Abstract
Background
Increased heart rate (HR) and decreased heart rate variability (HRV) are evident in some dogs with degenerative mitral valve disease (DMVD).
Objectives
Evaluation of the factors influencing HR and HRV (assessed by the vasovagal tonus index; VVTI) and their change over time in dogs with DMVD.
Animals
Client‐owned dogs (n = 257) with DMVD recruited from first opinion practice.
Methods
Prospective longitudinal follow‐up at six‐monthly intervals of dogs with DMVD. Dogs followed up for at least 18 months (n = 102) were grouped according to their outcome as dogs dying/euthanized because of cardiac disease (n = 28; Group 1), noncardiac disease (n = 40; Group 2) and dogs alive (n = 34; Group 3). HR and VVTI were measured on 1‐minute ECG recordings. Repeated measures linear models were constructed to investigate the factors that influence HR and VVTI and their changes over time.
Results
Heart rate and VVTI were affected by disease severity and were different in Cavaliers compared to other breeds. Group 1 and Group 2 dogs underwent an increase in HR and decrease in VVTI, evident at least 18 months before death. Group 1 had a further decrease in VVTI followed by an increase in HR approximately 1 year and 6 months before death, respectively.
Conclusions and Clinical Importance
Dogs with DMVD have an increase in HR and decrease in HRV over a year before death, with greater changes in those dogs dying/euthanized because of cardiac disease. Both HR and VVTI can potentially be regarded as biomarkers for all‐cause mortality.
Keywords: Autonomic nervous system, Heart rate variability, Vasovagal tonus index
Abbreviations
- AUC
area under the curve
- CHF
congestive heart failure
- CKCS
Cavalier King Charles Spaniels
- cTnI
cardiac troponin I
- DMVD
degenerative mitral valve disease
- ECG
electrocardiogram
- FS
fractional shortening
- HR
heart rate
- HRV
heart rate variability
- LA : Ao
left atrium to aortic diameter ratio
- LVEDD(N)
left ventricular end‐diastolic internal dimension (normalized)
- LVESD(N)
left ventricular end‐systolic internal dimension (normalized)
- NT‐proBNP
N‐terminal probrain natriuretic peptide
- ROC
receiver operator characteristic
- VVTI
vasovagal tone index
Degenerative mitral valve disease (DMVD) is the most common cause of cardiac disease in dogs.1, 2 Middle‐ to old‐aged, male dogs of small breeds are over‐represented.3 Degeneration and loss of leaflet coaptation lead to progressive cardiac volume overload, increased diastolic filling pressures, and eventual development of congestive heart failure (CHF).4 Neural and endocrine compensatory mechanisms, beyond a point, contribute to the progression of the disease; hence the term maladaptive compensation.5 Dogs with DMVD that develop heart failure suffer parasympathetic withdrawal6 and sympathetic activation, caused by both direct adrenergic stimulation and adrenergic postganglionic liberation of catecholamines mediated by angiotensin.7 Both the autonomic nervous system and the renin‐angiotensin‐aldosterone system affect heart rate (HR) and heart rate variability (HRV).8
The time‐domain analysis of HRV is the overall quantification of the variability in the electrocardiogram (ECG) R‐R intervals and serves as indirect indicator of autonomic tone.9 The vasovagal tone index (VVTI), measured over 20 intervals, was first described in veterinary medicine to evaluate the severity and predict decompensation of DMVD in Cavalier King Charles Spaniels (CKCS).10 This first study concluded that VVTI differentiates dogs in CHF, but stress also influenced the results, lowering its sensitivity.
Vasovagal tone index is negatively correlated with HR and CHF class,10, 11 and successful treatment decreases the HR.12 Similarly, several Holter monitor derived HRV estimates covary with echocardiographic indices of DMVD severity in CKCS.13 Of all‐cause mortality predictors in dogs affected with DMVD, lower VVTI is associated with shorter survival at the univariable level.14 VVTI at presentation in dogs with dilated cardiomyopathy correlates with HR, fractional shortening, and survival time but does not correlate with age, sex, or ventricular or atrial internal dimensions; furthermore, VVTI at presentation was the only independent predictor of survival in a multivariable model.15 However, to the best of the authors' knowledge, longitudinal evaluation of the progression of HR and VVTI over time in dogs with cardiac disease has not been reported to date.
