Abstract
Background
Boxer arrhythmogenic right ventricular cardiomyopathy (ARVC) is a disease that may result in sudden death or heart failure.
Hypothesis/objectives
To prospectively study the natural history of Boxer ARVC.
Animals
72 dogs (49 ARVC, 23 controls).
Methods
Boxers >1 year of age were recruited for annual reevaluation. Controls were defined as being ≥6 years of age and having <50 ventricular premature complex (VPCs)/24 h. ARVC was defined as ≥300 VPCs/24 h in the absence of other disease. Dogs were genotyped for the striatin deletion when possible. Descriptive statistics were determined for age; VPC number; annual change in VPC number; and left ventricular (LV) echocardiographic dimensions. Survival time was calculated.
Results
Controls: median age of 7 years (range, 6–10); number of VPCs 12 (range, 4–32). Median time in study of 6 years (range, 2–9). Seventeen of 23 were genotyped (5 positive, 12 negative).
ARVC: median age of diagnosis of 6 (range, 1–11). Median time in study 5 years (range, 3–8). A total of 33% were syncopal and 43/49 were genotyped (36 positive, 7 negative). Yearly change in VPCs was 46 (range, −7,699 to 33,524). Annual percentage change in LV dimensions was 0, and change in fractional shortening (FS%) was 2%. Two dogs had FS% <20%. Although ARVC dogs died suddenly, there was no difference in survival time between groups. ARVC median age of survival was 11 years, and for controls was 10 years.
Conclusions/Clinical Importance
Arrhythmogenic right ventricular cardiomyopathy is a disease of middle age and frequently is associated with the striatin deletion. Syncope occurs in approximately 1/3 of affected dogs; systolic dysfunction is uncommon. The prognosis in many affected dogs is good.
Keywords: Boxer, Holter, Striatin, Sudden death, Ventricular premature complex
Abbreviations
- AECG
ambulatory electrocardiography
- AFLP
amplified fragment length polymorphism
- ARVC
arrhythmogenic right ventricular cardiomyopathy
- FS
fractional shortening
- LVIDD
left ventricular internal diastolic dimension
- LVIDS
left ventricular internal systolic dimension
- PCR
polymerase chain reaction
- VPC
ventricular premature complex
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is a familial myocardial disease observed in the adult Boxer dog.1, 2 It is characterized by the presence of ventricular tachyarrhythmias, syncope and, in a small percentage of dogs, myocardial dysfunction. It has been shown to be a familial trait that is associated with a deletion in the striatin gene, in at least some cases.3 Although affected dogs may experience sudden cardiac death or progress to congestive heart failure, many dogs appear to live for years with stable disease. The disease has been well characterized from familial, clinical, and pathological perspectives.1, 2, 3, 4, 5, 6 However, the natural history of the disease is less well described.
The objective of this study was to prospectively characterize the natural history of ARVC in the adult Boxer dog. Boxer dogs >1 year of age were recruited for participation in a study requiring annual reevaluation. Affected status for ARVC was defined by the detection of at least 300 ventricular premature complexes (VPCs) per day in the absence of other systemic or cardiovascular disease.
Materials and Methods
This study was conducted in accordance with the “Position of the American Heart Association on Research Animal Use” and under the guidelines of the Animal Care and Use Committee of The Ohio State University.
Boxer dogs >1 year of age were prospectively recruited for participation in a study to evaluate the natural history of Boxer ARVC. Evaluation included obtaining a clinical history, which sought a history of syncope, signs consistent with congestive heart failure or both, and other systemic illness. A physical examination, echocardiogram, and 24‐hour ambulatory electrocardiogram (AECG) using a 3‐channel transthoracic system were performed. Owners were asked to return with their dogs on an annual basis for a follow‐up evaluation to include physical examination, echocardiogram, and AECG until the time of death. Date and cause of death were recorded when possible.
