Abstract
Background
Neutrophil gelatinase–associated lipocalin (NGAL) is a protein that is used in human medicine as a real‐time indicator of acute kidney injury (AKI).
Hypothesis
Dogs with AKI have significantly higher plasma NGAL concentration and urine NGAL‐to‐creatinine ratio (UNCR) compared with healthy dogs and dogs with chronic kidney disease (CKD).
Animals
18 healthy control dogs, 17 dogs with CKD, and 48 dogs with AKI.
Methods
Over a period of 1 year, all dogs with renal azotemia were prospectively included. Urine and plasma samples were collected during the first 24 hours after presentation or after development of renal azotemia. Plasma and urine NGAL concentrations were measured with a commercially available canine NGAL Elisa Kit (Bioporto® Diagnostic) and UNCR was calculated. A single‐injection plasma inulin clearance was performed in the healthy dogs.
Results
Median (range) NGAL plasma concentration in healthy dogs, dogs with CKD, and AKI were 10.7 ng/mL (2.5–21.2), 22.0 ng/mL (7.7–62.3), and 48.3 ng/mL (5.7–469.0), respectively. UNCR was 2 × 10−8 (0–46), 1,424 × 10−8 (385–18,347), and 2,366 × 10−8 (36–994,669), respectively. Dogs with renal azotemia had significantly higher NGAL concentrations and UNCR than did healthy dogs (P < .0001 for both). Plasma NGAL concentration was significantly higher in dogs with AKI compared with dogs with CKD (P = .027).
Conclusions and Clinical Importance
Plasma NGAL could be helpful to differentiate AKI from CKD in dogs with renal azotemia.
Keywords: AKI, CKD, Lipocalin‐2, renal function
Abbreviations
- AKI
acute kidney injury
- CKD
chronic kidney disease
- ELISA
enzyme‐linked immunosorbent assay
- GFR
glomerular filtration rate
- JLU
Justus‐Liebig University Giessen
- MAT
microagglutination test
- NGAL
neutrophil gelatinase‐associated lipocalin
- UNCR
urine NGAL to creatinine ratio
- UPC
urine protein‐to‐creatinine ratio
- USG
urine specific gravity
- UTI
urinary tract infection
- VFUB
Vetsuisse Faculty University of Bern
Acute kidney injury (AKI) is characterized by a sudden loss of renal function.1 Early recognition of AKI, especially in critically ill patients, is essential for adequate therapy in human and veterinary patients. Therefore, differentiation from prerenal azotemia or CKD is important.2 Urea and creatinine are insensitive markers of renal function and cannot distinguish acute from chronic disease. In addition, azotemia lags behind the actual injury (initial phase of AKI), but an early therapeutic intervention can impede further progression.3 Despite advances in renal replacement therapy, AKI still has a high case fatality rate in human and in animal patients.4, 5 In this setting, renal biomarkers can be sensitive indicators of kidney damage.6 In the past, different renal biomarkers to detect tubulointerstitial injury have been evaluated in dogs with CKD compared with healthy dogs.7, 8 Of 28 dogs, urinary excretion of retinol‐binding protein (RBP), a protein indicating proximal tubular damage, could only be detected in dogs with renal disease (n = 16) and Tamm‐Horsfall protein was reduced in dogs with kidney disease.7 The disease status (acute or chronic) was not reported. Of 25 dogs with X‐linked hereditary nephropathy and 19 unaffected littermates, dogs with progressive renal disease had a marked increase in all measured biomarkers compared with their unaffected littermates early in the disease process.9 However, neither study reported renal biomarkers in dogs with AKI.
In human patients, plasma and urine NGAL concentrations are used as a marker of AKI.2, 10, 11, 12 NGAL is a neutrophil‐derived protein whose concentration increases in plasma and urine with ongoing renal damage.12 In children, plasma and urinary concentration of NGAL increase as early as 2 hours after cardiopulmonary bypass surgery, whereas creatinine only rises after 1–3 days.10 There is also evidence that an increase in serum NGAL concentration can predict AKI even in people with underlying CKD, and it can be used as marker to decide if renal replacement therapy is necessary.1, 2 NGAL plays a role in innate immunity to bacterial infections by binding iron‐sederophore complexes, and can be used as a marker of urinary tract infection (UTI).12, 13 Few data concerning plasma or urine NGAL concentration of dogs exist.3, 4, 9, 14, 15, 16 This study, therefore, was designed to evaluate whether dogs with AKI have higher plasma NGAL and UNCR compared with healthy dogs or dogs with CKD.
