Abstract
Background
Quantitative and semiquantitative methods have been proposed for the assessment of MR severity, and though all are associated with limitations. Measurement of vena contracta width (VCW) has been used in clinical practice.
Objective
To measure the VCW in dogs with different levels of MR severity.
Animals
Two hundred and seventy‐nine dogs were classified according to 5 levels of MR severity.
Methods
This was a retrospective study. EROA and regurgitant volume calculated by the PISA method, were measured and indexed to BSA. Descriptive statistics were calculated for VCW and VCW index for all categories of MR severity. Spearman's rank correlation coefficients (ρs) were calculated to compare the results of the different methods (VCW and VCW index vs RV PISA, RV PISA index, EROA, EROA index), and between VCW and VCW index versus MR severity.
Results
All Spearman's rank correlation coefficients were significant (P < .001). The median values of VCW resulted of 2.9 mm (IQR 3.4–2.5) and of 4.6 mm (IQR 5.4–4.1) in the groups previously classified as mild‐to‐moderate and moderate‐to‐severe, respectively. The median values of VCW index resulted of 4.4 mm/m2 (IQR = 5.5–4.2) in mild‐to‐moderate MR and of 10.8 mm/m2 (IQR = 12.8–9.4) in moderate‐to‐severe MR.
Conclusion and Clinical Importance
This is not a validation study against any previously validated invasive gold standard, the VCW method has proved easy to employ and it might be an additional tool in quantifying disease severity that supports, rather than replace, data coming from other techniques in daily clinical practice and research.
Keywords: Congestive heart failure, Degenerative mitral valve disease, Vena contracta width
Abbreviations
- 2‐D
2‐dimensional
- ARJ/LAA
ratio area of the regurgitant jet area signal to left atrium area
- BSA
body surface area
- CFD
color flow Doppler
- CV
coefficient of variation
- EROA index
effective regurgitant orifice area index
- EROA
effective regurgitant orifice area
- IQR
interquartile range
- Km
constant (10.1 for dog)
- LA
left atrium
- L
mild
- LM
mild‐to‐moderate
- M
moderate
- MR
mitral regurgitation
- MS
moderate‐to‐severe
- MVD
mitral valve disease
- PISA index
proximal isovelocity surface area index
- PISA
proximal isovelocity surface area
- RV
regurgitant volume
- S
severe
- VCW index
vena contracta width index
- VCW
vena contracta width
- V
mitral valve regurgitation jet velocity
- VTI
mitral valve velocity time‐integral
- ρs
Spearman's correlation coefficient
The diagnosis of mitral regurgitation (MR) caused by degenerative mitral valve disease (MVD) is frequently straightforward, because the clinical and echocardiographic findings are often obvious and in agreement. However, there is currently no “gold standard” for reliably determining MR severity.1 However, quantification of MR severity is essential for clinical decision making, and many studies have indicated the importance of assessing MR severity for monitoring the progression of MVD, evaluating the effect of treatments, and predicting patient outcomes.2, 3, 4, 5, 6
Various semiquantitative methods by using color flow Doppler (CFD) mode (eg, area of regurgitant jet area signal to left atrium area ratio (ARJ/LAA), and quantitative methods (eg, proximal isovelocity surface area method (PISA), also called flow convergence) have been proposed for detecting MR severity in humans and dogs.1, 2, 3, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17 However, all these methods have some limitations, and none of them are simple enough to be universally recognized and safely applied without restrictions.
To overcome some of the limitations of these techniques, semiquantitative vena contracta width (VCW) analysis was introduced into clinical practice. VCW, as determined by transthoracic CFD mapping, has been introduced into human medicine as a simple echocardiographic marker of MR severity. VCW can predict the angiographic severity of MR and correlates well with catheterization‐derived regurgitant volume (RV) and transesophageal CFD mapping.1, 5, 6, 9, 10, 18
Hemodynamically, VCW represents a contraction in the edges of the flow streamlines as they move through an orifice.18, 19, 20 The method directly measures the diameter of the vena contracta, defined as the narrowest cross‐sectional area of the jet, just downstream from the regurgitant orifice. The VCW is the area of the jet as it leaves the regurgitant orifice; it thus reflects the regurgitant orifice area and has been shown to be related directly to the severity of the regurgitant lesion, and with both RV and the effective regurgitant orifice area (EROA).1, 6, 7, 13, 21
Using a canine animal model, Zhou et al. compared measurements of VCW with indicators of eccentric MR severity, as determined by electromagnetic flow meters, and obtained good correlations between VCW and RV, as well as regurgitant fraction (RF), the percentage of stroke volume ejected into the left atrium (LA) during systole.22
The VCW is generally easy to measure in clinical settings, though an extra zoom lens may be required to assess the size of the regurgitant jet with greater accuracy.22, 23 It should be noted that any errors in measurement will result in either over‐ or underestimation of MR severity, because of the small diameter of the jet in the regurgitant orifice.23
Various echocardiographic views can be used to measure VCW in humans, but measurements are usually made from the parasternal long axis and from the apical 4‐chamber view.6, 7, 13, 18
The VCW method is widely used in human medicine but is not applied in veterinary medicine, though to the best of our knowledge a range of measures of VCW is not available in large canine populations.2, 8, 10, 11, 22, 24, 25
The goal of this study was to measure the VCW in a population of dogs with MVD and different levels of MR severity graded by other noninvasive quantitative and semiquantitative techniques.
