosis in dogs with TBI. MRI can help to predict prognosis in dogs with TBI.
Keywords: Dogs, MRI, Prognostic, Traumatic brain injury
Abbreviations
- CT
computed tomography
- MGCS
modified Glasgow coma scale
- MRI
magnetic resonance imaging
- PTE
postraumatic epilepsy
- RTA
road traffic accident
- TBI
traumatic brain injury
Traumatic brain injury (TBI) is defined as structural injury or physiological disruption of the brain induced by an external force. In human medicine, TBI is indicated clinically by the acute onset of at least one of the following: a period of loss of or decreased consciousness, alteration in mental status, neurologic deficits, presence of an intracranial lesion, or some combination of these.1 Not all the patients exposed to head trauma will suffer from TBI.1 A recent single‐center retrospective study reported that up to 25% of dogs with severe blunt trauma suffer from TBI.2 Dogs with TBI are assessed clinically using the modified Glasgow coma scale (MGCS), which evaluates 3 categories: motor activity, brainstem reflexes, and level of consciousness.3, 4 Each category is scored from 1 to 6 (lower score assigned to worse neurologic status), and the category scores are added together to determine the patient's total modified Glasgow coma score (Table 1). The MGCS total score ranges from 3 to 18, with the lowest score representing the worst neurologic status and the lowest probability of survival within the first 48 hours after head trauma.4
Table 1.
Scoring system of the current Modified Glasgow Coma Scale.4
| Modified Glasgow Coma Scale | Score |
|---|---|
| Motor activity | |
| Normal gait, normal spinal reflexes | 6 |
| Hemiparesis, tetraparesis, or decerebrate activity | 5 |
| Recumbent, intermittent extensor rigidity | 4 |
| Recumbent, constant extensor rigidity | 3 |
| Recumbent, constant extensor rigidity with opisthotonus | 2 |
| Recumbent, hypotonia of muscles, depressed or absent spinal reflexes | 1 |
| Brainstem reflexes | |
| Normal pupillary light reflexes and vestibulo‐ocular reflex | 6 |
| Slow pupillary light reflexes and normal to reduced vestibulo‐ocular reflex | 5 |
| Bilateral unresponsive miosis with normal to reduced vestibulo‐ocular reflex | 4 |
| Pinpoint pupils with reduced to absent vestibulo‐ocular reflex | 3 |
| Unilateral, unresponsive mydriasis with reduced to absent vestibulo‐ocular reflex | 2 |
| Bilateral, unresponsive mydriasis with reduced to absent vestibulo‐ocular reflex | 1 |
| Level of consciousness | |
| Occasional periods of alertness and responsive to environment | 6 |
| Depression or delirium, capable of responding but response may be inappropriate | 5 |
| Semicomatose, responsive to visual stimuli | 4 |
| Semicomatose, responsive to auditory stimuli | 3 |
| Semicomatose, responsive only to repeated noxious stimuli | 2 |
| Comatose, unresponsive to repeated noxious stimuli | 1 |
Posttraumatic epilepsy (PTE) is among the most common form of acquired epilepsy in humans.5 The onset of seizures after TBI has been categorized into immediate (<24 hours after TBI), early (within a week after TBI), and late (>1 week after TBI) onset.5 Focal brain lesions in the frontal, temporal and parietal lobes, bilateral contusions, depressed fractures, midline shift >5 mm, subdural hematomas, decreased brain volume, intraparenchymal hemorrhage, and occurrence of seizures within the first week after TBI are important risk factors for PTE in humans.5, 6, 7, 8 Traumatic brain injury in dogs recently has been associated with significant risk of developing PTE with an incidence up to 6.8%.9, 10 Moreover, the risk of developing PTE increases with the severity of TBI.9, 10
The role of neuroimaging in human patients with TBI is still emerging.1 Currently, computed tomography (CT) is the modality of choice as a diagnostic tool for acute TBI.1 However, MRI (1.5 Tesla) has sensitivity superior to that of CT for small focal traumatic intracranial lesions in patients with TBI and also results in fewer artifacts when assessing the brainstem.11, 12, 13 Recent studies in humans have determined the value of MRI in patients with TBI.14, 15, 16, 17, 18 More severe brain contusion has been associated with poorer 3‐month outcome based on MRI in a recent prospective multicenter observational study in humans.17
To the best of the authors' knowledge, the prognostic value of early MRI after TBI in dogs has not been studied previously. The aim of this study was to determine whether MRI findings are associated with prognosis after TBI in dogs.