Materials and Methods
Healthy Dogs
Twenty dogs owned by staff members of the small animal clinic of the Justus‐Liebig University of Giessen (JLU) were recruited. The dogs were considered healthy based on physical exam, complete blood count, biochemistry profile, and urinalysis including urine culture. Glomerular filtration rate (GFR) was measured by means of a plasma inulin clearance procedure described previously.17 The samples for plasma and urine NGAL measurement were taken immediately before GFR measurement. The study was approved by the state ethics and welfare committee Hessia (number 35/2011).
Dogs with Renal Azotemia
Dogs presented between November 2010 and November 2011 with renal azotemia, or which developed renal azotemia during hospital stay, were eligible for the study. Urinary tract infection (UTI) was excluded based on urinalysis and negative urine culture. In dogs with AKI, urine culture was not performed in dogs with anuria or if dogs were pretreated with antibiotics. Two university clinics were involved: the internal medicine service of the JLU and the Small Animal Clinic of the Vetsuisse Faculty of the University of Bern (VFUB). Renal azotemia was defined as a serum creatinine concentration >1.4 mg/dL (upper laboratory reference value) and serum urea concentration >54 mg/dL (upper reference value) persisting at least 24 hours after correction of prerenal factors together with a urine specific gravity <1.025.18 If no initial urine was available, response to fluid therapy was used to determine if a dog had prerenal azotemia. If azotemia resolved within 24 hours of fluid therapy, the dog was considered to have prerenal azotemia and was subsequently excluded. Dogs with known or suspected acute‐on‐chronic kidney disease were excluded.
In all dogs, plasma and urine samples were obtained in the first 24 hours after presentation or after development of azotemia. The plasma and the supernatant of the urine samples were frozen within 6 hours, stored at −80°C, and batched for analysis.
Based on history, clinical course, laboratory, and ultrasonographic findings, dogs were assigned either to have CKD or AKI. Assignment was performed by the investigators, which were blinded to the NGAL results. Criteria used to assign a dog to the AKI group were acute onset of clinical signs and azotemia in a prior healthy dog, signs of acute tubular injury on urinalysis (eg, glucosuria, urinary casts), imaging findings compatible with AKI such as perirenal free fluid or enlarged kidneys, as well as absence of chronic changes or resolution or marked improvement of azotemia within 30 days of discharge (in survivors). Criteria used to assign a dog to the CKD group were previous history of CKD in the absence of acute deterioration, laboratory findings compatible with chronic disease (eg, nonregenerative anemia), imaging findings compatible with chronic disease (eg, cystic lesions, irregular kidneys), or stable azotemia within 1 month after discharge.3, 19 Dogs with CKD were not staged according to the IRIS guidelines for this study, as most of them were newly diagnosed with CKD, but they were followed up for at least 30 days to confirm stable disease. On the basis of the cause for AKI, dogs in this group were further subdivided in having leptospirosis or not. All dogs with leptospirosis were presented to the VFUB. Dogs were diagnosed with leptospirosis based on a positive PCR on urine, a 4‐fold titer increase in paired microagglutination tests (MAT), a single MAT titer ≥1 : 800 for nonvaccinal serovars or a single MAT titer ≥1 : 3,200 for vaccinal serovars as previously published.18 Dogs that were suspected to have leptospirosis based on laboratory results and clinical course but died before diagnosis could be proven were considered not to have leptospirosis (n = 4).