Materials and Methods
In this retrospective study, medical records for 896 privately owned dogs that underwent cardiologic examination from January 2007 to February 2011 were reviewed. The records were selected according to the following inclusion criteria: diagnosis of MVD; complete medical records referring to the first visit; thoracic radiography in presence of clinical signs2, 11; electrocardiographic examination2, 11; complete data for echocardiographic and Doppler examinations as recommended by the authors to achieve grading of MR severity (ARJ/LAA ratio), mitral inflow pattern analyzed by pulsed wave (PW), signal intensity (jet density) of the continuous wave (CW), and envelope of the MR jet assessment of MR severity achieved by quantitative PISA and semiquantitative methods.1, 8, 11, 21, 22, 24, 26 We first graded the disease by means of quantitative methods and semiquantitative methods (different from VCW) and then observed how VCW values were distributed among different grade of MR severity (average based on ≥ 3 measurements for each parameter).1, 2, 3, 10, 11, 12, 13 Exclusion criteria included presence of congenital heart disease; presence of atrial fibrillation, any other arrhythmias, or both that severely altered the beat to beat time interval11; VCW, PISA, or both not clearly visualized11; presence on CFD imaging of more than 1 mitral regurgitant jet evidenced through accurate recording at the time of the examination.8
Hemodynamic Measurements
EROA and RV were calculated by using the PISA method (EROA (cm2) = flow rate (mL/s)/V (cm/s); RV (mL) = EROA (cm2) × VTI cm) 2, 11 (Fig 1). To take account of animal size, the parameters were indexed according to body surface area (BSA m2 = K m × BWgr 0.67/10−4) to give EROA index (measured as cm2/m2) and RV index (measured as mL/m2).3, 27, 28
Figure 1.
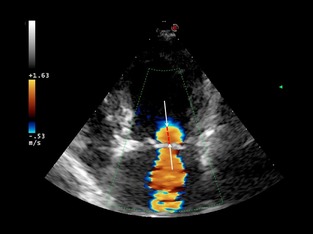
Apical 4‐chamber view. Color flow Doppler image showing the semicircle of flow of convergence on the left ventricular side of the mitral valve. The radius of the proximal isovelocity region (dotted line) should be measured from the ventricular side of the mitral valve leaflets to the edge of the hemisphere (arrows).
Vena contracta width was determined in each subject from the left parasternal apical 4‐ and 5‐chamber views. The narrowest sector angle that allowed visualization of the MR jet was used to maximize the color flow imaging frame rate.18 The transducer was angled to optimize visualization of the area of proximal flow acceleration, the VCW and the downstream expansion of the jet. For each echocardiographic window, zoom mode was used to optimize the visualization of VCW (mm), and an average value was calculated based on its largest diameter during systole measured for at least 3 cardiac cycles.14, 22, 23, 29, 30 To take account of the effect of body weight on the intrinsic dimensions of the valvular apparatus, VCW was indexed for BSA to give the VCW index (mm/m2).27
All the echocardiographic examinations were performed on awake dogs by the same experienced investigator (MDM), by using standardized thoracic imaging planes, in accordance with the recommendations of the American Society of Echocardiography, and by using two‐dimensional (2‐D), M‐mode, and CFD and spectral Doppler.14 The gain settings for CFD and spectral Doppler were adjusted as required to optimize the signal, and the settings were not changed when recording the images and measuring VCW.
All dogs were examined using a vet echocardiograph with 2.5–3.5 MHz, 3.5–5 MHz, and 7.5–10 MHz mechanical phased array transducers.1 MR severity was graduated on the basis of the area of the regurgitant jet projecting into the left atrium, the jet density of the CW Doppler signal, the mitral inflow pattern analyzed by PW Doppler, and EROA dimensions.1, 7, 8, 10, 11, 12, 21, 22, 24, 31 The grade of MR was classified as mild (L), mild‐to‐moderate (LM), moderate (M), moderate‐to‐severe (MS), or severe (S).6, 10, 14, 15, 16, 21, 32
Within‐day and between‐day intraobserver variability in the studied parameters were determined by reanalysing the parameters measured by the same observer (MDM) 3 times after the first measurement on a subset of 10 randomly selected exams from the database.
