Abstract
Background
Iatrogenic hypothyroidism is associated with an increased incidence of azotemia after treatment of hyperthyroidism, and decreased survival time in azotemic hyperthyroid cats.
Hypothesis
Restoration of euthyroidism will decrease plasma creatinine concentrations.
Animals
Nineteen client‐owned, methimazole‐ or carbimazole‐treated, hyperthyroid cats with documented iatrogenic hypothyroidism (based on subnormal plasma total thyroxine concentrations [TT4] and increased plasma thyroid‐stimulating hormone concentrations).
Methods
Prospective interventional study. Doses of antithyroid medication were reduced until euthyroidism was restored (TT4 10–40 nmol/L). Plasma creatinine concentration and selected other clinicopathologic variables were evaluated before and after restoration of euthyroidism and compared by nonparametric statistics. Data are presented as median [25th, 75th percentile].
Results
Restoration of euthyroidism was associated with a significant decrease in plasma creatinine concentrations (2.61 [1.90, 3.26] mg/dL versus 2.07 [1.42, 2.82] mg/dL; P < .001) and body weight (4.03 [3.59, 4.53] kg versus 3.89 [3.34, 4.18] kg; P = .019), and a significant increase in packed cell volume (30 [28, 39]% versus 34 [29, 39]%; P = .038), heart rate (174 [163, 201] bpm versus 190 [164, 202] bpm; P = .009), and plasma alkaline phosphatase activity (26.6 [17.0, 33.0] IU/L versus 38.0 [23.5, 46.5] IU/L; P < .001).
Conclusions and Clinical Importance
Restoration of euthyroidism in medically treated hyperthyroid cats with iatrogenic hypothyroidism causes a reduction in plasma creatinine concentrations, and thus might improve renal function; however, this could be influenced by concurrent changes in body weight.
Keywords: Azotemia, Anemia, CKD, PCV, ALP
Abbreviations
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- BCS
body condition score
- CKD
chronic kidney disease
- GFR
glomerular filtration rate
- HR
heart rate
- PCV
packed cell volume
- SBP
systolic blood pressure
- TSH
thyroid stimulating hormone
- TT4
plasma total thyroxine concentration
Iatrogenic hypothyroidism is associated with an increased incidence of azotemia in hyperthyroid cats after treatment.1 Thyroid hormone replacement treatment is associated with an improvement in renal function in dogs with primary hypothyroidism2; however, the effect of restoration of euthyroidism on renal function in cats with iatrogenic hypothyroidism has only been investigated in 1 small previous study of cats that had been treated with radioactive iodine, which demonstrated no improvement in glomerular filtration rate (GFR).3
The aim of the present prospective study was to investigate the effect of restoration of euthyroidism on renal function, and selected other clinicopathologic variables, in a cohort of medically treated hyperthyroid cats with iatrogenic hypothyroidism.
Materials and Methods
Hyperthyroid cats receiving antithyroid medication (methimazole [n = 18] or carbimazole [n = 1]) with documented iatrogenic hypothyroidism were prospectively recruited into the study at 2 London based 1st opinion practices (Peoples' Dispensary for Sick Animals, Bow and Beaumont Sainsbury Animal Hospital, Camden) between July 2009 and October 2013. Blood samples were collected with the informed consent of the owner. Iatrogenic hypothyroidism was defined as a plasma total thyroxine (TT4) <10 nmol/L in combination with plasma thyroid stimulating hormone (TSH) >0.15 ng/mL (the upper limit of the reference interval derived from 90 healthy geriatric cats in a previous study4). The Ethics and Welfare Committee of the Royal Veterinary College approved the diagnostic protocol. Jugular venous blood samples were collected and transferred to heparinized tubes. Samples were kept at 4°C before processing which occurred within 6 hours of collection. Blood samples were placed in a centrifuge at 2016 × g for 10 minutes to enable separation of plasma from cellular components. Heparinized plasma was submitted to an external laboratory1 for routine biochemical analysis (including TT4 concentration). Plasma creatinine concentrations were measured by using the Jaffe (picric acid) method and plasma total thyroxine concentrations were determined by using the Immulite chemiluminescent assay as validated for use in cats previously.5, 6 Plasma TSH concentrations were measured at the Royal Veterinary College laboratory2 by using the Immulite canine TSH assay (Siemens, Camberley, UK) as previously validated.7 Systolic blood pressure (SBP) measurements were made with an 8.1 MHz Doppler ultrasound probe according to the protocol previously described.8
After confirmation of iatrogenic hypothyroidism, dose reductions were made in 1.25 mg average daily methimazole dose increments (usually by reducing the dose in 2.5 mg methimazole every other day increments) every 4 weeks until euthyroidism was established (plasma TT4 between 10 and 40 nmol/L). During the study period, no new medications or diets were instituted and the doses of all existing medications (except antithyroid medication) were not changed. Body weight, body condition score (BCS), SBP, packed cell volume (PCV), heart rate (HR), and plasma concentrations of urea, creatinine, cholesterol, total calcium and plasma activities of alanine aminotransferase (ALT) and alkaline phosphatase (ALP) were recorded at baseline and once euthyroidism was achieved.