NGAL ELISA
Plasma and urine NGAL concentrations were measured with a commercially available sandwich enzyme‐linked immunosorbent assay (ELISA) kit.5 After thawing of the batched plasma and urine samples, they were diluted according to the manufacturer's instructions and added in duplicate to 96‐well plates coated with a monoclonal mouse antibody against canine NGAL. After incubation at room temperature for 1 hour, the detection antibody, HRP‐conjugated straptavidin, and a color‐forming substrate were added each after a 1‐hour incubation period. After 10 minutes, the enzymatic reaction was stopped by diluted sulfuric acid. The color intensity was read with an ELISA reader at 450 nm.6 The concentration of NGAL in each sample was calculated from a standard curve, obtained by a 4‐point logistic regression model.
The detection limit of the ELISA test lies between 0.4 ng/mL and 40 ng/mL. NGAL samples above the detection limit were further diluted (up to 1 : 800 for plasma samples and up to 1 : 6,400 for urine samples). For statistical calculations, values below the detection limit were assigned a value of 0.2 ng/mL.
UNCR
Urine creatinine was measured with an automatic analyzer7 using an enzymatic assay8 and UNCR was calculated.
Intra‐assay Variability
Intra‐assay variability was calculated using the standard deviation of the 2 duplicate measurements and dividing that by the duplicate mean.
Statistics
Statistical analysis was performed by statistical software Prism 6.9 As most data were not normally distributed, nonparametric methods were used. Spearman's correlation was calculated between plasma NGAL concentration and GFR in healthy dogs, as well as between plasma NGAL concentration and creatinine in the entire cohort and in dogs with and without azotemia. Plasma NGAL, urinary NGAL, and UNCR between healthy dogs and dogs with renal azotemia were compared by the Mann‐Whitney U‐test. Relevant clinical data between groups (healthy, CKD, AKI), plasma NGAL concentration, urinary NGAL concentration, and UNCR of healthy and renal azotemic dogs were compared by the Kruskal‐Wallis test. Posthoc assessment to calculate significant differences among the 3 groups was performed by the Dunn's Test with correction of alpha for multiple comparisons. Within the AKI group, plasma concentration of NGAL from dogs with and without leptospirosis was compared by the Mann‐Whitney U‐test. Statistical significance was set at P < .05. An ROC analysis was performed to calculate sensitivity and specificity to differentiate CKD from AKI.
Results
A total of 108 dogs were included in the study, 20 healthy dogs and 88 dogs with azotemia. Fifty‐two dogs (including the healthy controls) were recruited at the JLU and 56 at the VFUB. Sex was recorded for 98 dogs, and 44 dogs were female, 54 dogs were male. Of the 20 healthy dogs, 2 were found to have an asymptomatic UTI (E. coli and Pseudomonas aeruginosa, respectively) and were excluded from further analysis. Median (range) GFR in the healthy dogs was 3.6 mL/kg/min (2.2–5.5). Plasma NGAL (Fig 1), urine NGAL and UNCR (Fig 2) were significantly lower in the healthy dogs compared with dogs with renal azotemia (P < .0001 for all three).
Figure 1.
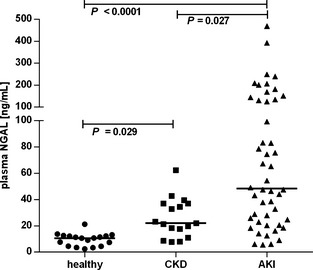
Plasma NGAL concentration in healthy dogs, dogs with chronic kidney disease (CKD), and dogs with acute kidney injury (AKI). Horizontal lines represent the median value.
Figure 2.
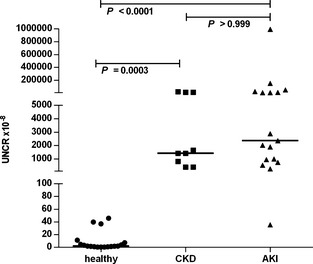
Urine NGAL‐to‐creatinine ratio (UNCR) in healthy dogs, dogs with chronic kidney disease (CKD), and dogs with acute kidney injury (AKI). Horizontal lines represent the median value.