Material and Methods
Study Population and Clinical Evaluation
The medical records of all dogs presented to the Animal Health Trust from April 2000 to August 2012 were reviewed. Inclusion criteria were as follows: (1) TBI based on clinical history, (2) no known history of intracranial central nervous system disease unrelated to the TBI, (3) neurologic examination documented in the medical record and MGCS recorded at presentation,3 (4) minimum follow‐up of 48 hours, and (5) 1.5 T MRI performed in the first 14 days after TBI.
Data retrieved from the medical records included signalment, history, physical and neurologic examination findings at presentation, presence of seizures after TBI, treatment, and outcome. Seizures were classified as immediate when the first seizure occurred <24 hours after TBI, early when it occurred within a week after TBI and late when it occurred >1 week after TBI.
Imaging Protocol and Analysis
Magnetic resonance imaging was performed using a 1.5 Tesla scanner.1 T2‐weighted (T2W) fast spin echo (FSE) images were acquired in dorsal, sagittal, and transverse planes. Additional sequences included T2* gradient echo, T2‐fluid attenuation inversion recovery, and T1‐weighted FSE sequences before and after bolus administration of gadopentetate dimeglumine2 at a dosage of 0.1 mL/kg of body weight. Slice thickness ranged from 2 to 4 mm.
The MRI findings were graded by three of the authors (E.B., F.M., and R.D.) who were blinded to the clinical findings. Disagreement was solved by consensus. Severity and location of lesions were classified based on a modified MRI grading system,19 as follows: Grade I—normal parenchyma; Grade II—lesions only affecting the cerebral hemisphere, cerebellar parenchyma, or both without midline shift; Grade III—lesions only affecting the cerebral hemisphere, cerebellar parenchyma, or both and causing midline shift; Grade IV—lesions affecting corpus callosum, thalamus, or basal nuclei with or without any of the foregoing lesions of lesser grades; Grade V—unilateral lesions in the brainstem with or without any of the foregoing lesions of lesser grades; Grade VI—bilateral lesions affecting the brainstem with or without any of the foregoing lesions of lesser grades (Fig 1).
Figure 1.
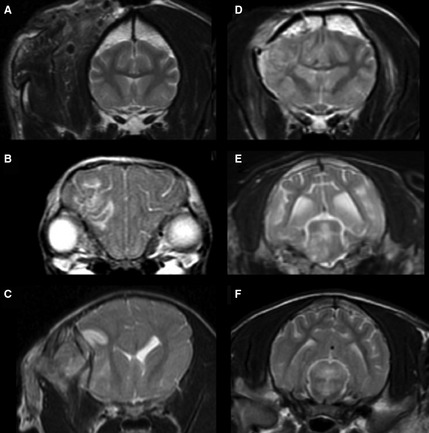
Brain magnetic resonance imaging (MRI) of dogs with traumatic brain injury. Transverse T2‐weighted images Fast Spin Echo (A–F) showing the MRI grading system. (A) Grade I—normal parenchyma. (B) Grade II—lesions only affecting the cerebral hemisphere, cerebellar parenchyma without midline shift or both. (C) Grade III—lesions only affecting the cerebral hemisphere, cerebellar parenchyma, or both and causing midline shift. (D) Grade IV—lesions affecting corpus callosum, thalamus, or basal nuclei with or without any of the foregoing lesions of lesser grades. (E) Grade V—unilateral lesions in the brainstem with or without any of the foregoing lesions of lesser grades. (F) Grade VI—bilateral lesions affecting the brainstem with or without any of the foregoing lesions of lesser grades.
The percentage of midline shift was calculated on transverse T2W‐FSE images. The midline shift was measured as the distance of maximum shift from the midline using commercial software.3 This value was multiplied by 100 and divided by the distance from the midline to the convexity surface at the same level to calculate the percentage of midline shift.