Statistical Analysis
Statistical analysis was performed by using SPSS (v. 17). The normal distributions of the measurements of interest (RV PISA, RV PISA index, EROA, EROA index, VCW, VCW index) were tested using the Shapiro‐Wilk test. Means, standard deviations, medians, and Interquartile Ranges were calculated for VCW and VCW index for all categories of MR severity (L, LM, M, MS, S). If the distribution was not normal, the significance of the differences among categories were calculated using the Kruskal‐Wallis test (P < 0.001).33
Spearman's rank correlation coefficients (ρs) were calculated to compare the results of the different methods (VCW and VCW index vs RV PISA, RV PISA index, EROA, EROA index). The relationships between VCW and VCW index, and MR severity were also evaluated.
Results
A total of 279 dogs were selected. The mean age was 11.4 ± 2.9 years and the mean weight was 11.7 ± 10 kg. Most of the dogs were mongrels (44%) and among the breeds, the most common were Poodles (13%), Yorkshire Terriers (8%), and Shih‐tzus (5%).
The study population was classified into 5 groups on the basis of MR severity.24, 26, 29, 34, 35, 36 Most dogs had severe MR (97 dogs), followed by moderate (78 dogs), and moderate‐to‐severe (47 dogs), mild (30 dogs) and mild‐to‐moderate (27 dogs) MR (Figs 2, 3, 4).
Figure 2.
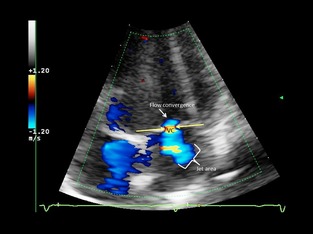
Apical 4‐chamber view. Semiquantitative assessment of mitral regurgitation severity using vena contracta width (VC) in a dog with mild mitral regurgitation. Color Doppler at the level of the mitral valve during systole. The components of the regurgitant jet (flow convergence zone, vena contracta, jet turbulence) were obtained.
Figure 3.
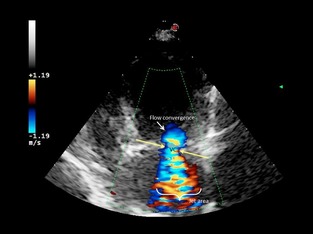
Apical 4‐chamber view. Semiquantitative assessment of mitral regurgitation severity using vena contracta width (VC) in a dog with moderate mitral regurgitation. Color Doppler at the level of the mitral valve during systole. The components of the regurgitant jet (flow convergence zone, vena contracta, jet turbulence) were obtained.
Figure 4.
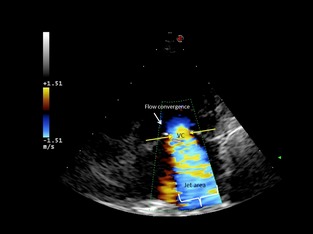
Apical 4‐chamber view. Semiquantitative assessment of mitral regurgitation severity using vena contracta width (VC) in a dog with severe mitral regurgitation. Color Doppler at the level of the mitral valve during systole. The components of the regurgitant jet (flow convergence zone, vena contracta, jet turbulence) were obtained.
The intraobserver coefficients of variation (CV range in %) were <10% for each tested variable.
There were significant correlations between VCW and VCW index and other quantitative and semiquantitative parameters (Table 1). The highest correlation coefficients occurred among dimensionally similar parameters (indexed values with indexed values, and nonindexed ones with nonindexed ones). Semiquantitative MR severity correlated better with VCW (ρs = 0.76) than with VCW index (Table 1). All Spearman's rank correlation coefficients were statistically significant (P < 0.001).
Table 1.
Spearman's correlation coefficients (ρs) measure the association between 2 scale of variables (P < .000001)
| RV PISA (mL) | RV PISA Index (mL/m2) | EROA (cm2) | EROA Indexed (cm2/m2) | Severity of MR | |
|---|---|---|---|---|---|
| VCW (mm) | 0.82 | 0.65 | 0.82 | 0.66 | 0.76 |
| VCW indexed (mm/m2) | 0.59 | 0.84 | 0.66 | 0.86 | 0.73 |
VCW, vena contracta width; VCWIX, vena contracta width indexed; RV, regurgitant volume; PISA, proximal isovelocity surface area; PISA, proximal isovelocity surface area index; EROA, effective regurgitant orifice area; EROA index, effective regurgitant orifice area index.
Regression analysis demonstrated positive relationships for RV PISA, RV PISA index, EROA, EROA index to VCW and VCW index. The best relationships were between VCW IX and RV PISA IX and EROA IX (ρs = 0.836 and 0.857, respectively) (Figs 5, 6, 7, 8).
Figure 5.
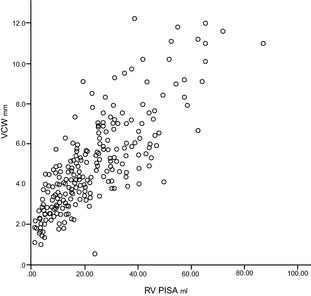
Scatter plot showing positive, direct, and significant relationship between vena contracta width (mm) and regurgitant volume (mL) by the proximal isovelocity surface area method (ρs = 0.82; P < .001).