In the 1‐year period, blood samples of 88 dogs with azotemia have been obtained. Four dogs with prerenal and 1 dog with postrenal azotemia, as well as 7 dogs with UTI and 11 dogs with acute deterioration of CKD were excluded. Of the 65 dogs with renal azotemia, 48 were classified as having AKI (74%) and 17 as having CKD (26%). In the AKI group, 23 dogs were diagnosed with leptospirosis. Polyuria and oligoanuria at presentation was reported in 10 and 24 dogs with AKI, respectively. Eleven dogs did not show a change in urine production according to the owners and for 3 dogs, the information was unavailable. In the CKD group, 9 dogs were polyuric and 7 dogs did not show a change in urine production according to the owners. For 1 dog, no information was available. Two dogs in the AKI group had a urine specific gravity >1.025 assessed as falsely elevated: 1 dog was glucosuric because of acute tubular injury; the other dog had marked proteinuria with a urine protein‐to‐creatinine (UPC) ratio of >10. For relevant clinical and clinicopathologic data, see Table 1. Follow‐up data were available in all but 3 dogs.
Table 1.
Relevant clinical and clinicopathologic data at inclusion for all dogs.
| Parameter [reference range] | Healthy Median (range) | Chronic Kidney Disease Median (range) | Acute Kidney Injury Median (range) |
|---|---|---|---|
| Age (years) |
3.5 (1.0–11) n = 18 |
8.5 (1.0–15) n = 16 |
7.0 (1.0–12.0) n = 46 |
| Body weight (kg) |
27.5 (8.1–58) n = 18 |
15.4 (2.4–43.0) n = 16 |
22.4 (1.9–64.0) n = 47 |
|
Urea (mg/dL) [19.82–58.96] |
40.7 (25.0–75.6) n = 18 |
117.9 (54.1–701.9) n = 17 |
305.9 (67.1–656.5) n = 48 |
|
Creatinine (mg/dL) [0.60–1.38] |
0.94 (0.58–1.18) n = 18 |
2.3 (1.1–12.7) n = 17 |
7.3 (2.3–22.9) n = 48 |
|
Potassium (mEq/L) [3.35–4.37] |
4.02 (3.70–4.66) n = 18 |
4.13 (3.40–5.8) n = 17 |
4.6 (2.9–7.87) n = 48 |
|
Phosphorus (mg/dL) [2.45–6.50] |
4.15 (2.57–5.42) n = 18 |
5.14 (1.92–19.76) n = 17 |
11.86 (1.83–30.32) n = 48 |
| USG |
1.038 (1.021–1.050) n = 18 |
1.014 (1.006–1.024) n = 14 |
1.016 (1.008–1.035) n = 24 |
| UPC |
0.1 (0.0–0.4) n = 11 |
2.5 (0.0–7.7) n = 10 |
2.3 (0.1–23) n = 13 |
| Plasma NGAL (ng/mL) |
10.7 (2.5–21.2) n = 18 |
22.0 (7.7–62.3) n = 17 |
48.3 (5.7–469.0) n = 48 |
| UNCR (×10−8) |
2.1 (1–46) n = 18 |
1,424 (385–18,347) n = 9 |
2,366 (36–994,864) n = 17 |
| Urine NGAL (ng/mL) |
0.4 (0.4–11.7) n = 18 |
43.6 (7.2–256.2) n = 9 |
59.8 (0.4–1,026.7) n = 17 |
UNCR, urine NGAL‐to‐creatinine ratio; USG, urine specific gravity; UPC, urine protein‐to‐creatinine ratio.
Plasma and urine NGAL concentration for the duplicate measurements showed an intra‐assay variability of 3.1 and 4.8%, respectively. Creatinine was significantly correlated with plasma NGAL in the entire cohort and in dogs with renal azotemia (r = 0.69; P < .0001 and r = 0.49; P < .0001, respectively), but not in healthy dogs (r = −0.20; P = .42). Plasma NGAL and GFR in healthy dogs were not significantly correlated (r = 0.19; P = .46).
Within the AKI group, no difference was detected in plasma NGAL concentration (median; range) between dogs with leptospirosis (47.6 ng/mL; 9.0–469 ng/mL) and dogs without leptospirosis (65.3 ng/mL; 5.7–392.4 ng/mL); P = .62).