M‐mode echocardiographic measurements were recorded from 2‐dimensional guided right parasternal short axis views obtained with either a 3.5 or 5‐MHz transducer with the dog positioned in right lateral recumbency. Measurements of the left ventricular internal dimensions and fractional shortening (FS%) were made in accordance with the American Society of Echocardiography standards by board‐ certified veterinary cardiologists or their residents under supervision of a veterinary cardiologist.7 Dogs were classified as having myocardial dysfunction or ventricular dilatation, if measurements exceeded chamber size or function as defined for normal adult Boxers as a left ventricular FS% <21%, left ventricular internal diastolic dimension (LVIDD) >4.8 cm and a left ventricular systolic internal dimension (LVIDS) >3.3 cm. These values were chosen because they either exceed (LVIDD and LVIDS) or fail to meet (FS%) the reference range as defined for normal adult Boxer dogs.8
Dogs with echocardiographic abnormalities suggestive of congenital heart disease were excluded from the study. In addition, dogs with indicatons of possible systemic disease were more thoroughly evaluated based on clinical suspicion (physical examination or historical findings). Degree of evaluation was dependent on the clinician's opinion and may have included a CBC and serum biochemistry, thoracic radiography, and abdominal ultrasonography. Dogs with evidence of serious systemic disease were excluded from the study. Serum thyroid hormone measurements were not required for inclusion in this investigation.
The AECG was placed, and the dog was sent home in order to allow monitoring of the dog's cardiac electrical activity in its normal environment. The owners were encouraged to maintain the dog's normal activity level and the monitor was removed after 25 hours. The analysis was performed by a technician in a prospective, user‐interactive method under the guidance of a veterinary cardiologist.1 Recordings with <20 hours of readable data were excluded. The total number of VPCs/24 h was tabulated and the ventricular arrhythmia complexity was graded numerically from 0 to 4 as follows: 0 = no VPCs, 1 = single VPCs, 2 = bigeminy, trigeminy, 3 = couplets, triplets, 4 = R on T phenomenon or ventricular tachycardia. The highest grade observed was the grade assigned.
Dogs were considered affected with ARVC if they had ≥300 VPCs/24 h on at least 1 Holter monitoring without an obvious cause for the arrhythmia and had been evaluated at least 2 years sequentially. The number of VPCs used for diagnosis was based on Animal Registry of Certified Health (ARCH) (http://www.archcertify.org/) guidelines presented at the American College of Veterinary Internal Medicine Forum in 2009 (unpublished data). Briefly, the guidelines are as follows: the presence of 0–50 VPCs/24 h, predominantly single, monomorphic is interpreted as normal; the presence of 50–300 VPCs/24 h suggests that Boxer ARVC cannot be conclusively diagnosed or ruled out at this time and the presence of runs of ventricular tachycardia or >300 VPCs/24 h suggests Boxer ARVC. Dogs were considered unaffected by ARVC (controls) if they were ≥6 years of age, had been followed up for at least 2 consecutive years and never had >50 VPCs/24 h on any Holter monitoring.
Dogs were placed on antiarrhythmic medication at the discretion of the evaluating cardiologist. The decision to start an antiarrhythmic drug typically was made based on the presence of syncope and the number of VPCs or complexity (grade) of the arrhythmia. In some cases, owners elected not to treat the dogs. There was no attempt to randomize or control treatment.
A cohort of 16 ARVC Boxers that had never received antiarrhythmic medications was evaluated as part of the combined group as well as on an individual basis to evaluate annual variation in VPC number that occurred without the effect of antiarrhythmic medication.
Genotyping for the Boxer ARVC striatin deletion was performed with DNA samples obtained from blood samples collected in EDTA tubes. DNA was extracted from the sample and evaluated by amplified fragment length polymorphism (AFLP)‐polymerase chain reaction (PCR) for the striatin deletion.3 Standard PCR amplifications were carried out using a cocktail of NH4SO4 amplification buffer, Taq DNA polymerase (0.1 U/μL of reaction volume), 2.5 mM MgCl2, 12.5 μM of each dNTP and 2.5 mM of each primer (the 5′ end of the forward primer labeled fluorescently with 56‐FAM). Amplicons then were evaluated by AFLP on a DNA analyzer.2 Alleles were scored using peak scanner software2 according to fragment size (352 bp for wildtype, 343 bp and 352 bp for positive heterozygote fragments and 343 bp for positive homozygous deletion fragment).
Descriptive statistics were determined for age of the control group, maximum number of VPCs and maximum grade of the arrhythmia for the control group for all Holter monitoring performed on an individual dog, age at detection of ≥300 VPCs in each ARVC dog, number of VPCs detected the first time ≥300 VPCs were observed, annual change in VPC number for each Holter monitoring in ARVC dogs, number of VPCs in years before, and after the year in which ≥300 VPCs were detected for dogs not on antiarrhythmic medication and percentage annual change in FS%, LVIDD and LVIDS for the duration of participation in the study.