The type of brain herniation20 also was recorded as: herniation through the foramen magnum, caudal transtentorial herniation (CTH), rostral transtentorial herniation, subfalcine, and herniation through a fracture site (HFS). The extent of intraparenchymal damage (lesion[s] size) was measured using the software and recorded as 0, <25, 25–50, or >50% based on transverse, dorsal, and sagittal T2W‐FSE images. Skull fractures were classified as open or closed, and depressed or nondepressed.
Outcome Measures
Short‐term outcome was defined as survival or nonsurvival at 48 hours after TBI. Long‐term outcome was assessed, when possible, at 3, 6, 12, and 24 months after TBI. Follow‐up information at 3, 6, 12, and 24 months was obtained by telephone consultation with the owner, referring veterinarian, or both and combined with information from medical records. The outcome scale comprised 4 scores: (score 0) dead or euthanized because of the severity of the clinical presentation; (score 1) poor recovery: the dog had severe neurologic deficits or did not improve; (score 2) good recovery: the dog improved but still had visible mild neurologic deficits, required antiepileptic medication, or both; (score 3) excellent recovery: the dog was considered normal and did not require antiepileptic medication.
Statistical Analysis
Spearman correlation was used to test for correlations of MGCS at presentation and follow‐up at 3, 6, 12, and 24 months with the following risk factors: MRI grade, intraparenchymal lesion percentage, and percentage of midline shift. Mann‐Whitney U‐tests were used to compare MGCS at presentation and follow‐up scores between various binomial (yes/no) risk factor groups (presence of midline shift, brain herniation, brain herniation types, fractures, fracture types [depressed or nondepressed], open or closed fractures, surgical decompression, or seizures). Chi‐square was used to test for association of binomial risk factors with death by 48 hours or seizures. Mann‐Whitney U‐tests were used to compare risk factors (MRI grade, intraparenchymal lesion percentage, percentage of midline shift) between dogs that were alive or dead at 48 hours and between dogs with and without seizures. All tests were 2‐sided and the significance level was α = 0.05.
Results
A total of 50 dogs fulfilled the inclusion criteria. Of these, 11 (22%) were intact females, 12 (24%) were intact males, 17 (34%) were spayed females, and 10 (20%) were castrated males. The median age at presentation was 3 years (range, 2 months to 12 years). The median body weight was 8 kg (range, 0.9–45).
Breeds represented included crossbreed (n = 5), Toy poodle (4), Yorkshire Terrier (3), Dachshund (2), Jack Russell Terrier (2), Pomeranian (2), Labrador (2), Hungarian Vizsla (2), Cavalier King Charles Spaniel (2), Chihuahua (2), Lurcher (2), Staffordshire Bull Terrier (2), West Highland White Terrier (1), Rhodesian Ridgeback (1), Border Terrier (1), Dalmatian (1), Golden Retriever (1), Cocker Spaniel (1), West Highland White Terrier (1), Cairn Terrier (1), Labradoodle (1), Bearded Collie (1), Great Dane (1), Miniature Bull Terrier (1), Greyhound (1), Doberman (1), Tibetan Spaniel (1), Miniature Schnauzer (1), Beagle (1), Lhasa Apso (1), Weimaraner (1), and English Springer Spaniel (1).
The most common cause of TBI was road traffic accident (RTA) in 20 dogs (40%) followed by canine attack in 9 dogs (18%), a fall in 9 dogs (18%), being hit by an object in 8 dogs (16%), and kicked by a horse in 4 dogs (8%).
The median MGCS at presentation was 16 (range, 8–18). All dogs were hospitalized and received standard treatment for stabilization after TBI.1, 21 Two dogs were presented in status epileptics and therefore MGCS could not be assessed at presentation.
Imaging Findings
Median time interval between TBI and MRI was 1 day (range, <1 day to 14 days). Thirty‐three dogs (66%) had an abnormal MRI. The median MRI grade was III (range, I‐VI). Seventeen dogs presented with grade I, 7 dogs presented with grade II, 4 dogs presented with grade III, 11 dogs presented with grade IV, 8 dogs presented with grade V, and 3 dogs presented with grade VI MRI findings. The frequency of agreement among radiologists was 82%. Twenty‐eight dogs had <25% of intraparenchymal lesions and 5 dogs had 25–50% of intraparenchymal lesions. No dogs had >50% of intraparenchymal lesions.