Figure 6.
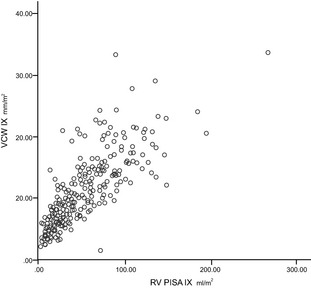
Scatter plot showing positive, direct, and significant relationship between vena contracta width indexed (mm/m2) and regurgitant volume indexed (mL/m2) by proximal isovelocity surface area method (ρs = 0.84; P < .01).
Figure 7.
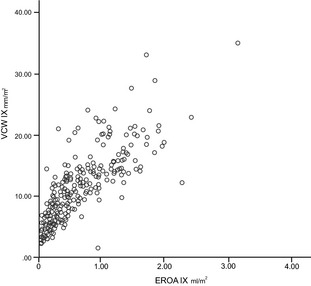
Scatter plot showing positive, direct, and significant relationship between vena contracta width indexed (mm/m2) and EROA index (mL/m2) (ρs = 0.86; P < .01).
Figure 8.
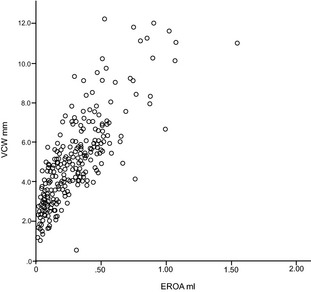
Scatter plot showing positive, direct, and significant relationship between vena contracta width (mm) and EROA (mL) (ρs = 0.825; P < 0.01).
The indexed and unindexed VCW were calculated with respect to the semiquantitative method in subjects with different classes of MR severity (Figs 9, 10).
Figure 9.
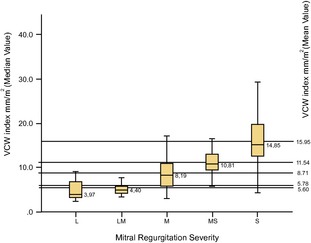
Box and whisker plot showing median, mean, and interquartile ranges of VCW index (mm/m2) in the 5 different classes of mitral regurgitation severity. The ends of the box are the 25th and 75th quartiles. The line across the box identifies the median sample value. VCWIX, vena contracta width indexed; L, mild (30 dogs); LM, mild‐to‐moderate (27 dogs); M, moderate (78 dogs); MS, moderate‐to‐severe (40 dogs); S, severe (97 dogs).
Figure 10.
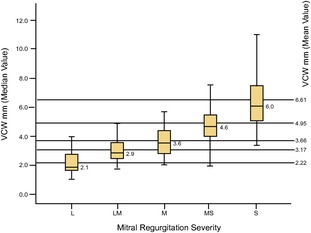
Box and whisker plot showing mean, median, and interquartile ranges of vena contracta width (mm) in the 5 different classes of mitral regurgitation severity. The ends of the box are the 25th and 75th quartiles. The line across the box identifies the median sample value. VCW, vena contracta width; L, mild (30 dogs); LM, mild‐to‐moderate (27 dogs); M, moderate (78 dogs); MS, moderate‐to‐severe (40 dogs); S, severe (97 dogs).
A proportional increase in VCW and VCW index was observed with worsening mitral insufficiency. Even in the event of an overlap among adjacent categories, there was no overlap between the medians and IQR values for mild‐to‐moderate and moderate‐to‐severe MR in terms of VCW and VCW index, and the differences among the classes of MR severity resulted statistically significant (P < 0.001).
The median values of VCW, irrespective of body size, resulted of 2.9 mm (IQR 3.4–2.5) and of 4.6 mm (IQR 5.42–4.08) in the groups previously classified as mild‐to‐moderate and moderate‐to‐severe, respectively (Table 2, Fig 10).21, 31, 32
Table 2.
Mean, standard deviation, median, and interquartile range of vena contracta width and vena contracta width indexed in the 5 different classes of mitral regurgitation severity
| Mitral Regurgitation Severity | VCW | IQR | VCW IX | IQR | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | ±SD | Median | 75°–25° VCW | Mean | ±SD | Median | 75°–25° | |
| L | 2.22 | 0.85 | 2.1 | 2.65–1.63 | 5.78 | 4.31 | 3.97 | 6.59–3.33 |
| LM | 3.17 | 1.01 | 2.9 | 3.4–2.5 | 5.6 | 2.28 | 4.40 | 5.55–4.16 |
| M | 3.66 | 1.34 | 3.6 | 4.3–2.90 | 8.71 | 3.64 | 8.19 | 10.79–6.09 |
| MS | 4.95 | 1.76 | 4.6 | 5.42–4.08 | 11.54 | 4.16 | 10.81 | 12.81–9.45 |
| S | 6.61 | 2.1 | 6 | 7.5–5.1 | 15.95 | 5.24 | 14.85 | 19.66–12.7 |
VCW, vena contracta width; VCWIX, vena contracta width indexed; IQR, interquartile range; SD, standard deviation.