Survival was calculated for both the control and ARVC dogs with Kaplan–Meier curves. Survival also was calculated for the control dogs and compared to that of ARVC dogs that had at ≥1,000 VPCs at some point during the study. Because of the small group size and large number of dogs censored, survival was not calculated separately for the cohort of 16 dogs that had received not on an antiarrhythmic medication. Dogs were censored if they were still alive at final contact, died of noncardiac cause, or were euthanized for noncardiac reasons. To determine if there was bias in censoring of the 2 groups, a Fisher's test was done to compare censoring between the 2 groups. An alpha of P = .05 was considered to be significant.
Results
A total of 551 dogs were evaluated over of the span of this 8‐year study, although the number of annual follow‐up examinations varied with each subject.
Twenty‐three dogs (4 male [1 intact, 3 castrated] and 19 female ([8 intact, 11 spayed]) made up the unaffected control group. Median time for participation in the study was 6 years (range, of 2–9). Median age for dogs classified as unaffected was 7 years (range, 6–10). The median maximum number of VPCs for all controls for the duration of their participation in the study was 12 (range, 4–32). The median grade of the arrhythmia was 3 (range, 1–4). Ventricular size and function were within normal limits as described for adult Boxers.8 Seventeen of the 23 control Boxers were genotyped, 5 (29%) were heterozygous for the striatin mutation and 12 (71%) were negative.
Forty‐nine (18 male [8 intact, 10 castrated] and 31 female [10 intact, 21 spayed]) dogs had ≥300 VPCs. Sixteen (33%) of the 49 Boxers were syncopal at some point during the study.
Median time for participation in the study was 5 years (range, 3–8) and dogs were followed up for a median of 3 years (range, 0–7) after the detection of ≥300 VPCS. Median age for detection of ≥300 VPCs was 6 years (range, 1–11). Twenty‐eight of the dogs were followed up for ≥1 year (range, 0–7 years) before they developed ≥300 VPCs. Median age for detection of ≥300 VPCs in this group also was 6 (range, 2–11). Median grade at first detection of ≥300 was 3 (range,1–4). Median number of VPCs at first detection of ≥300 VPCs was 1,823 (range, 340–23,943) with 46 (94%) dogs eventually having ≥1000 VPCs/24 h.
Forty‐three of 49 ARVC Boxers were genotyped. Thirty‐six were positive for the striatin deletion. Twenty‐six (61%) were heterozygous for the deletion, 10 (23%) were homozygous for the deletion, and 7 (16%) were negative.
Thirty‐three (67%) ARVC Boxers were on some type of antiarrhythmic medication (eg, atenolol, metoprolol, sotalol, procainamide, mexiletine, or a combination of ≥2 of these drugs) at some time during the study and were excluded from additional arrhythmia evaluation.
Sixteen Boxers with ARVC never received antiarrhythmic treatment during the course of the study and were used for additional evaluation of annual change in VPC number. The maximum number of VPCs the year ≥300 VPCs were detected ranged from 333 to 14,848/24 h (median 1,190). The number of VPCs in the year preceding detection of ≥300 ranged from 0 to 201 (median 45). The number of VPCs detected 1 year after ranged from 1 to 29,240 (median 256) (Fig 1). The annual variation in VPC number for these dogs for all years evaluated ranged from a decrease of 7,699 VPCs/24 h to an increase in 33,524 VPCs/24 h (median annual change 46 VPCs/24 h). Eight dogs of the 16 had <25 VPCs (0–23) on an annual Holter monitoring at some point in the years after initial detection of ≥300 VPCs, despite receiving no antiarrhythmic treatment (Fig 2).
Figure 1.
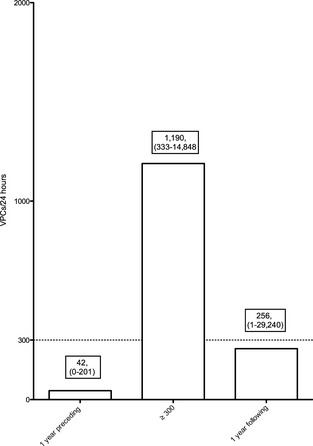
Median number of ventricular premature complex (VPCs) in the year before and after the year in which arrhythmogenic right ventricular cardiomyopathy (ARVC) dogs had ≥300 VPCs/24 h as well as the year in which ≥300 VPCs were first detected. Median and ranges are presented in the boxes above each bar.