On the basis of previous studies, we hypothesized that HR will increase progressively while VVTI will progressively decrease in dogs with DMVD before them succumbing or being euthanized because of their cardiac disease. By contrast, we hypothesized that dogs with DMVD that die of noncardiac causes and dogs with DMVD that do not go on to die in the following year, would not show such changes in HR or VVTI. The objectives of this were (1) to evaluate factors that influence HR and VVTI, (2) to investigate changes in these ECG parameters observed in a cohort of dogs followed longitudinally. In addition, we aimed to evaluate whether the determination of VVTI over a greater number of R‐R intervals (instead of the standard 20 intervals) would be superior in discriminating between groups.
Materials and Methods
Study Population
This study was approved by the Royal Veterinary College Welfare and Ethics Committee and the owners gave informed consent for enrollment. Dogs were prospectively recruited from December 2004 and data collected up to January 2013 from 2 first opinion practices. The inclusion criteria were the presence of a left apical systolic murmur with echocardiographic evidence of mitral regurgitation and characteristic abnormalities of the valve leaflets (eg, thickening, prolapse, or both). Dogs were excluded if they had concomitant noncardiac diseases or cardiac diseases other than DMVD at the time of enrollment and if limited follow‐up was available (less than 1 revisit). Dogs receiving heart failure medication (different combinations of angiotensin‐converting enzyme inhibitors, furosemide and pimobendan) and animals that developed comorbidities during the study period were not excluded. Poor quality ECG traces were also excluded.
The owners were invited to bring their dogs from the first detection of the cardiac murmur and every 6 months thereafter until the animal died or was euthanized or was withdrawn from the study. All the investigations always followed the same routine and order for consistency of data collection: history was obtained at each consultation, followed by physical examination, blood pressure measurement, blood sample collection for measurement of NT‐proBNP and cTnI, 1 minute ECG recording, and echocardiographic examination as previously reported.14, 16
Electrocardiograms were recorded with the dog in right lateral recumbency after a variable period of adaptation to the environment, depending on the animal's character. The paper speed was set at 25 mm/s. The HR was calculated as the number of QRS complexes in 1 minute. The R‐R interval durations were measured with a ruler to the nearest quarter of a millimeter (equivalent to the nearest 10 milliseconds). The measurements were entered into a spread sheet programmed to automatically convert the measurements to milliseconds using the formula (measurement/0.025). VVTI was calculated as the natural logarithm of the variance of the R‐R intervals for 20 and 60 consecutive QRS complexes (VVTI20 and VVTI60, respectively). Nonsinus rhythms and ectopic beats were not included in the analysis; note was made of the presence of any rhythm abnormality and the preceding and following R‐R intervals eliminated from calculations.
For the assessment of the progression of HR and VVTI over time, 3 groups of dogs were assessed. Dogs in Group 1 died or were euthanized because of their cardiac disease; dogs in Group 2 died or were euthanized because of noncardiac causes; dogs in Group 3 were alive for at least 6 months after their last ECG included in the study was recorded. To be included in this part of the study, an ECG should have been recorded on the last 3 consultations included. The cause and date of death was obtained either from the records of the 2 practices or by direct communication with the owners. According to the reasons given, these were divided into cardiac and noncardiac causes. Dogs that died suddenly were assigned to the cardiac death group unless a clear alternative reason for their death was known. The six‐monthly interval consultations obtained from dogs in Groups 1 and 2 were coded numerically as ‐1 for the last visit (<180 days before demise), ‐2 for the penultimate visit (between 180 and 360 days), ‐3 for visit more than 360 days but not more than 540 days, and so on, up to visit ‐6 for the oldest visits. To ensure that Group 3 dogs did not die in the subsequent 6 months, dogs needed to have been presented for an additional consultation after visit ‐1 and were therefore known to have survived at least an additional 6 months following their last included visit. Again, an ECG from the last 3 consultations prior the last revisit recorded needed to be available to be included in the study. The consultations from dogs in Group 3 were coded numerically as ‐1 for the visit before the last one recorded (between 180 and 360 days before the last recorded revisit), ‐2 for the previous revisit (360–540 days before the last recorded revisit), ‐3 for the next revisit included (540–720 days before the last recorded revisit), and so on, until visit ‐6.