ROC analysis of plasma NGAL had an area under the curve of 0.752 (Fig 3). Specificity of plasma NGAL >42.9 ng/mL and >63.8 ng/mL was 94 and 100%, respectively, to diagnose AKI. A plasma NGAL value <13.4 ng/mL excluded AKI with a sensitivity of 90%. A plasma NGAL concentration >37.4 ng/mL had 65% sensitivity and 82% specificity to differentiate AKI from CKD in dogs with renal azotemia.
Figure 3.
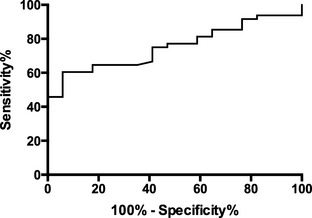
Receiving operator characteristic curve of plasma NGAL in dogs with chronic kidney disease compared against dogs with acute kidney injury (AKI). An NGAL value >37.4 ng/mL had a sensitivity of 65% and a specificity of 82% to diagnose AKI.
Discussion
This study showed that dogs with renal azotemia had higher concentrations of NGAL in plasma and urine as well as UNCR compared with healthy dogs. Plasma NGAL might be helpful to differentiate AKI from CKD in dogs with renal azotemia, especially when using a cutoff of >37.4 ng/mL (sensitivity 65%, specificity 82%).
NGAL (also known as lipocalin‐2, siderocalin, or uterocalin) has been rediscovered in the setting of AKI in human medicine. The 25‐kDa protein belonging to the lipocalin superfamily was first isolated from human neutrophils in 1993.20 More recently, up‐regulation of the NGAL gene after ischemic kidney injury has been found in a mouse model. Increased induction of NGAL mRNA and overexpression of NGAL in proximal tubules and urine were present after ischemic insult to the kidney. Furthermore, urinary NGAL was also increased after mild ischemia not followed by azotemia.21 Subsequent studies in human patients proved the usefulness of urinary NGAL as a real‐time indicator of AKI.1 , 2 , 2, 10, 12
Despite the availability of a NGAL ELISA Kit for dogs, NGAL results in dogs are sparse and describe mostly healthy dogs and dogs with CKD.3 , 4 , 9 However, 3 recent studies investigated NGAL as a marker of AKI in dogs.14, 15, 16 The only study presenting data on plasma NGAL in dogs was designed to evaluate plasma and urinary NGAL as an early marker for AKI in dogs after surgery. This study found plasma NGAL to be less sensitive compared with urinary NGAL.14 Direct comparison of this study with the study of Lee et al14 is somewhat difficult, as the study design was completely different. It might be that urinary NGAL is an earlier marker of AKI compared with plasma NGAL. The marked increase in plasma NGAL in dogs with AKI observed in this study can be explained by 2 mechanisms. It is known that AKI leads to up‐regulation of various inflammatory genes, including the lipocalin‐2 gene, which codes for NGAL.22 In addition, the reduced filtration capacity of the kidney during AKI leads to a reduced clearance of NGAL and therefore to systemic accumulation.23 In agreement with results in human medicine, this study showed that plasma NGAL and UNCR are increased in dogs with CKD compared with healthy dogs, but to a lesser extent than in dogs with AKI.23, 24 It is hypothesized that NGAL is a real‐time marker for active kidney damage rather than a functional parameter such as serum creatinine or GFR.25 This finding is supported by our study, which found no correlation between GFR or plasma creatinine concentration and plasma NGAL in the healthy dogs. In contrast, both serum NGAL and UNCR were found to be correlated with GFR in a study evaluating 69 humans with CKD and 32 healthy controls.26 This study found a positive, but weak correlation of creatinine and plasma NGAL in the whole cohort of dogs (healthy, CKD, AKI). Further studies are necessary to evaluate the relation of NGAL and GFR in dogs with different levels of azotemia.