Figure 2.
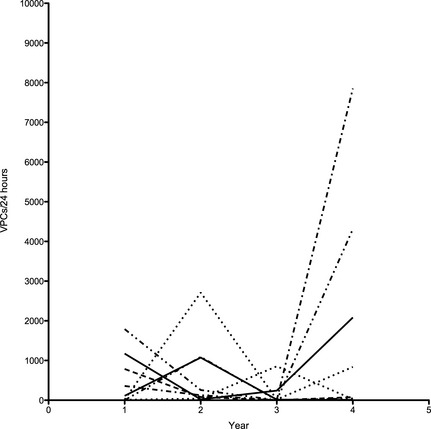
Change in number of ventricular premature complex (VPCs)/24 h over time. Each line represents 1 of 8 dogs that had an annual VPC number of <25 VPCs after the initial detection of ≥300 VPCs, despite receiving no antiarrhythmic treatment. Large year‐to‐year variability is noted.
Forty‐seven of the 49 dogs had normal myocardial function for the duration of the study. Median annual percentage change was 0 for both LVIDD (−33 to 47.9% change) and LVIDS (−57 to 62% change). Median annual percentage change in FS was 2% (−57 to 62%). Two dogs had myocardial dysfunction as defined by decreased FS (11 and 17%, respectively) without left ventricular dilatation. Both dogs had been evaluated for at least 3 years before the decrease in myocardial function and had been observed to have gradual cardiac enlargement and a gradual decrease in systolic function. Both of these dogs died suddenly. One of these dogs was heterozygous for the striatin mutation and the other was not genotyped. Four dogs developed both diastolic and systolic dilatation (2 were not genotyped, 1 was negative, and 1 was homozygous for the deletion mutation) but maintained an FS ≥25% for the duration of the study.
There was no difference in survival between the control and ARVC groups. The control group had a median survival of 10 years of age and the ARVC group had a median survival of 11 years of age (Fig 3). Sixty‐four percent of the ARVC dogs were still alive at the age of 9 years regardless of whether ≥300 or ≥1,000 VPCs was used as criteria for being affected. The median survival time after detection of ≥300 VPCs was 1528 days, and median survival after detection of at least 1,000 VPCs was 1,754 days. Fewer dogs were censored in the control group (4/23) than in the ARVC group (15/49) but this difference was not statistically significant (P = .77).
Figure 3.
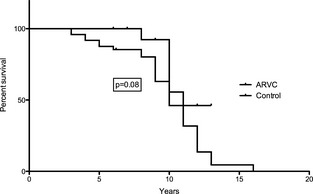
Survival for control and arrhythmogenic right ventricular cardiomyopathy (ARVC) dogs. Dogs were censored if they were still alive at final contact, died of noncardiac cause, or were euthanized for noncardiac reasons. There was no difference in survival between the control and ARVC groups. The control group had a median survival of 10 years and the ARVC group had a median survival of 11 years.
Fifteen ARVC dogs died suddenly, including the 2 dogs with decreased systolic function. One additional dog with progressive ventricular dilatation and an FS of 23% was euthanized for congestive heart failure. Five dogs were euthanized for neoplastic causes and 5 dogs were euthanized for degenerative myelopathy or other neurologic diseases. One dog each was euthanized for refractory diabetes mellitus, liver failure, renal failure, and 2 for other unknown causes. None of the control dogs died of a cardiac cause but 4 were euthanized because of degenerative myelopathy or neoplasia.
Discussion
The objective of this study was to prospectively evaluate the natural history of Boxer dog ARVC. Although Boxer ARVC is a familial disease and many dogs have a causative genetic mutation that is present since birth, it appears that most dogs do not develop the phenotype until middle age. The evaluation of 28 of the dogs in this study for ≥1 year before they met the affected criteria illustrated that the detection of ≥300 VPCs generally was not apparent before 6 years of age. This observation is consistent with the age‐related penetrance of ARVC observed in human beings, where it been suggested that age‐related penetrance may be because of progressive exposure of the abnormal myocardium to mechanical stress.9, 10 It also has been determined in both human beings and animals that endurance training may exacerbate disease, and it has been suggested that the disease may progress more slowly in individuals leading a sedentary life, with myocardial injury accumulating as part of the aging process.10, 11, 12
Our data also indicate that the development of arrhythmias in Boxer ARVC may be fairly abrupt. Affected dogs progressed from having a median of 41 VPCs/24 h, which generally would be interpreted as normal in this breed, to a median of 1,823 VPCs within a year. Once the arrhythmia was detected, a considerable yearly variation was observed. Several dogs returned to what is considered normal at some point during the study. These findings are consistent with a previous study that identified >80% day‐to‐day variability in VPC number in Boxers that had ≥500 VPCs.13 These findings emphasize the difficulty in diagnosing Boxer ARVC on a single Holter monitoring and suggest that a single Holter monitoring may not be enough to conclusively rule out the presence of disease for the purposes of breed screening or diagnosis of a dog with suspicious clinical signs. The development of more comprehensive diagnostic criteria for this disease is warranted.