Statistical Analysis
Data were entered into a spreadsheet1 and statistical analyses performed by commercially available software.2 , 3 After graphic assessment of normality, basic descriptive statistics were calculated. Group comparisons for continuous data were performed with Kruskal‐Wallis (and Mann‐Whitney U with Bonferroni correction for posthoc comparison) or ANOVA (and least significant difference [LSD] for posthoc comparison) and chi‐square to compare proportions as indicated. For the construction of the different models, the assumptions were tested and confirmed as required. Circulating cardiac biomarker levels below the lower or above the upper limit of detection of the assay were assigned the same value as the corresponding limit of detection. Associations between the different continuous parameters studied were assessed by means of the Pearson's correlation coefficient and Spearman's rank correlation; an association was suspected when the absolute value for the correlation coefficient r > 0.70.
For the assessment of the factors that influence HR and VVTI, repeated measures linear models were constructed, including the dogs' identification number as random effect and the different variables as fixed factors. A first evaluation of each variable allowed univariable selection of variables significant at the 10% level to be included in the final model. The final model was constructed in a manual stepwise backward fashion until all the remaining variables were significant at the 5% level.
For the assessment of the progression of HR and VVTI over time firstly, a graphic assessment was performed (Figs 1, 2, 3). According to the inclusion criteria, only the last 3 visits were included in the statistical analysis for the 3 study groups. Repeated measures linear models were constructed including the visit code and cause of death as fixed factors and the animal identification number as a random effect. These models were then constructed again including age and breed (CKCS: yes/no) to assess their possible confounding effect. Posthoc analysis of the estimated marginal means for each group at each consultation was subsequently assessed with the LSD multiple comparisons correction. Receiver operator characteristic (ROC) curves were generated to assess the performance of HR and both VVTIs for discrimination of dogs that would go on to experience death (all‐cause mortality) and cardiac‐related death from those that survived at the 3 visits. The negative predictive value of the test to predict mortality was then calculated from the ROC with greatest estimated area under the curve (AUC).
Figure 1.
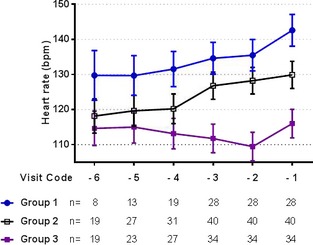
Graphic assessment of the progression of the mean heart rate (±SE of the mean) over time in the 3 study groups.
Figure 2.
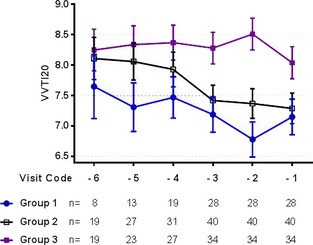
Graphic assessment of the progression of the mean VVTI20 (±SE of the mean) over time in the 3 study groups.
Figure 3.
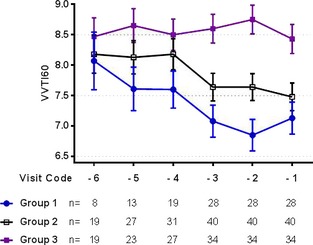
Graphic assessment of the progression of the mean VVTI60 (±SE of the mean) over time in the 3 study groups.