Recently published data on urinary NGAL after gentamicin administration showed that an increase in total urinary NGAL concentration could be measured before an increase in serum creatinine was evident.10, 16 The measurement of total urinary NGAL is of questionable accuracy, though, as these values are likely to be influenced by urinary concentration ability, hydration status, and use of diuretics or aggressive fluid therapy, especially in a clinical setting. In human medicine, UNCR and absolute urinary NGAL concentration are used interchangeably, but UNCR shows less intraindividual variation and is independent of urine concentration.11, 27 The significantly higher urine NGAL concentration and UNCR in dogs with renal azotemia compared with healthy dogs found in this study were also reported in human patients with various stages of CKD compared with individuals with nonrenal disease.26 In patients undergoing liver transplantation, UNCR was significantly higher in those developing AKI after the procedure.27 The finding that urine NGAL and UNCR were not significantly different in dogs with AKI compared with dogs with CKD is contradictory to a recently published study, which found a significant difference in UNCR between dogs with CKD and dogs with different stages of AKI.15 In this study, we also included dogs with anuric AKI, which obviously led to missing urinalysis. This might have rendered the analysis underpowered. Given that dogs with AKI are at high risk for anuria, plasma NGAL seems to be more valuable to support a differentiation of AKI from CKD in dogs.
Plasma NGAL concentration is not only increased in human patients with renal disease but also with other disease conditions, such as acute infections,28 different types of neoplasia,24 or in obstructive lung disease.29 It remains unclear how much concomitant disease conditions could have influenced the results of this study. Only 1 dog with CKD had evidence of neoplasia (multiple myeloma). As both university clinics are referral institutions, preinclusion treatment as well as the time from disease onset to inclusion may have additionally influenced plasma and urine NGAL levels. However, further assessment of these factors was not possible. Further limitations of the study are the small number of animals included. It would have been interesting to evaluate dogs with acute‐on‐chronic kidney disease. However, to definitively differentiate a mild prerenal component from AKI can be difficult, and misclassification confounding the results would have been possible. Dogs with UTI were excluded from analysis in this study. It would have been valuable to compare these with dogs without UTI; however, the small number of dogs with UTI precluded significant statistical analysis. Another limitation is that IRIS staging was not possible. However, dogs were followed up for a minimum of 30 days and only 3 animals were lost to follow‐up.
The definitive test to differentiate AKI from CKD is renal histopathology. Because these were not performed in this study, it is possible that some dogs might have been falsely assigned to the CKD or AKI groups and therefore confounded the results. However, 48% of the dogs with AKI had a clear diagnosis, having been tested positive for leptospirosis. In addition, only 3 dogs were lost to follow‐up and for the remainder, the clinical course and the available clinicopathologic data during the follow‐up period were used to validate the initial assignment. A major limitation is that in some dogs with CKD, the samples were obtained at their first visit. Despite 30‐day follow‐up data, it is possible that these dogs were presented because of an acute deterioration of kidney function not necessarily associated with an increase in creatinine concentration, making misclassification possible.
In conclusion, NGAL is a promising renal biomarker to distinguish dogs suffering from AKI from dogs with CKD. Further studies are now needed to assess if NGAL could help to distinguish renal from prerenal azotemia, if patients at risk could be detected at an earlier stage, or if NGAL can also be used as a prognostic indicator.
Acknowledgment
Conflict of Interest Declaration: Authors disclose no conflict of interest.
The study is a collaborative project of the Small Animal Clinic of the Justus‐Liebig University Giessen, Germany and the Vetsuisse Faculty University of Bern, Switzerland. All work was performed at these two institutions.
These results were presented at the 22th ECVIM‐CA Congress, Maastricht, Netherlands, 2012.