Both human beings and dogs with ARVC can develop decreased systolic function and congestive heart failure, but this was an uncommon finding in the dogs studied here.6, 9, 10, 14 In fact, the median percentage annual change in LVIDD and LVIDS was zero. However, 2 dogs (4%) did develop progressive systolic dysfunction. Both of these dogs died suddenly, which is consistent with studies in both human beings and dogs with ARVC that found that echocardiographically apparent left ventricular involvement is associated with an adverse outcome.6, 15, 16
Although dogs with Boxer ARVC do die suddenly and do develop congestive heart failure, the majority of dogs in this study were still alive at 9 years of age and did not have shorter survival than the control dogs.1, 2, 4, 6 The median survival after detection of ≥300 or ≥1,000 VPCs was 1,528 and 1,754 days, respectively. This is quite a bit longer than what was previously described in a population of affected Boxers from Spain, where the median survival time was 1 year (range, 7–1,961 days).4 In that study, a retrospective evaluation was performed by evaluating cases referred to 3 hospitals in Spain, where a diagnosis of ARVC was based on the presence of ≥1,000 VPCs/24 h. Dogs were brought to the hospital for medical attention by their owners and therefore may already have developed clinical signs or showed some indication of having more advanced disease. In comparison, the dogs in this study were recruited because of signalment (Boxer dog >1 year of age) and over half of them were evaluated at least 1 year before they met the diagnostic criteria of ARVC. Therefore, the dogs in this study may have been diagnosed at the earliest onset of disease and may have had relatively longer survival. In addition, the dogs in the study from Spain may have had a different etiology for ARVC than did the dogs in this US study, which may be more likely to have a poor prognosis. Finally, some of the dogs in this study were receiving antiarrhythmic medication and this may or may not have impacted prognosis. Because the study was not designed to prospectively evaluate the impact of treatment and a variety of different drugs and dosages were used, it is difficult to determine the impact of antiarrhythmic treatment on survival. Additional studies in this area are warranted.
Forty‐three of the 49 ARVC dogs in this study were genotyped for the striatin deletion. Thirty‐six (84%) of the dogs were positive for the mutation, but 7 (16%) dogs did not carry the mutation indicting at least 1 other cardiac or systemic cause for the VPCs in these dogs. In addition, 5 of the control dogs were heterozygous for the striatin deletion, but did not demonstrate the ARVC phenotype. This finding is consistent with the variable penetrance of the disease observed in both humans and dogs.3, 9 In some human kindreds with ARVC mutations, the penetrance is as low as 30% (ie, only 30% of humans with the known causative mutation show the disease).17 We have shown previously that the penetrance of the striatin mutation in Boxer dogs with ARVC is approximately 80%, so it is not surprising that 5 dogs in the control group were positive for the mutation but did not show the phenotype.3 The mechanism associated with incomplete penetrance in ARVC and other forms of cardiomyopathy are poorly understood, but likely involve a variety of factors including both genetic modifiers and environmental influences.17
This study has several limitations. Although >500 dogs were enrolled in the study, many dogs did not fit the criteria for inclusion in either the affected or unaffected groups and could not be included in the final analysis. In many cases, the number of VPCs on the Holter monitoring did not place the dog clearly in either the affected or unaffected group, or they appeared to be unaffected but died from noncardiac causes (eg, trauma, lymphoma) before they reached 6 years of age (the cut‐off age for considering a dog unaffected). Some dogs were lost to follow‐up for various reasons (eg, owner moved, dog was lost, dog was sold) and did not return for annual evaluation. These factors markedly limited the number of dogs that had more than 1 annual evaluation, and ultimately the numbers of dogs in the affected and control groups were quite small. Evaluation of a larger number of dogs in each group may have impacted the findings.