Statistical Analysis
Continuous data are presented as median [25th, 75th percentile]. Clinicopathologic parameters were compared at baseline and after restoration of euthyroidism using the Wilcoxon signed rank test. Correlations were made using Spearman's correlation coefficient. Statistical significance was defined as P < .05.
Results
Nineteen cats with iatrogenic hypothyroidism were recruited into the study. Baseline plasma TT4 and TSH concentrations were <4.0 [<4.0, 4.8] nmol/L and 4.3 [0.9, 7.2] ng/mL, respectively. At baseline, 14/19 cats (74%) were azotemic (defined as plasma creatinine concentration >2 mg/dL [the upper reference limit for creatinine at the external laboratory1]). Three of these cats were azotemic before or at the time of diagnosis of hyperthyroidism. The reference limits for plasma creatinine concentration were determined by the external laboratory1 in an internal unpublished study (Federico Sacchini, personal communication). Urine specific gravity (USG) was <1.035 in 8 of the azotemic cats (consistent with renal azotemia). In the other 6 azotemic cats, concurrent USG measurement was not performed (as the cats did not have a palpable bladder). Five cats had a plasma creatinine >250 μmol/L (the lower limit of IRIS stage 3) and 14 cats had plasma creatinine concentrations between 177 and 249 μmol/L. The average daily methimazole dose given to cats at the hypothyroid visit was 5 [5, 7.5] mg.
Euthyroidism was established 32 [28, 68] days (maximum 218 days) after diagnosis of iatrogenic hypothyroidism and required a dose adjustment of 2.5 [1.25, 2.5] mg methimazole (or methimazole equivalent) daily. Eight cats required every other day dosing. The average daily methimazole dose given to cats after restoration of euthyroidism was 3.75 [2.5, 5] mg. Plasma TT4 concentration after dose adjustment was 20.8 [14.7, 26.1] nmol/L. Plasma TSH concentrations were not determined after restoration of euthyroidism. Restoration of euthyroidism resulted in a significant decrease (median 20.7%) in plasma creatinine concentration (Fig 1), and azotemia resolved in 7/14 cats (50%). After restoration of euthyroidism, 3 cats had a plasma creatinine concentration >250 μmol/L (the lower limit of IRIS stage 3), 2 of which had concentrations >250 μmol/L at the hypothyroid visit, and all other cats had plasma creatinine concentrations between 140 and 249 μmol/L (corresponding to lower and upper limits of IRIS stage 2). Restoration of euthyroidism also resulted in a significant increase in plasma ALP activity, HR, and PCV, and a significant decrease in body weight (Table 1). No significant changes in BCS, SBP, plasma concentrations of urea, cholesterol, total calcium, or plasma ALT activity were observed after restoration of euthyroidism (Table 1). Plasma creatinine concentrations were not correlated (P > .05) with body weight or TT4 at either the hypothyroid or euthyroid visits.
Figure 1.