Results
A total of 859 ECGs from 257 dogs were recorded from December 2004 to January 2013. From these, the overall rhythm in 421 ECGs (421/859 = 49.0%) from 170 dogs (170/257 = 66.1%) was sinus arrhythmia; 432 ECGs (432/859 = 50.3%) from 179 dogs (179/257 = 69.6%) demonstrated sinus rhythm or sinus tachycardia. Seventy‐eight ECGs (78/859 = 9.1%) from 49 dogs (49/257 = 19.1%) demonstrated a rhythm abnormality during the study period. From these, 5 ECGs (5/859 = 0.58%) from 4 dogs (4/257 = 1.6%) showed atrial fibrillation; and 1 ECG (1/859 = 0.1%) from 1 dog (1/257 = 0.4%) atrioventricular dissociation; 45 ECGs (45/859 = 5.2%) from 28 dogs (28/257 = 10.9%) showed occasional atrial premature complexes (APCs) (36 ECGs presented 1 to 3 APCs/min and 9 ECGs presented from 4 to 18 APCs/min), 2 ECGs (2/859 = 0.2%) from 2 dogs (2/257 = 0.8%) showed paroxysms of atrial tachycardia with isolated APCs; 19 ECGs (19/859 = 2.2%) from 14 dogs (14/257 = 5.4%) showed occasional ventricular premature complexes (14 ECGs presented 1 to 3 VPCs/min and 5 ECGs presented 5 to 9 VPCs/min) and 8 ECGs (8/859 = 0.9%) from 6 dogs (6/257 = 2.3%) showed 2nd degree atrioventricular block associated with sinus bradycardia (two of them also showing escape complexes).
For the assessment of variables associated with HR and VVTI, only the remaining 853 ECGs with an underlying sinus rhythm, sinus tachycardia, and sinus arrhythmia were included in the analysis. These were obtained from 257 dogs (Table 1), including 99 CKCS (38.5%), 25 each cross breed and Jack Russell Terrier (9.7% each), 20 Yorkshire Terrier (7.8%), 18 Collie (7.0%), 8 Shih Tzu (3.1%), 6 each Chihuahua and Poodle (2.3% each), 5 Lurcher (1.9%), 4 Bichon Frisé (1.5%), 3 each Whippet and Pomeranian (1.2% each), 2 each Border Collie, Chinese Crested, English Bullterrier, Maltese, Norfolk Terrier, Springer Spaniel, Staffordshire Bullterrier, Tibetan Terrier, and West Highland White Terrier (0.8% each), and 1 each Beagle, Bedlington Terrier, Cockapoo, Cocker Spaniel, Dachshund, Greyhound, Norfolk, Irish Terrier, Japanese Chin, King Charles Spaniel, Lhasa Apso, Miniature Schnauzer, Papillon, Pekinese, Saluki (0.4% each).
Table 1.
Descriptive data for the overall population at presentation and for the 3 study groups at visit ‐3, expressed as median (interquartile range). A significant difference between the groups is indicated by different letters.
| Overall (n = 257) | Group 1 (n = 28) | Group 2 (n = 40) | Group 3 (n = 34) | P‐Value | |
|---|---|---|---|---|---|
| Age (years) | 9.8 (7.5–11.8) |
8.3 (7.4–10.5) A |
11.5 (9.7–13.7) B |
9.11 (8.2–11.2) A |
<.001 |
| Weight (kg) | 10.5 (7.7–13.9) | 10.0 (8.3–12.4) | 9.7 (7.2–12.8) | 9.9 (7.3–12.5) | .90 |
| Murmur intensity | III/VI (II–IV) |
IV/VI (IV–V) A |
III/VI (II–IV) B |
III/VI (II–IV) B |
.038 |
| cTnI (ng/mL) | 0.03 (0.02–0.07) | 0.03 (0.04–0.07) | 0.03 (0.02–0.06) | 0.03 (0.02–0.06) | .23 |
| NT‐proBNP (pmol/L) | 626 (368–1,061) |
1,278 (608–2,559) A |
606 (287–933) B |