A significant negative correlation was identified between MGCS at presentation and MRI grade (r = −0.63, P < .001) and for MGCS and size of intraparenchymal lesions (r = −0.58, P < .001).
The risk of developing seizures increased with severity of TBI. The presence of seizures was significantly associated with higher size (>25%) of intraparenchymal lesions (P = .0054). Twenty‐three (46%) dogs had skull fractures. Twelve of these 23 (52%) dogs developed seizures. Twelve dogs (52%) had open fractures and 11 (48%) had closed fractures. Seventeen dogs (74%) had depressed fractures.
Midline shift was present in 19 dogs (38%). The median percentage of midline shift was 8.3% (range, 2.6–22.8). Brain herniation was present in 10 dogs (20%). Eight dogs had brain HFS, 3 had FM, 3 had CTH, and 2 had SFH.
Seizure occurrence was significantly associated with HFS (P = .0122), CTH (P = .0172), and skull fractures (P = .0279).
The MGCS score at presentation was significantly lower in dogs with brain herniation than in those without herniation (P = .0191), and specifically in dogs with HFS than in those without HFS (P = .04).
Outcome
Eighteen dogs (36%) had seizures at some point after TBI (median, 0.5 days; range, 0–427). Eight dogs developed seizures immediately, 6 dogs developed early seizures, and 4 dogs developed late onset seizures. A total of 5 dogs exhibited recurrent seizures (10%) indicating development of PTE. None of these dogs had experience seizures before head trauma. The median number of days before PTE onset was 119 (range, 0–427). Fifteen dogs with seizures were started on antiepileptic medication: 11 dogs on phenobarbitone, 2 dogs on potassium bromide, 1 dog on phenobarbitone and potassium bromide, and 1 dog on levetiracetam. Antiepileptic medication was discontinued in 7 dogs within a year after TBI because of the lack of seizure activity. Three dogs were lost to follow‐up. The remaining 4 dogs were in the group with PTE. The owner of 1 dog with PTE declined to start antiepileptic medication because of the low frequency of epileptic events (3 episodes per year). Three dogs were started on prophylactic antiepileptic medication (phenobarbitone) immediately after TBI and the medication was discontinued in 2 dogs after 1 year because of lack of seizure activity. The remaining dog developed severe cluster seizures 3 months after TBI and was euthanized.
Five dogs (10%) underwent surgical decompression. The prevalence of seizures was higher in dogs that underwent surgical decompression (4/5; 80%) compared to dogs that did not undergo surgical decompression (14/45; 31.1%; P = .0307). Two of these 4 dogs had seizures after TBI and before surgical intervention. The other 2 remaining dogs with surgical decompression developed late onset seizures.
Three dogs (6%) were dead (nonsurvivors) 48 hours after TBI. Death at 48 hours was significantly associated with herniation (P < .001), HFS (P = .0135), FM (P < .001), CTH (P = .0398), SFH (P = .0075), and depressed fractures (P = .0128). The size of intraparenchymal lesions was significantly higher (>25%) in dogs that were dead at 48 hours (P = .0420) compared to those that were alive at 48 hours.
Forty‐one dogs had follow‐up at 3 months with a median outcome scale score of 2 (range, 1–3). One dog was euthanized at 4 months because of severe neurologic signs. Forty‐two dogs had follow‐up at 6 months with a median score of 2 (range, 0–3). Thirty‐eight dogs had follow‐up at 12 months with a median score of 2 (range, 2–3). Thirty‐five dogs had follow‐up at 24 months with a median score of 3 (range, 2–3).
A significant negative correlation was identified between MRI grade and scores at 3 (r = −0.44, P = .0027) and 6 months (r = −0.37, P = .0160; Fig 2). A significant negative correlation was identified between percentage of midline shift and outcome score at 6 (r = −0.53, P = .0353), 12 (r = −0.56, P = .0291), and 24 months (r = −0.81, P < .001). A significant negative correlation was identified between size of intraparenchymal lesions and follow‐up scores at 3 (r = −0.43, P = .0037), 6 (r = −0.49, P = .001), 12 (r = −0.47, P = .0027), and 24 months (r = −0.36, P = .0332; Fig 3). A positive correlation was identified between follow‐up scores at 3 (r = 0.46, P = .0018) and 6 (r = 0.32, P = .0403) months with MGCS at presentation.