The median values of VCW index resulted of 4.4 mm/m2 (IQR 5.5–4.1) in dogs with mild‐to‐ moderate mitral insufficiency and of 10.8 mm/m2 (IQR 12.8–9.4) in dogs with moderate‐to‐severe MR (Table 2; Fig 9).
Discussion
The combination of 2‐D and Doppler echocardiography represents an important method for the noninvasive detection and evaluation of the severity of MR.2, 22 Calculation of the ARJ/LAA ratio using CFD mapping is commonly used as a noninvasive method for assessing the severity of MR in veterinary medicine.2, 12 The major advantages of this method are its rapidity and the ease of data acquisition.1 However, jet area evaluation is limited by a variety of hemodynamic (eg, systemic hypertension, ventricular‐atrial pressure gradient reduction, loading conditions, left atrial compliance) and technical factors (eg, gain setting, angle between the jet and the ultrasound beam, orientation of the jet).1, 11, 13, 22 Doppler color flow mapping may also markedly underestimate the severity of MR in eccentric jets: a flow jet directed against the atrial wall appears smaller than a central jet of the same regurgitant volume (Coanda effect), which could lead to an underestimation of the severity of MR.1, 6, 11, 12, 13, 18, 22, 31, 37
Other proposed semiquantitative methods in human and veterinary medicine include the CW Doppler evaluation of jet characters and intensity, the PW Doppler mitral inflow pattern, and the estimation of variables related to the compensatory changes in the heart secondary to MR such as pulmonary artery pressure by tricuspid regurgitation velocity, pulmonary venous inflow pattern, and left atrial size.2, 15, 22 However, these techniques have several limitations: the interpretation of CW Doppler patterns of MR can be highly operator dependent, thus blurring the distinction between moderate and severe disease, and eccentric jets tend to underestimate whereas central jets overestimate the severity of MR.32
Quantitative Doppler echocardiographic assessment of MR severity is based on the evaluation of RV and RF realized by the volumetric and PISA methods, as the product of EROA and RV integral.3, 6, 7, 8, 10, 11, 14, 37, 38, 39 Several veterinary studies have quantified MR severity using PISA and compared the results with those obtained by using the volumetric method, as well as highlighting their relevance for follow‐up evaluations.2, 10, 11, 25 The volumetric method has limitations: it is time‐consuming, is unable to distinguish between the severity of MR and that of aortic regurgitation in patients with both lesions, and it is not effective in the presence of mitral stenosis, aortic stenosis, and intraventricular septal defects.1, 9, 10, 19
The PISA method is currently the most common method for quantifying MR severity in dogs.2, 7, 9, 10, 11 However, this method also has limitations: it is time‐consuming, requires multiple measurements, and the evaluation of the variables is subjected to both method‐ and operator‐dependent errors (intra‐ and interobserver variability are high), and it requires the skill of a well‐trained operators.2, 3, 9, 10, 12, 36 The PISA method is more accurate for regurgitations with circular rather than noncircular orifices, and it may be difficult to judge the precise location of the orifice and the flow convergence shape.1, 2, 6, 7, 8 Lastly, PISA is more accurate for central than for eccentric jets, and the presence of multiple regurgitant jets, which can occur in dogs with severe MVD, precludes the use of the PISA method.2, 7, 8
Measurement of VCW is less technically demanding than the previously mentioned methods and is relatively independent of instrument settings.7, 18, 35 Determination of VCW can be easily performed by a trained operator, and is feasible in a high percentage of subjects (>98%) in our experience, with the exception of poorly compliant patients or under poor imaging conditions. Unlike PISA, VCW measurements have been validated in human beings for assessing eccentric jets, which are a common characteristic of mitral valve prolapse or flail leaflets in dogs.18 However, opinions on how the position of the jets influences the VCW evaluation vary: some authors state that VCW has been validated for assessing both central and eccentric jets and its value is proportional to the effective orifice area.13, 40 The correlation between VCW and EROA, both indexed or not, was significant in our study (ρs = 0.825 and ρs = 0.857). Others, however, have suggested that VCW may be overestimated in the case of eccentric jets, and in cases with a nonconcentric regurgitant area, the VC diameter obtained in M‐mode and 2‐D echocardiographic views may over‐ or underestimate the regurgitant area.40, 41