644 (339–956) B |
<.001 |
| Systolic BP (mmHg) | 155 (136–177) | 144.8 (130.7–156.0) | 158.4 (142.0–177.8) | 160.0 (140.6–180.1) | .09 |
| LA : Ao ratio | 1.26 (1.13–1.45) | 1.44 (1.22–1.56) | 1.29 (1.17–1.52) | 1.25 (1.13–1.48) | .11 |
| LVEDDN | 1.69 (1.52–1.90) |
1.92 (1.75–2.22) A |
1.65 (1.46–1.80) B |
1.69 (1.52–1.96) B |
<.001 |
| FS (%) | 39.0 (32.2–44.4) | 39.9 (37.7–47.8) | 39.9 (32.4–45.7) | 38.0 (33.4–43.7) | .44 |
| E wave velocity (m/s) | 0.84 (0.73–1.06) |
1.03 (0.95–1.24) A |
0.78 (0.65–0.97) B |
0.81 (0.69–1.06) B |
.001 |
| A wave velocity (m/s) | 0.71 (0.59–0.84) | 0.76 (0.68–0.90) | 0.84 (0.72–0.95) | 0.74 (0.60–0.87) | .052 |
| E : A ratio | 1.24 (1.02–1.43) |
1.38 (1.22–1.49) A |
0.97 (0.79–1.09) B |
1.19 (0.99–1.47) A |
<.001 |
| Wall stress | 1.96 (1.68–2.26) |
2.20 (1.97–2.46) A |
1.87 (1.46–2.21) B |
1.86 (1.69–2.37) B |
.001 |
| Cardiac treatment (yes/no) | 25.1%/74.9% |
39%/61% A |
32.5%/67.5% A |
11.7%/88.3% B |
.035 |
| Sex |
Male 59.9% (ME = 18.7% + MN = 41.2%) Female 40.1% (FE = 6.1% + FN = 34.0%) |
Male 50% (ME = 17.8% + MN = 32.2%) Female 50% (FE = 7.2% + FN = 42.8%) |
Male 55% (ME = 10% + MN = 45%) Female 45% (FE = 0% + FN = 45.0%) |
Male 58.8% (ME = 14.7% + MN = 44.1) Female 41.2% (FE = 2.9% + FN = 38.3%) |
.79 |
cTnI, cardiac troponin I; NT‐proBNP, N‐terminal probrain natriuretic peptide; BP, blood pressure; LA : Ao, left atrium to aorta ratio; LVEDDN, left ventricular end‐diastolic internal dimension normalized; FS, fractional shortening; ME, entire male; MN, neutered male; FE, entire female; FN, neutered female.
Variables that were significantly associated were VVTI20 with VVTI60 (r = 0.92, P < .001), VVTI20 with HR (r = −0.69, P < .001), VVTI60 with HR (r = −0.71, P < .001), LVEDDN with E wave velocity (r = 0.72, P ≤ .001), and LVEDDN with wall stress (r = 0.82, P ≤ .001) (data not shown). Taking account of these results, multivariable models were constructed excluding E wave velocity and wall stress to avoid effects of multi‐colinearity with VVTI or LVEDDN.
Univariable repeated measures modeling to assess the factors that affect HR and VVTI are shown in Table 2. The final multivariable model for the HR showed that being a CKCS, receiving cardiac medication and having increased NT‐proBNP and FS are independent predictors of increased HR (Table 3). The final multivariable model for both VVTIs showed that independent predictor variables for lower VVTI20 and VVTI60 values included being a CKCS and having higher NT‐proBNP, E : A ratio values, and FS (Table 3).
Table 2.
Estimate of the fixed effects affecting heart rate and VVTI at the univariable level.
| n | HR | VVTI20 | VVTI60 | ||||
|---|---|---|---|---|---|---|---|
| Estimate (95% CI) | P‐Value | Estimate (95% CI) | P‐Value | Estimate (95% CI) | P‐Value | ||
| Age (years) | 257 | 1.08 (0.37–1.78) | .003 | −0.06 (−0.11 to −0.01) | .009 | −0.06 (−0.11 to −0.02) | .004 |
| CKCS (yes/noa) | 257 | 14.58 (8.87–20.28) | <.001 | −0.76 (−1.08 to −0.44) | <.001 | −0.43 (−0.58 to −0.32) | <.001 |