Figure 2.
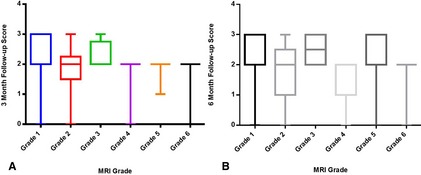
Box and whisker diagram displaying follow‐up scores at 3 months (A) and at 6 months (B) with magnetic resonance imaging grade.
Figure 3.
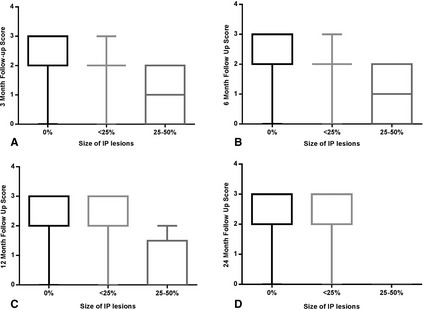
Box and whisker diagram displaying follow‐up scores at 3 months (A), 6 months (B), 12 months (C), and 24 months (D) with size of intraparenchymal lesions (IP).
Follow‐up scores at 3, 6, 12, and 24 months were significantly lower in dogs that had brain herniation or fractures (Fig 4). Follow‐up scores in dogs that had HFS were significantly lower at 6 and 12 months. Follow‐up scores in dogs that had CTH were significantly lower at 6, 12, and 24 months. Follow‐up scores in dogs that had FM herniation were significantly lower at 3 and 6 months. Follow‐up scores in dogs that had surgical decompression were significantly lower at 3, 6, and 12 months.
Figure 4.
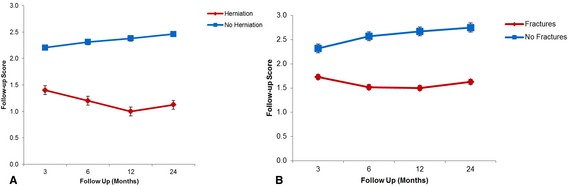
Line graph of follow‐up scores at 3, 6, 12, and 24 months in dogs with brain herniation and in dogs without brain herniation (A). Line graph of follow‐up scores at 3, 6, 12, and 24 months in dogs with skull fractures and in dogs without skull fractures (B).
Discussion
Traumatic brain injury is an important cause of morbidity and mortality in dogs.2 In agreement with previous studies in dogs, RTA was the most common cause of TBI in our study.2, 9, 10
There is growing recognition that prediction of outcome in humans after TBI based only on the Glasgow coma scale is very limited.22 Recent studies have focused on MRI findings as predictors of clinical outcome because of its high sensitivity for the detection of nonhemorrhagic contusions, brainstem lesions, and diffuse axonal injuries.17, 18, 23, 24, 25, 26 MRI analysis and grading reported in this study appear to have clinical relevance because of their significant correlation with outcome. This study used a grading system modified from human medicine, where the severity and location of the lesions were taken into account.19 Brainstem lesions have been associated with poor outcome in humans with caudal and bilateral brainstem injuries detected on MRI having the poorest prognosis.19, 23, 25 Similarly in this study, unilateral and bilateral brainstem lesions were respectively classified with the higher MRI grade (grade V and VI) and had significant negative correlation with MGCS at presentation and follow‐up scores, which is consistent with previous studies in humans.23, 25
The findings of this study also are in agreement with previous work in humans where poor outcome was significantly associated with brain herniation, skull fractures, and increased size of intraparenchymal lesions.17, 27
Recent studies support the use of advanced MRI techniques in human patients with TBI, including diffusion tensor imaging and resting state functional MRI, which may help to better define the type of injury (diffuse axonal injury) and predict outcome.28, 29, 30 Functional MRI was not performed in our study, but its use in veterinary medicine also may advance the ability to prognosticate and should be considered in the future.