The VCW is considered to be relatively unaffected by flow rate and driving pressure within the clinically encountered flow range.22 This technique may have a limited ability to obtain reliable measures with multiple regurgitant jets; in the case of multiple MR jets, the fact that the respective widths of the VC are not additive must be taken into account, and in these cases the use VCW method is not recommended.21, 31, 42
Our results showed a high correlation between VCW and the semiquantitative and quantitative evaluating methods of mitral insufficiency as seen in human patients, and the values for mild and severe regurgitant jets are also similar.1, 5, 20 The absolute values of VCW we found in dog are consistent with the results from human studies, where a VCW <3–4 mm is associated with mild mitral insufficiency, whereas values >4–6 mm are associated with severe mitral insufficiency.21, 28, 31 In dogs, as in children, VCW indexed to account for BSA appears to be also an useful measure of MR severity, as shown by its high correlation with semiquantitative and quantitative stadiation.43, 44
However, the analysis of regurgitant area based on VCW has some limitations relating to the difficulty in identifying the plane perpendicular to the MR flow, the narrowest neck of the jet in any imaging plane available from the transthoracic windows may cut obliquely across the flow stream and include portions of the expanding jet, thus overestimating the VCW.20 Three‐dimensional color Doppler echocardiography has recently been suggested for the direct measurement of VCW and may overcome some of the limitations of the 2‐D echocardiographic techniques.21, 40, 41
Even if direct relationships exist between VCW index and RV index and PISA index, the scatter is so wide that VCW determination alone seems unsuitable for assessing the severity of mitral insufficiency. Its results should be confirmed by other methods,5, 8 including qualitative (ie, ARJ/LAA ratio, CW signal density of MR jet), semiquantitative (ie, VCW, mitral inflow, pulmonary vein flow), and quantitative parameters (eg, PISA method, EROA, RV) for grading MR severity.8, 21 In dogs, as in humans, it should be possible to develop a score of MR severity suitable for use in daily clinical practice and research.1, 16, 19, 21 The evaluation of MR severity should therefore be recommended in dogs with MVD to identify and track alterations over time and to detect worsening of the disease.8
This was a retrospective study, and although the inclusion and exclusion criteria were very strict, some errors may have occurred. The reliabilities of the analyses of VCW and VCW index were not tested when multiple regurgitant jets were present, which could represent a limitation of this study. It may be advisable to avoid using the technique under these circumstances until further studies have been performed. Many of the dogs in this study had received medical treatment for heart failure (eg, furosemide, spironolactone, angiotensin converting enzyme inhibitors), which might have interfered with the studied variables. Prospective and invasive studies are needed to evaluate and compare the sensitivity, specificity, and predictive values of the VCW method with other methods, and examine differences in the regurgitant volume before and after therapies.
Conclusions
Characterization of the severity of regurgitant lesions is among the most difficult problems in valvular heart disease. The search for a suitable quantitative technique for evaluating MR severity has led to the development of many tools, however no “gold standard” has yet been established.5 Cardiac computed tomographic angiography slightly overestimates mild MR and slightly underestimates severe MR.45 At the moment, a “stand‐alone” technique for accurate quantification of mitral insufficiency is not available neither in human nor in veterinary medicine. The intention of the authors was to introduce the technique as an additional tool in quantifying disease severity that supports, rather than replace, data coming from other techniques.
Even if the present study is not a VCW validation study, as the technique was not assessed against any previously validated invasive gold standard in dog, and although the VCW method alone is not adequate and has some limitations, it appears to provide a valuable tool for the rapid identification of belonging to a group of moderate‐to‐severe mitral insufficiency (VCW = 4.6 mm; IQR = 5.4–4.1 and VCW IX = 10.8; IQR = 12.8–9.4) and of mild‐to‐moderate insufficiency (VCW = 2.9 mm; IQR = 3.4–2.5 and VCW IX = 4.4; IQR = 5.5–4.2), while intermediate VCW values need further confirmation.
Vena contracta width analysis should thus be considered as a helpful tool for the evaluation of MR severity in light of its ease of use which makes it valuable for determining MR severity when other semiquantitative techniques cannot be applied.23
Acknowledgments
We thank Marco Maria Colombo for his technical artwork in the preparation of the digital format of figures and graphs.