Footnotes
Doi K, Urata M, Katagiri D, et al. Plasma Neutrophil Gelatinase‐Associated Lipocalin Predicts Acute‐on‐Chronic Kidney Injury after Adult Cardiac Surgery: A Multicenter Prospective Study. J Am Soc Nephrol 2011;22:123A (abstract)
Tiranathanagu K, Amornsuntorn S, Susantitaphong P, Eiam‐Ong S. Plasma and Urine Neutrophil Gelatinase‐Associated Lipocalin (pNGAL and uNGAL) as a Novel Indicator for Early Identifying Critically Ill Acute Kidney Injury (AKI) Patients Who Subsequently Require Replacement Therapy. J Am Soc Nephrol 2011;22:124A (abstract)
LeRoy B, Kowalkowski K, Blomme E, et al. Neutrophil Gelatinase‐Associated Lipocalin (Ngal) Concentrations in Urine From Normal Dogs and Dogs with Kidney Injury. Vet Clin Pathol. 2011;40(4):601–2 (abstract)
Cobrin A, Blois S, Abrams‐Ogg A, Kruth S, Dewey C. Serum and Urine Neutrophil Gelatinases‐Associated Lipocalin (Ngal) Concentration in Healthy Dogs and Dogs with Chronic Kidney Disease. Proceedings of the Advanced Renal Therapies Symposium, New York, 2012, 131 pp.
Dog NGAL ELISA Kit 043, Bioporto® Diagnostics A/S, Gentofte, Denmark
PR 3100 TSC Microplate Reader, Bio‐Rad Laboratories, Inc, Hercules, CA
PENTRA 400, ABX Horiba, Darmstadt, Germany
CREA PAP, Axon Lab GmbH, Stuttgart, Germany
Prism 6 for Mac OS X, GraphPad Software Inc, La Jolla, CA
Palm C, Westropp J, LeRoy B, Segev G, Cowgill L. Urinary NGAL: a Biomarker for Early Identification of Acute Kidney Injury in Dogs. J Vet Intern Med. 2012;26(3):799 (abstract)
Cavalier E, Bekaer A‐C, Legrand D, et al. Urinary NGAL: Use of absolute value or ratio to creatinine? Acta Clinica Belgica. 2012;65(3):13 (abstract)
References
- 1. Ross L. Acute kidney injury in dogs and cats. Vet Clin North Am Small Anim Pract 2011;41:1–14. [DOI] [PubMed] [Google Scholar]
- 2. Nickolas TL, O'Rourke MJ, Yang J, et al. Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase‐associated lipocalin for diagnosing acute kidney injury. Ann Intern Med 2008;148:810–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Langston C. Acute uremia In: Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Internal Medicine: Diseases of the Dog and the Cat, 7th ed St. Louis, MO: Saunders Elsevier; 2010:1969–1984. [Google Scholar]
- 4. Chertow GM, Burdick E, Honour M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 2005;16:3365–3370. [DOI] [PubMed] [Google Scholar]
- 5. Segev G, Kass PH, Francey T, Cowgill LD. A novel clinical scoring system for outcome prediction in dogs with acute kidney injury managed by hemodialysis. J Vet Intern Med 2008;22:301–308. [DOI] [PubMed] [Google Scholar]
- 6. Price R. Early markers of nephrotoxicity. Comp Clin Pathol 2002;11:2–7. [Google Scholar]
- 7. Forterre S, Raila J, Schweigert FJ. Protein profiling of urine from dogs with renal disease using proteinchip analysis. J Vet Diagn Invest 2004;16:271–277. [DOI] [PubMed] [Google Scholar]
- 8. Smets PMY, Meyer E, Maddens BEJ, et al. Urinary markers in healthy young and aged dogs and dogs with chronic kidney disease. J Vet Intern Med 2010;24:65–72. [DOI] [PubMed] [Google Scholar]
- 9. Nabity MB, Lees GE, Cianciolo R, et al. Urinary biomarkers of renal disease in dogs with X‐linked hereditary nephropathy. J Vet Intern Med 2012;26:282–293. [DOI] [PubMed] [Google Scholar]
- 10. Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase‐associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 2005;365:1231–1238. [DOI] [PubMed] [Google Scholar]
- 11. Bolignano D, Lacquaniti A, Coppolino G, et al. Neutrophil gelatinase‐associated lipocalin (NGAL) and progression of chronic kidney disease. Clin J Am Soc Nephrol 2009;4:337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Haase M, Haase‐Fielitz A, Bellomo R, Mertens PR. Neutrophil gelatinase‐associated lipocalin as a marker of acute renal disease. Curr Opin Hematol 2011;18:11–18. [DOI] [PubMed] [Google Scholar]