One of the limitations of using MRI is that usually it is performed in referral centers and requires patients that are hemodynamically stable enough to undergo general anesthesia. This necessity could suggest that patients that undergo MRI are more likely to survive and that patients with severe TBI may not reach the referral center. A recent study reported that the risk of developing severe perianesthetic complications in dogs with intracranial lesions was nonsignificant compared with dogs without intracranial lesions and the overall severe complication rate was considered low (1.3%).31 The ultimate anesthesia management goal in dogs with intracranial lesions is to maintain cerebral perfusion by controlling factors that have an effect on cerebral blood flow. This goal can be successful with the use of multimodal techniques, which are beyond the scope of our study.31, 32 The mean time of performing MRI in this study was 1 day after TBI, but the number of dogs with severe TBI that did not reach our referral center is unknown.
Posttraumatic seizures can cause secondary brain injury, contributing to morbidity and mortality.33 Previous studies have reported the incidence of epilepsy as 0.55–2% of all the dogs referred to veterinary hospitals.34, 35, 36 Our study demonstrated that 10% of dogs with TBI develop PTE. Therefore, we can consider dogs with TBI to have a 5‐ to 18‐fold increased risk of developing epilepsy compared to the general hospital population and based on the expected incidence of epilepsy. These results are similar to a recent study in which 6.6% of dogs with TBI developed PTE and had an 11.6‐fold increased risk of developing epilepsy compared to the general hospital population.9 Another recent study reported that late onset of seizures in dogs after TBI is greater than early onset of seizures (6.8% versus 3.5%).10 This finding differs from our study in which the onset of immediate or early onset of seizures was greater than late onset of seizures (28% versus 8%). Four of the 5 dogs that developed PTE did not have any immediate or early onset of seizures. Therefore, in contrast to what is reported in the human literature, the early onset of seizures after TBI in dogs does not seem to be an important risk factor for development of PTE.5
According to the 3rd edition of the guidelines of management of TBI in humans,37 the use of antiepileptic medication is indicated to decrease the incidence of early posttraumatic seizures, but early posttraumatic seizures have not been associated with poorer outcome. In human medicine, the role of prophylactic antiepileptic medication in moderate to severe TBI is still debatable, but there are some recent studies that support their use.38, 39 Randomized controlled double‐blinded trials may be necessary to investigate whether treatment (such as prophylactic antiepileptic medication) of these cases with increased risk of developing PTE can alter outcome.
This study is retrospective with heterogeneous design, including different ages of patients, antiepileptic treatments, and multiple forms of TBI (mild to severe TBI). Therefore, these results should be validated with prospective and multicenter studies. An additional limitation of this study is that outcome assessment was not performed blindly, because some information about the patient history was known. However, the authors do not think this situation affected the overall results of this study.
In summary, this study supports the prognostic value of MRI in dogs with TBI. The MRI classification proposed here could help identify dogs at higher risk of poorer outcome, which may allow modification of the management of these patients and assist in informing owners about the prognosis for survival and the possibility of developing PTE.
Acknowledgment
This study was not financially supported by any organization or grant.
Conflict of Interest: Authors disclose no conflict of interest.
Cases were collected from the Animal Health Trust. The results of this study were presented as a clinical research abstract at the 26th annual symposium of the European College of Veterinary Neurology, 26–28 September, 2013, Paris, France.