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Footnote
Pie Medical 300S Pandion: Esaote, Florence, Italy
References
- 1. Grayburn PA, Bhella P. Grading severity of mitral regurgitation by echocardiography: Science or art? JACC Cardiovasc Imaging 2010;3:244–246. [DOI] [PubMed] [Google Scholar]
- 2. Gouni V, Serres FJ, Pouchelon JL, et al. Quantification of mitral valve regurgitation in dogs with degenerative mitral valve disease by use of the proximal isovelocity surface area method. J Am Vet Med Assoc 2007;231:399–406. [DOI] [PubMed] [Google Scholar]
- 3. Jacob R, Stewart WJ. A practical approach to the quantification of valvular regurgitation. Curr Cardiol Rep 2007;9:105–111. [DOI] [PubMed] [Google Scholar]
- 4. Kahlert P, Plicht B, Schenk IM, et al. Direct assessment of size and shape of noncircular vena contracta area in functional versus organic mitral regurgitation using real‐time three‐dimensional echocardiography. J Am Soc Echocardiogr 2008;21:912–921. [DOI] [PubMed] [Google Scholar]
- 5. Khanna D, Miller AP, Nanda NC, et al. Transthoracic and transesophageal echocardiographic assessment of mitral regurgitation severity: Usefulness of qualitative and semiquantitative techniques. Echocardiography 2005;22:648–669. [DOI] [PubMed] [Google Scholar]
- 6. Irvine T, Li XK, Sahn DJ, Kenny A. Assessment of mitral regurgitation. Heart 2002;88(Suppl IV):iv11–iv19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Biner S, Rafique A, Rafii F, et al. Reproducibility of proximal isovelocity surface area, vena contracta, and regurgitant jet area for assessment of mitral regurgitation severity. JACC Cardiovasc Imaging 2010;3:235–243. [DOI] [PubMed] [Google Scholar]
- 8. Chetboul V, Tissier R. Echocardiographic assessment of canine degenerative mitral valve disease. J Vet Cardiol 2012;14:127–148. [DOI] [PubMed] [Google Scholar]
- 9. Fernandes FP, Manlhiot C, McCrindle BW, et al. Usefulness of mitral regurgitation as a marker of increased risk for death or cardiac transplantation in idiopathic dilated cardiomyopathy in children. Am J Cardiol 2011;107:1517–1521. [DOI] [PubMed] [Google Scholar]
- 10. Hojung C, Kichang L, Heechun L, et al. Quantification of mitral regurgitation using proximal isovelocity surface area method in dogs. J Vet Sci 2004;5:163–171. [PubMed] [Google Scholar]
- 11. Kittleson MD, Brown WA. Regurgitant fraction measured by using the proximal isovelocity surface area method in dogs with chronic myxomatous mitral valve disease. J Vet Intern Med 2003;17:84–88. [DOI] [PubMed] [Google Scholar]
- 12. Muzzi RAL, de Araújo RB, AL Muzzi L, et al. Regurgitant jet area by Doppler color flow mapping: Quantitative assessment of mitral regurgitation severity in dogs. J Vet Cardiol 2003;5:33–38. [DOI] [PubMed] [Google Scholar]
- 13. Roberts BJ, Paul A, Grayburn MD. Color flow imaging of the vena contracta mitral regurgitation: Technical considerations. J Am Soc Echocardiogr 2003;16:1002–1006. [DOI] [PubMed] [Google Scholar]
- 14. Thomas WP, Gaber CE, Jacobs GJ, et al. Recommendations for standards in transthoracic two‐dimensional echocardiography in the dog and cat. J Vet Intern Med 1993;7:247–252. [DOI] [PubMed] [Google Scholar]
- 15. Thomas L, Foster E, Schiller NB. Peak mitral inflow velocity predicts mitral regurgitation severity. J Am Coll Cardiol 1998;31:174–179. [DOI] [PubMed] [Google Scholar]
- 16. Thomas L, Foster E, Hoffman JIE, Schiller NB. The mitral regurgitation index: An echocardiographic guide to severity. J Am Coll Cardiol 1999;33:2016–2022. [DOI] [PubMed] [Google Scholar]
- 17. Dujardin KS, Enriquez‐Sarano M, Bailey KR, et al. Grading of mitral regurgitation by quantitative doppler echocardiography calibration by left ventricular angiography in routine clinical practice. Circulation 1997;96:3409–3415. [DOI] [PubMed] [Google Scholar]
- 18. Hall SA, Brickner ME, DuWayne LW, et al. Assessment of mitral regurgitation severity by Doppler color flow mapping of the vena contracta. Circulation 1997;95:636–642. [DOI] [PubMed] [Google Scholar]
- 19. Thomas L, Foster E, Hoffman JIE, Schiller NB. Prospective validation of an echocardiographic index for determining the severity of chronic mitral regurgitation. Am J Cardiol 2002;90:607–612. [DOI] [PubMed] [Google Scholar]
- 20. Fehske W, Omran H, Manz M, et al. Color‐coded Doppler imaging of the vena contracta as a basis for quantification of pure mitral regurgitation. Am J Cardiol 1994;73:268–274. [DOI] [PubMed] [Google Scholar]
- 21. Lancellotti P, Moura L, Pierard LA, et al. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: Mitral and tricuspid regurgitation (native valve disease). Eur J Echocardiogr 2010;11:307–332. [DOI] [PubMed] [Google Scholar]