- 13. Yilmaz A, Sevketoglu E, Gedikbasi A, et al. Early prediction of urinary tract infection with urinary neutrophil gelatinase associated lipocalin. Pediatr Nephrol 2009;24:2387–2392. [DOI] [PubMed] [Google Scholar]
- 14. Lee YJ, Hu YY, Lin YS, et al. Urine neutrophil gelatinase‐associated lipocalin (NGAL) as a biomarker for acute canine kidney injury. BMC Vet Res 2012;8:248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Segev G, Palm C, Leroy B, et al. Evaluation of neutrophil gelatinase‐associated lipocalin as a marker of kidney injury in dogs. J Vet Intern Med 2013;27:1362–1367. [DOI] [PubMed] [Google Scholar]
- 16. Kai K, Yamaguchi T, Yoshimatsu Y, et al. Neutrophil gelatinase‐associated lipocalin, a sensitive urinary biomarker of acute kidney injury in dogs receiving gentamicin. J Toxicol Sci 2013;38:269–277. [DOI] [PubMed] [Google Scholar]
- 17. Haller M, Müller W, Estelberger W, Arnold P. Single‐injection inulin clearance ‐ a simple method for measuring glomerular filtration rate in dogs. Res Vet Sci 1998;64:151–156. [DOI] [PubMed] [Google Scholar]
- 18. Geigy CA, Schweighauser A, Doherr M, Francey T. Occurrence of systemic hypertension in dogs with acute kidney injury and treatment with amlodipine besylate. J Small Anim Pract 2011;52:340–346. [DOI] [PubMed] [Google Scholar]
- 19. Polzin DJ. Chronic kidney disease In: Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Internal Medicine: Diseases of the Dog and the Cat, 7th ed St. Louis, MO: Saunders Elsevier; 2010:1990–2021. [Google Scholar]
- 20. Kjeldsen L, Johnsen AH, Sengelov H, Borregaard N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J Biol Chem 1993;268:10425–10432. [PubMed] [Google Scholar]
- 21. Mishra J. Identification of neutrophil gelatinase‐associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol 2003;14:2534–2543. [DOI] [PubMed] [Google Scholar]
- 22. Grigoryev DN, Liu M, Hassoun HT, et al. The local and systemic inflammatory transcriptome after acute kidney injury. J Am Soc Nephrol 2008;19:547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Devarajan P. Review: Neutrophil gelatinase‐associated lipocalin: A troponin‐like biomarker for human acute kidney injury. Nephrology 2010;15:419–428. [DOI] [PubMed] [Google Scholar]
- 24. Bolignano D, Donato V, Lacquaniti A, et al. Neutrophil gelatinase‐associated lipocalin (NGAL) in human neoplasias: A new protein enters the scene. Cancer Lett 2010;288:10–16. [DOI] [PubMed] [Google Scholar]
- 25. Mori K, Nakao K. Neutrophil gelatinase‐associated lipocalin as the real‐time indicator of active kidney damage. Kidney Int 2007;71:967–970. [DOI] [PubMed] [Google Scholar]
- 26. Bolignano D, Lacquaniti A, Coppolino G, et al. Neutrophil gelatinase‐associated lipocalin reflects the severity of renal impairment in subjects affected by chronic kidney disease. Kidney Blood Press Res 2008;31:255–258. [DOI] [PubMed] [Google Scholar]
- 27. Wagener G, Minhaz M, Mattis FA, et al. Urinary neutrophil gelatinase‐associated lipocalin as a marker of acute kidney injury after orthotopic liver transplantation. Nephrol Dial Transplant 2011;26:1717–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fjaertoft G, Foucard T, Xu S, Venge P. Human neutrophil lipocalin (HNL) as a diagnostic tool in children with acute infections: A study of the kinetics. Acta Paediatr 2005;94:661–666. [DOI] [PubMed] [Google Scholar]
- 29. Keatings VM, Barnes PJ. Granulocyte activation markers in induced sputum: Comparison between chronic obstructive pulmonary disease, asthma, and normal subjects. Am J Respir Crit Care Med 1997;155:449–453. [DOI] [PubMed] [Google Scholar]