Footnotes
1.5 Tesla MR scanner, GE Signa, GE Medical System, Milwaukee, WI
Multi‐Hance, Bracco Imaging SpA or Gadovist, Bayer Schering Pharma, Berlin, Germany
Osirix version 4.1.2 DICOM viewer, Pixmeo SARL, Bernex, Switzerland
References
- 1. Management of Concussion/mTBI Working Group . VA/DoD clinical practice guideline for management of concussion/mild traumatic brain injury. J Rehabil Res Dev 2009;46:1–68.19533516 [Google Scholar]
- 2. Simpson SA, Syring R, Otto CM. Severe blunt trauma in dogs: 235 cases (1997‐2003). J Vet Emerg Crit Care (San Antonio) 2009;19:588–602. [DOI] [PubMed] [Google Scholar]
- 3. Shores A. Craniocerebral trauma In: Kirk RW, ed. Current Veterinary Therapy X. Philadelphia, PA: WB Saunders; 1983:847–854. [Google Scholar]
- 4. Platt SR, Radaelli ST, McDonnell JJ. The prognostic value of the modified Glasgow Coma Scale in head trauma in dogs. J Vet Intern Med 2001;15:581–584. [DOI] [PubMed] [Google Scholar]
- 5. Lowenstein DH. Epilepsy after head injury: An overview. Epilepsia 2009;50(Suppl 2):4–9. [DOI] [PubMed] [Google Scholar]
- 6. Asikainen I, Kaste M, Sarna S. Early and late posttraumatic seizures in traumatic brain injury rehabilitation patients: Brain injury factors causing late seizures and influence of seizures on long‐term outcome. Epilepsia 1999;40:584–589. [DOI] [PubMed] [Google Scholar]
- 7. Frey LC. Epidemiology of posttraumatic epilepsy: A critical review. Epilepsia 2003;44(Suppl 10):11–17. [DOI] [PubMed] [Google Scholar]
- 8. Englander J, Bushnik T, Duong TT, et al. Analyzing risk factors for late posttraumatic seizures: A prospective, multicenter investigation. Arch Phys Med Rehabil 2003;84:365–373. [DOI] [PubMed] [Google Scholar]
- 9. Steinmetz S, Tipold A, Löscher W. Epilepsy after head injury in dogs: A natural model of posttraumatic epilepsy. Epilepsia 2013;54:580–588. [DOI] [PubMed] [Google Scholar]
- 10. Friedenberg SG, Butler AL, Wei L, et al. Seizures following head trauma in dogs: 259 cases (1999‐2009). J Am Vet Med Assoc 2012;241:1479–1483. [DOI] [PubMed] [Google Scholar]
- 11. Orrison WW, Gentry LR, Stimac GK, et al. Blinded comparison of cranial CT and MR in closed head injury evaluation. Am J Neuroradiol 1994;15:351–356. [PMC free article] [PubMed] [Google Scholar]
- 12. Mittl RL, Grossman RI, Hiehle JF, et al. Prevalence of MR evidence of diffuse axonal injury in patients with mild head injury and normal head CT findings. Am J Neuroradiol 1994;15:1583–1589. [PMC free article] [PubMed] [Google Scholar]
- 13. Byrne JV, Kendall BE, Kingsley DPE, Moseley IF. Lesions of the brain stem: Assessment by magnetic resonance imaging. Neuroradiology 1989;31:129–133. [DOI] [PubMed] [Google Scholar]
- 14. Bigler ED. Neuroimaging biomarkers in mild traumatic brain injury (mTBI). Neuropsychol Rev 2013;23:169–209. [DOI] [PubMed] [Google Scholar]
- 15. Lannsjö M, Raininko R, Bustamante M, et al. Brain pathology after mild traumatic brain injury: An exploratory study by repeated magnetic resonance examination. J Rehabil Med 2013;45:721–728. [DOI] [PubMed] [Google Scholar]
- 16. Lagares A, Ramos A, Pérez‐Nuñez A, et al. The role of MR imaging in assessing prognosis after severe and moderate head injury. Acta Neurochir 2009;151:341–356. [DOI] [PubMed] [Google Scholar]
- 17. Yuh EL, Mukherjee P, Lingsma HF, et al. Magnetic resonance imaging improves 3‐month outcome prediction in mild traumatic brain injury. Ann Neurol 2013;73:224–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chew BG, Spearman CM, Quigley MR, Wilberger JE. The prognostic significance of traumatic brainstem injury detected on T2‐weighted MRI. J Neurosurg 2012;117:722–728. [DOI] [PubMed] [Google Scholar]
- 19. Firsching R, Woischneck D, Klein S, et al. Classification of severe head injury based on magnetic resonance imaging. Acta Neurochir 2001;143:263–271. [DOI] [PubMed] [Google Scholar]
- 20. Kornegay JN, Oliver JE, Gorgacz EJ. Clinicopathologic features of brain herniation in animals. J Am Vet Med Assoc 1983;182:1111–1116. [PubMed] [Google Scholar]