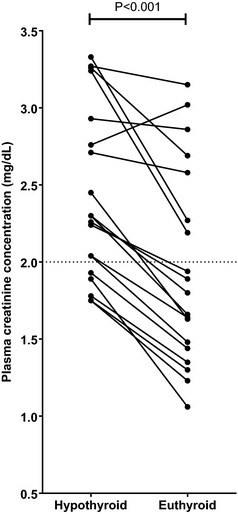
Change in plasma creatinine concentration before and after establishment of euthyroidism in 19 cats with iatrogenic hypothyroidism. Dotted line represents the upper limit of the laboratory1 reference interval for plasma creatinine concentration (2 mg/dL). Change in plasma creatinine concentration between the hypothyroid and euthyroid visits was assessed by Wilcoxon signed rank test with statistical significance defined as P < .05. Plasma creatinine concentration decreased significantly after restoration of euthyroidism (P < .001).
Table 1.
Selected clinicopathologic variables before and after establishment of euthyroidism in 19 cats with iatrogenic hypothyroidism. N/A, not applicable. Wilcoxon signed rank test was used to compare variables between the hypothyroid and euthyroid visits. Variables highlighted in bold were significantly different between the 2 visits (P < .05)
| Variable | Laboratory1 Reference Interval | Hypothyroid Visit | Euthyroid Visit | P‐Value |
|---|---|---|---|---|
| Median [25th, 75th Percentile] | Median [25th, 75th Percentile] | |||
| Plasma urea concentration (mg/dL) | 15.1–59.6 | 42.3 [37.2, 57.1] | 43.4 [31.4, 64.7] | .19 |
| Plasma creatinine concentration (mg/dL) | 0.22–2.00 | 2.61 [1.90, 3.26] | 2.07 [1.42, 2.82] | <.001 |
| Plasma alanine aminotransferase activity (IU/L) | 5.0–60.0 | 74.7 [42.7, 85.6] | 69.6 [52.1, 135.8] | .077 |
| Plasma alkaline phosphatase activity (IU/L) | <60.0 | 26.6 [17.0, 33.0] | 38.0 [23.5, 46.5] | <.001 |
| Plasma cholesterol concentration (mg/dL) | 85.1–154.7 | 189.2 [166.0, 281.9] | 200.8 [146.7, 247.1] | .47 |
| Plasma total calcium concentration (mg/dL) | 8.2–11.8 | 10.6 [10.0, 11.6] | 10.4 [9.9, 11.1] | .14 |
| Packed cell volume (%) | 26–45 | 30 [28, 39] | 34 [29, 39] | .038 |
| Body weight (kg) | N/A | 4.03 [3.59, 4.53] | 3.89 [3.34, 4.18] | .019 |
| Body condition score (/9) | N/A | 4 [3, 5] | 5 [3, 6] | .37 |
| Mean systolic blood pressure (mmHg) | N/A | 130.6 [122.0, 140.7] | 135.4 [129.8, 140.7] | .81 |
| Heart rate (bpm) | N/A | 174 [163, 201] | 190 [164, 212] | .009 |
Discussion
The aim of the present study was to assess the effect of restoring euthyroidism on renal function, using plasma creatinine concentration as a marker of GFR. Thyroid hormone replacement therapy is associated with an improvement in renal function in canine patients with primary hypothyroidism2; however, 1 small previous study reported that restoration of euthyroidism in radioiodine‐treated cats with iatrogenic hypothyroidism and azotemia did not improve GFR.3 In the present study, restoration of euthyroidism in cats with iatrogenic hypothyroidism resulted in a decrease in plasma creatinine concentrations in 18 of 19 cats, and resolution of azotemia in 50% of hypothyroid azotemic cats. However, restoration of euthyroidism also resulted in a significant decrease in body weight; therefore, if this was also associated with a reduction in body muscle mass, this could account for the reduction in plasma creatinine concentrations. Body condition score was recorded in the present study, and did not change significantly after restoration of euthyroidism; however, specific muscle condition scoring systems are currently being developed and validated, which might be a more sensitive indicator of changes in body muscle mass. These could be incorporated into future studies to assess the changes in muscle mass that occur after restoration of euthyroidism in cats with iatrogenic hypothyroidism. However, it is likely that these scoring methods will always be insensitive to small changes and hampered by interindividual variability in assessment, making it difficult to detect changes in body weight and body muscle mass accurately.