Females were overrepresented in both the affected and unaffected groups. The reason for this finding is unknown. Boxers were widely recruited from referring veterinarians in the region as well as from local breed clubs. Some of the participating dogs came from breeding kennels where the number of female dogs tends to exceed the number males.
The criterion used for inclusion in this study was the presence of ≥300 VPCs/24 h. This number was based on guidelines developed by a committee of board certified veterinary cardiologists for the Animal Health Registry of Certified Health (ARCH) (http://www.archcertify.org/) program and was presented at the ACVIM Forum in 2009. However, the diagnosis of ARVC in human beings typically is based on the presence of a combination of major or major and minor findings from several different diagnostic categories including global or regional myocardial dysfunction and structural abnormalities, tissue characterization of the ventricular wall, repolarization abnormalities, depolarization or conduction abnormalities, arrhythmias, and family history.18 Such diagnostic criteria have not yet been established for veterinary medicine, and access to some forms of diagnostic testing may be limited and impractical. Many veterinary cardiologists rely on the combination of signalment (adult Boxer) and detection of an increased number of VPCs in the absence of other underlying causes for the arrhythmia. Although for the purposes of this study, we used detection of ≥300 VPCs as a criterion, we reassessed the survival data using a cut‐off of ≥1,000 VPCs (as used in a previous study) and 46/49 dogs still were considered affected.4 It has been suggested that a number much lower than 300 VPCs/24 h may indicate disease.15 Holter monitoring in normal adult large breed dogs identified no more than a median of 2 VPCs/24 h.19 In addition, a study in normal asymptomatic Boxers identified a median of 97 VPCs.5, 19 Although it therefore is possible that a lower number of VPCs actually may indicate disease, we believe that using a cut‐off of ≥300 VPCs/24 h was likely to identify dogs that were truly abnormal. The inclusion criteria did not weigh the impact of grade of arrhythmia, although it was recorded. The median grade of arrhythmia for both the control and ARVC dogs was 3 (range, 1–4). The substantial overlap between the 2 groups with regard to grade of arrhythmia could suggest that some of the control dogs actually were affected. The presence of a small amount of a high‐grade ventricular arrhythmia in apparently normal dogs is not uncommon. Two previous Holter studies of apparently healthy (nonboxer) dogs have identified some high grade (4) ventricular arrhythmias in normal dogs.19, 20 The identification of higher grade arrhythmias in apparently normal dogs is a poorly understood finding that deserves further study. Importantly, both the number of VPCs and the grade of arrhythmia should be taken into consideration when evaluating an individual dog for disease status.
Finally, there was no attempt to control antiarrhythmic treatment in the dogs in this study. Use of different antiarrhythmic drugs and different dosages of the antiarrhythmic drugs could have impacted survival results and deserves further study.
Despite the limitations of this study, these results confirm that Boxer ARVC is a disease that typically develops in the middle‐aged Boxer dog and is frequently associated with the striatin gene deletion. The previously reported incomplete penetrance and age‐related penetrance of the striatin mutation were further supported by this data, similar to what is observed in mutations in human beings affected with ARVC. Clinical signs including syncope are common in approximately 1/3 of the dogs and development of systolic myocardial dysfunction is uncommon. In fact, most dogs do not have any changes in cardiac size or function for the duration of their disease. Finally, although cause of death is more frequently cardiac nature, the prognosis of Boxer ARVC in many dogs actually is quite good, with no apparent difference in overall survival from a control population.
Acknowledgment
Funding: American Kennel Club – Canine Health Foundation and American Boxer Charitable Foundation.
Conflict of Interest Declaration: Dr Meurs has the potential to receive royalties from the striatin mutation test. None are currently received.