- 21. Sande A, West C. Traumatic brain injury: A review of pathophysiology and management. J Vet Emerg Crit Care 2010;20:177–190. [DOI] [PubMed] [Google Scholar]
- 22. Zuercher M, Ummenhofer W, Baltussen A, Walder B. The use of Glasgow Coma Scale in injury assessment: A critical review. Brain Inj 2009;23:371–384. [DOI] [PubMed] [Google Scholar]
- 23. Skandsen T, Kvistad KA, Solheim O, et al. Prognostic value of magnetic resonance imaging in moderate and severe head injury: A prospective study of early MRI findings and one‐year outcome. J Neurotrauma 2011;28:691–699. [DOI] [PubMed] [Google Scholar]
- 24. Lee S‐Y, Kim SS, Kim C‐H, et al. Prediction of outcome after traumatic brain injury using clinical and neuroimaging variables. J Clin Neurol 2012;8:224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hilario A, Ramos A, Millan JM, et al. Severe traumatic head injury: Prognostic value of brain stem injuries detected at MRI. Am J Neuroradiol 2012;33:1925–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shenton ME, Hamoda HM, Schneiderman JS, et al. A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav 2012;6:137–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tanoue K, Matsui K, Nozawa K, Aida N. Predictive value of early radiological findings in inflicted traumatic brain injury. Acta Paediatr 2012;101:614–617. [DOI] [PubMed] [Google Scholar]
- 28. Bazarian JJ, Zhong J, Blyth B, et al. Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: A pilot study. J Neurotrauma 2007;24:1447–1459. [DOI] [PubMed] [Google Scholar]
- 29. Wilde EA, McCauley SR, Hunter JV, et al. Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology 2008;70:948–955. [DOI] [PubMed] [Google Scholar]
- 30. McDonald BC, Saykin AJ, McAllister TW. Functional MRI of mild traumatic brain injury (mTBI): Progress and perspectives from the first decade of studies. Brain Imaging Behav 2012;6:193–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hicks JA, Kennedy MJ, Patterson EE. Perianesthetic complications in dogs undergoing magnetic resonance imaging of the brain for suspected intracranial disease. J Am Vet Med Assoc 2013;243:1310–1315. [DOI] [PubMed] [Google Scholar]
- 32. Wendt‐Hornickle EL, Johnson RA. Anesthesia case of the month. J Am Vet Med Assoc 2011;239:194–197. [DOI] [PubMed] [Google Scholar]
- 33. Vespa PM, Miller C, McArthur D, et al., et al. Nonconvulsive electrographic seizures after traumatic brain injury result in a delayed, prolonged increase in intracranial pressure and metabolic crisis. Crit Care Med 2007;35:2830–2836. [PMC free article] [PubMed] [Google Scholar]
- 34. Löscher W, Schwartz‐Porsche D, Frey HH, Schmidt D. Evaluation of epileptic dogs as an animal model of human epilepsy. Arzneimittelforschung 1985;35:82–87. [PubMed] [Google Scholar]
- 35. Kearsley‐Fleet L, O'Neill DG, Volk HA, et al. Prevalence and risk factors for canine epilepsy of unknown origin in the UK. Vet Rec 2013;172:338. [DOI] [PubMed] [Google Scholar]
- 36. Schwartz‐Porsche D. Epidemiological, clinical and pharmacokinetic studies in spontaneously epileptic dogs and cats. ACVIM 1986;16:161–163. [Google Scholar]
- 37. Brain Trauma Foundation; American Association of Neurological Surgeons; Congress of Neurological Surgeons , eds. Guidelines for the management of severe traumatic brain injury. J Neurotrauma 2007;24:S1–S106. [DOI] [PubMed] [Google Scholar]
- 38. Klein P. Results of phase 2 safety and feasibility study of treatment with levetiracetam for prevention of posttraumatic epilepsytreatment with levetiracetam for prevention of PTE. Arch Neurol 2012;69:1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pearl PL, McCarter R, McGavin CL, et al. Results of phase II levetiracetam trial following acute head injury in children at risk for posttraumatic epilepsy. Epilepsia 2013;54:e135–e137. [DOI] [PMC free article] [PubMed] [Google Scholar]