- 22. Zhou X, Jones M, Shiota T, et al. Vena contracta imaged by Doppler color flow mapping predicts the severity of eccentric mitral regurgitation better than color jet area. A chronic animal study. J Am Cardiol 1997;30:1393–1398. [DOI] [PubMed] [Google Scholar]
- 23. Lesniak‐Sobelga A, Olszowska M, Pienazek P, et al. Vena contracta width as a simple method of assessing mitral valve regurgitation. Comparison with Doppler quantitative methods. J Heart Valve Dis 2004;13:609–614. [PubMed] [Google Scholar]
- 24. Borgarelli M, Haggstrom J. Canine degenerative myxomatous mitral valve disease: Natural history, clinical presentation and therapy. Vet Clin North Am Small Anim Pract 2010;40:651–663. [DOI] [PubMed] [Google Scholar]
- 25. Paiva RMP, Garcia‐Guasch L, Manubens J, et al. Proximal isovelocity surface area variability during systole in dogs with mitral valve prolapsed. J Vet Cardiol 2011;13:267–270. [DOI] [PubMed] [Google Scholar]
- 26. Bonow RO, Carabello BA, Chatterjee K, et al. Guidelines for the management of patients with valvular heart disease. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2006;48:1–148.16814641 [Google Scholar]
- 27. Jacobs G, Mahyoob K. Multiple regression analysis, using body size and cardiac cycle length, in predicting chocardiographic variables in dogs. Am J Vet Research 49:1590–1594. [PubMed] [Google Scholar]
- 28. Boon J, Wingfield WE, Miller CW. Echocardiographic indices in the normal dog. Vet Radiol 1983;24:214–221. [Google Scholar]
- 29. Kizilbash AM, Willett DWL, Brickner ME, et al. Effects of afterload reduction on vena contracta width in mitral regurgitation. J Am Coll Cardiol 1998;32:427–431. [DOI] [PubMed] [Google Scholar]
- 30. Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M‐mode echocardiography: Results of a survey of echocardiographic measurements. Circulation 1978;6:1072–1083. [DOI] [PubMed] [Google Scholar]
- 31. Zoghbi WA, Enriquez‐Sarano M, Foster E, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two‐dimensional and Doppler echocardiography. J Am Soc Echocardiogr 2003;16:777–802. [DOI] [PubMed] [Google Scholar]
- 32. O'Gara P, Sugeng L, Lang R. The role of imaging in chronic degenerative mitral regurgitation. JACC Cardiovasc Imaging 2008;1:221–237. [DOI] [PubMed] [Google Scholar]
- 33. Kruskal WH, Wallis WA. A nonparametric test for several sample problem. Ann Math Stat 1952;23:525–540. [Google Scholar]
- 34. Norman GR, Streiner DL. Biostatistica. Milano: Casa Editrice Ambrosiana; 2004. [Google Scholar]
- 35. Brad JR, Grayburn PA. Color flow imaging of the vena contracta in mitral regurgitation: Technical considerations. J Am Soc Echocardiogr 2003;16:1002–1006. [DOI] [PubMed] [Google Scholar]
- 36. Yang W, Shim C, Kang M, et al. Vena contracta width as a predictor of adverse outcomes in patients with severe isolated tricuspid regurgitation. J Am Soc Echocardiogr 2011;24:1013–1019. [DOI] [PubMed] [Google Scholar]
- 37. Oh JK, Seward JB, Tajik AJ. The Echo Manual. 2nd ed. Philadelphia, PA: Lippincot W&W;1999. [Google Scholar]
- 38. Grayburn PA. How to measure severity of mitral regurgitation. Postgrad Med J 2008;84:395–402. [DOI] [PubMed] [Google Scholar]
- 39. Omran AS, Arifi AA, Mohamed AA. Echocardiographic atlas of the mitral regurgitation. J Saudi Heart Assoc 2011;23:163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Altiok E, Hamada S, van Halla S, et al. Comparison of direct planimetry of mitral valve regurgitation orifice area by three‐dimensional transesophageal echocardiography to effective regurgitant orifice area obtained by proximal flow convergence method and vena contracta area determined by color Doppler echocardiography. Am J Cardiol 2011;107:452–458. [DOI] [PubMed] [Google Scholar]
- 41. Marsan NA, Westenberg JJM, Ypenburg C, et al. Quantification of functional mitral regurgitation by real‐time 3D echocardiography comparison with 3D velocity‐encoded cardiac magnetic resonance. JACC Cardiovasc Imaging 2009;2:1245–1252. [DOI] [PubMed] [Google Scholar]
- 42. Hyodo E, Iwata S, Tugcu A, et al. Direct measurement of multiple vena contracta areas for assessing the severity of mitral regurgitation using 3D TEE. JACC Cardiovasc Imaging 2012;7:669–676. [DOI] [PubMed] [Google Scholar]
- 43. Sluysman T, Colan SD. Theoretical and empirical derivation of cardiovascular allometric relationships in children. J Appl Physiol 2005;99:445–457. [DOI] [PubMed] [Google Scholar]
- 44. Prakash A, Lacro RV, Sleeper LA, et al. Challenges in echocardiographic assessment of mitralregurgitation in children after repair of atrioventricular septal defect. Pediatr Cardiol 2012;33:205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Arnous S, Killeen RP, Martos R, et al. Quantification of mitral regurgitation on cardiac computed tomography: Comparison with qualitative and quantitative echocardiographic parameters. J Comput Assist Tomogr 2011;35:625–630. [DOI] [PubMed] [Google Scholar]