In addition, dogs with experimentally induced hypothyroidism have reduced GFR without significant increases in plasma creatinine concentration, perhaps because of a reduction in endogenous creatinine production.9 Therefore, it could be hypothesized that iatrogenic hypothyroidism in cats might also result in decreased endogenous creatinine production, which in turn might cause an overestimation of GFR (based on plasma creatinine concentrations) in these cats. Hence, the severity of renal dysfunction in iatrogenic hypothyroidism might have been underestimated in the present study. Direct measurement of GFR would be required to determine the true degree of renal dysfunction in cats with iatrogenic hypothyroidism, and to determine if renal function improved after restoration of euthyroidism; however, this was not performed in the present study.
In the present study, renal azotemia (defined as documentation of an increased plasma creatinine concentration and concurrent USG <1.035) was only confirmed in 8/14 azotemic cats; therefore, the presence of prerenal azotemia, which could have been transient, cannot be excluded in the remaining 6 azotemic cats. Nevertheless, plasma creatinine concentrations decreased after restoration of euthyroidism in all 8 cats with confirmed renal azotemia and 18/19 cats in the entire study cohort; therefore it is unlikely that the presence of transient prerenal azotemia could account for the observed changes in plasma creatinine concentrations after restoration of euthyroidism.
Previous studies have reported that up to 49% of initially nonazotemic hyperthyroid cats will develop azotemic CKD after treatment,10 which has led to the suggestion that hyperthyroid cats could be at increased risk of developing CKD. However, the results of the present study confirm that iatrogenic hypothyroidism is likely to increase plasma creatinine concentration and thus may have overexaggerated the prevalence of azotemic CKD in hyperthyroid cats in previous studies.
Restoration of euthyroidism resulted in a significant increase in PCV and HR of cats in the present study. These findings suggest that iatrogenic hypothyroidism has a negative effect on hematopoietic function, resulting in a reduction in red cell mass, and a concurrent negative effect on cardiovascular function, that together might increase morbidity (and thus perhaps mortality) in cats with iatrogenic hypothyroidism. Human patients with hypothyroidism are reported to have a macrocytic hypochromic anemia, which is associated with a reduced proliferative potential of hematopoietic stem cells.11 It is possible that a similar pathophysiologic mechanism could account for the reduced PCV that was observed in hypothyroid cats in the present study; however, further studies would be required to confirm this.
Hypothyroid azotemic cats have reduced survival times compared to hypothyroid nonazotemic cats,1 therefore it could be postulated that dose adjustments of antithyroid medication to restore euthyroidism will also improve the survival times of hypothyroid azotemic cats. Thyroid hormone replacement treatment in human patients with subclinical hypothyroidism attenuates the decline in renal function of patients with CKD,12 and could therefore also attenuate the decline in renal function in cats with iatrogenic hypothyroidism and CKD, thus perhaps increasing the survival time of these cats. However, it has also been suggested that mild hypothyroidism might be a beneficial adaptation in human patients with CKD, as hypothyroidism will lead to reduced protein nitrogen turnover and protein catabolism and hence reduced nitrogenous waste load for the kidney to excrete.13 Interestingly, plasma urea concentration did not decrease after restoration of euthyroidism in the present study, despite a concurrent significant reduction in plasma creatinine concentrations. This could suggest that iatrogenic hypothyroidism results in a decreased protein nitrogen turnover in cats, which could be beneficial in cats with CKD, although this beneficial effect is likely to be minimal. Alternatively, the lack of reduction in plasma urea concentrations despite a reduction in plasma creatinine concentrations could suggest that the reduction in plasma creatinine concentrations was secondary to changes in body weight and body muscle mass rather than changes in GFR. To improve the survival times of cats with CKD, the effect of decreasing nitrogenous waste load in the hypothyroid state would have to be more beneficial than the increase in GFR that would be associated with restoration of the euthyroid state. Prospective randomized, controlled, interventional studies would be required to evaluate the long‐term survival of azotemic cats with iatrogenic hypothyroidism after restoration of euthyroidism; however, they were not performed in the present study as it was not felt to be ethically justifiable to not adjust the dose of antithyroid medication given to some client‐owned azotemic cats with iatrogenic hypothyroidism in light of the current available data.1 Nevertheless, these studies would be required to definitively prove that restoration of euthyroidism results in improved survival times in azotemic cats with iatrogenic hypothyroidism.