Footnotes
Delmar Accuplus, Irvine, CA
Applied Biosystems, Foster City, CA
References
- 1. Harpster N. Boxer cardiomyopathy. Vet Clin North Am Small Anim Pract 1991;21:989–1004. [DOI] [PubMed] [Google Scholar]
- 2. Meurs KM. Cardiomyopathy in the Boxer dog In: Kirk RW, Bonagura JD. eds. Current Veterinary Therapy XV: Small Animal Practice. Philadelphia, PA: WB Saunders Elsevier; 2013:801–804. [Google Scholar]
- 3. Meurs KM, Mauceli E, Lahmers S, et al. Genome‐wide association identifies a mutation in the 3′ untranslated region of striatin, a desmosomal gene, in a canine model of arrhythmogenic right ventricular cardiomyopathy. Hum Genet 2010;128:315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Caro‐Vadillo A, Garcia‐Guasch L, Carreton E, et al. Arrhythmogenic right ventricular cardiomyopathy in Boxer dogs: a retrospective study of survival. Vet Rec 2013;172:268. Epub 20. [DOI] [PubMed] [Google Scholar]
- 5. Stern JA, Meurs KM, Spier AW, et al. Ambulatory electrocardiographic evaluation of a population of adult asymptomatic Boxer dogs. J Am Vet Med Assoc 2010;236:430–433. [DOI] [PubMed] [Google Scholar]
- 6. Palermo V, Stafford Johnson MJ, Sala E, et al. Cardiomyopathy in Boxer dogs: A retrospective study of the clinical presentation, diagnostic findings and survival. J Vet Cardiol 2011;13:45–55. [DOI] [PubMed] [Google Scholar]
- 7. Sahn DJ, DaMaria A, Kisslo J, et al. Recommendations regarding quantitation in M‐Mode echocardiography: Results of a survey of echocardiographic measurements. Circulation 1978;58:1072–1083. [DOI] [PubMed] [Google Scholar]
- 8. Cunningham SM, Rush JE, Freeman LM, et al. Echocardiographic ratio indices in overtly healthy Boxer dogs screened for heart disease. J Vet Intern Med 2008;22:924–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sen Chowdry S, Morgan RD, Chambers JC, et al. Arrhythmogenic cardiomyopathy: Etiology, diagnosis and treatment. Annu Rev Med 2010;61:233–253. [DOI] [PubMed] [Google Scholar]
- 10. Sen Chowdry S, Syrris P, McKenna WJ. Role of genetic analysis in the management of patients with arrhythmogenci right ventricular dysplasia/cardiomyopathy. J Am Coll Cardiol 2007;50:1813–1821. [DOI] [PubMed] [Google Scholar]
- 11. Sen Chowdry S, Syrris P, Ward D, et al. Clinical and genetic characterization of families with arrhythmogenic right ventricular dysplasia/cardiomyopathy provides novel insights into patterns of disease expression. Circulation 2007;115:1710–1720. [DOI] [PubMed] [Google Scholar]
- 12. Kirchhof P, Fabritz L, Zwiener M. Age‐ and training dependent development of arrhythmogenic right ventricular cardiomyopathy in heterozygous plakoglobin‐deficient mice. Circulation 2006;114:1799–1806. [DOI] [PubMed] [Google Scholar]
- 13. Spier AW, Meurs KM. Spontaneous variability in the frequency of ventricular arrhythmias in Boxers with arrhythmogenic cardiomyopathy. J Am Vet Med Assoc 2004;224:538–541. [DOI] [PubMed] [Google Scholar]
- 14. Meurs KM, Stern JA, Sisson D, et al. Association of dilated cardiomyopathy with the striatin mutation genotype in Boxer dogs. J Vet Intern Med 2013;27:1437–1440. [DOI] [PubMed] [Google Scholar]
- 15. Motskula PF, Linney C, Palermo V, et al. Prognostic value of 24‐hour ambulatory ECG (Holter) monitoring in Boxer dogs. J Vet Intern Med 2013;27:904–912. [DOI] [PubMed] [Google Scholar]
- 16. Lemola K, Brunckhorst C, Helfenstein U, et al. Predictors of adverse outcome in patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy: Long term experience of a tertiary care centre. Heart 2005;91:1167–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sen Chowdhry S, Syrris P, McKenna WJ. Genetics of right ventricular cardiomyopathy. J Cardiovasc Electrophysiol 2005;16:927–935. [DOI] [PubMed] [Google Scholar]
- 18. Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: Proposed modification of the Task Force Criteria. Eur Heart J 2010;31:806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Meurs KM, Spier AW, Wright NA, et al. Use of the ambulatory electrocardiogram for detection of ventricular premature complexes in healthy dogs. J Am Vet Med Assoc 2001;218:1291–1295. [DOI] [PubMed] [Google Scholar]
- 20. Rasmussen CE, Vesterholm S, Ludvigsen TP, et al. Holter monitoring in clinically healthy Cavalier King Charles Spaniels, Wire‐haired Dachshunds, and Cairn Terriers. J Vet Intern Med 2011;25:460–468. [DOI] [PubMed] [Google Scholar]