Corrections made after online publication April 28, 2014: citations in the final paragraph of the Discussion section have been updated.
Acknowledgment
Conflict of Interest: Authors disclose no conflict of interest.
This study was presented in abstract form at 2012 ACVIM Forum, New Orleans, LA
Footnotes
IDEXX Laboratories, Wetherby, UK
Royal Veterinary College Diagnostic Laboratories, North Mymms, Hatfield, UK
References
- 1. Williams TL, Elliott J, Syme HM. Association of iatrogenic hypothyroidism with azotemia and reduced survival time in cats treated for hyperthyroidism. J Vet Intern Med 2010;24:1086–1092. [DOI] [PubMed] [Google Scholar]
- 2. Gommeren K, van Hoek I, Lefebvre HP, et al. Effect of thyroxine supplementation on glomerular filtration rate in hypothyroid dogs. J Vet Intern Med 2009;23:844–849. [DOI] [PubMed] [Google Scholar]
- 3. van Hoek IM, Vandermeulen E, Peremans K, Daminet S. Thyroid stimulation with recombinant human thyrotropin in healthy cats, cats with non‐thyroidal illness and in cats with low serum thyroxin and azotaemia after treatment of hyperthyroidism. J Feline Med Surg 2010;12:117–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wakeling J, Elliott J, Syme H. Evaluation of predictors for the diagnosis of hyperthyroidism in cats. J Vet Intern Med 2011;25:1057–1065. [DOI] [PubMed] [Google Scholar]
- 5. Singh AK, Jiang Y, White T, Spassova D. Validation of nonradioactive chemiluminescent immunoassay methods for the analysis of thyroxine and cortisol in blood samples obtained from dogs, cats, and horses. J Vet Diagn Invest 1997;9:261–268. [DOI] [PubMed] [Google Scholar]
- 6. Jacobs RM, Lumsden JH, Taylor JA, Grift E. Effects of interferents on the kinetic Jaffé reaction and an enzymatic colorimetric test for serum creatinine concentration determination in cats, cows, dogs and horses. Can J Vet Res 1991;55:150–154. [PMC free article] [PubMed] [Google Scholar]
- 7. Wakeling J, Moore K, Elliott J, Syme H. Diagnosis of hyperthyroidism in cats with mild chronic kidney disease. J Small Anim Pract 2008;49:287–294. [DOI] [PubMed] [Google Scholar]
- 8. Syme HM, Barber PJ, Markwell PJ, Elliott J. Prevalence of systolic hypertension in cats with chronic renal failure at initial evaluation. J Am Vet Med Assoc 2002;220:1799–1804. [DOI] [PubMed] [Google Scholar]
- 9. Panciera DL, Lefebvre HP. Effect of experimental hypothyroidism on glomerular filtration rate and plasma creatinine concentration in dogs. J Vet Intern Med 2009;23:1045–1050. [DOI] [PubMed] [Google Scholar]
- 10. Boag AK, Neiger R, Slater L, et al. Changes in the glomerular filtration rate of 27 cats with hyperthyroidism after treatment with radioactive iodine. Vet Rec 2007;161:711–715. [DOI] [PubMed] [Google Scholar]
- 11. Kawa MP, Grymula K, Paczkowska E, et al. Clinical relevance of thyroid dysfunction in human haematopoiesis: Biochemical and molecular studies. Eur J Endocrinol 2010;162:295–305. [DOI] [PubMed] [Google Scholar]
- 12. Shin DH, Lee MJ, Kim SJ, et al. Preservation of renal function by thyroid hormone replacement therapy in chronic kidney disease patients with subclinical hypothyroidism. J Clin Endocrinol Metab 2012;97:2732–2740. [DOI] [PubMed] [Google Scholar]
- 13. Basu G, Mohapatra A. Interactions between thyroid disorders and kidney disease. Indian J Endocrinol Metab 2012;16:204–213. [DOI] [PMC free article] [PubMed] [Google Scholar]