| Sex (F/Ma) | 257 | 0.97 (−4.97 to 6.92) | .747 | 0.27 (−0.06 to 0.61) | .105 | 0.26 (−0.05 to 0.57) | .095 |
| Weight (kg) | 257 | −0.39 (−0.88 to 0.08) | .106 | 0.01 (−0.02 to 0.04) | .517 | 0.01 (−0.02 to 0.04) | .43 |
| Treatment (yes/noa) | 257 | 10.35 (6.29–14.42) | <.001 | −0.52 (−0.80 to −0.23) | <.001 | −0.54 (−0.80 to −0.29) | <.001 |
| Log(cTnI) (ng/mL) | 248 | 0.79 (−3.21 to 4.79) | .697 | −0.11 (−0.41 to 0.19) | .478 | −0.09 (−0.35 to 0.16) | .60 |
| Log(NT‐proBNP) (pmol/L) | 250 | 11.25 (6.86–15.65) | <.001 | −0.99 (−1.29 to −0.68) | <.001 | −0.93 (−1.20 to −0.67) | <.001 |
| Systolic BP (mmHg) | 239 | 0.01 (−0.04 to 0.05) | .750 | −0.01 (−0.004 to 0.003) | .761 | 0.01 (−0.003 to 0.003) | .97 |
| Rhythm ECG (SA/SRa) | 257 | −16.73 (−19.31 to −14.15) | <.001 | 1.65 (1.47 to 1.83) | <.001 | 1.56 (1.41 to 1.72) | <.001 |
| Ectopy ECG (yes/noa) | 257 | 5.50 (0.62–10.37) | .027 | −0.35 (−0.73 to 0.03) | .071 | −0.19 (−0.52 to 0.13) | .24 |
| LA : Ao ratio | 256 | 15.12 (9.25–20.99) | <.001 | −1.10 (−1.51 to −0.69) | <.001 | −1.07 (−1.44 to −0.69) | <.001 |
| LVEDDN | 252 | 9.02 (3.08–14.96) | .003 | −1.16 (−1.55 to −0.76) | <.001 | −1.23 (−1.59 to −0.87) | <.001 |
| FS (%) | 255 | 0.49 (0.28–0.71) | <.001 | −0.02 (−0.04 to −0.01) | .003 | −0.03 (−0.04 to −0.02) | <.001 |
| E wave velocity (m/s) | 237 | 20.19 (12.72–27.66) | <.001 | −1.77 (−2.28 to −1.26) | <.001 | −1.63 (−2.07 to −1.18) | <.001 |
| A wave velocity (m/s) | 236 | 14.18 (3.94–24.43) | .007 | −0.96 (−1.69 to −0.23) | .010 | −0.81 (−1.45 to −0.17) | .014 |
| E : A ratio | 236 | 6.11 (0.58–11.63) | .030 | −0.82 (−1.20 to −0.44) | <.001 | −0.77 (−1.10 to −0.43) | <.001 |
| Wall stress | 255 | 1.05 (−2.87 to 4.97) | .598 | −0.47 (−0.74 to −0.20) | .001 | −0.49 (−0.74 to −0.26) | <.001 |
HR, heart rate; CI, confidence interval; VVTI, vasovagal tone index; CKCS, Cavalier King Charles Spaniel; F, female; M, male; cTnI, cardiac troponin I; NT‐proBNP, N‐terminal probrain natriuretic peptide; BP, blood pressure; bpm, beats per minute; ECG, electrocardiogram; SA, sinus arrhythmia; SR, sinus rhythm; LA : Ao, left atrium to aorta ratio; LVEDDN, left ventricular end‐diastolic internal dimension normalized; FS, fractional shortening.
Shows the reference category for the dichotomous variables.
Table 3.
Final multivariable models for the assessment of independent factors associated with HR and both VVTIs.
| HR | VVTI20 | VVTI60 | ||||
|---|---|---|---|---|---|---|
| Estimate (95% CI) | P‐Value | Estimate (95% CI) | P‐Value | Estimate (95% CI) | P‐Value | |
| CKCS (yes/no*) | 12.99 (7.35–18.64) | <.001 | −0.54 (−0.89 to −0.17) | .003 | −0.61 (−0.92 to −0.30) | <.001 |
| Treatment (yes/no*) | 7.05 (2.89–11.21) | .001 | — | NS | — | NS |
| LogNTpro‐BNP | 5.68 (1.01–10.34) | .017 | −0.53 (−0.91 to −0.15) | .006 | −0.42 (−0.75 to −0.09) | .013 |
| E : A ratio | — | NS | −0.46 (−0.87 to −0.05) | .028 | −0.42 (−0.77 to −0.07) | .020 |
| FS | 0.37 (0.13–0.60) | .002 | −0.02 (−0.04 to −0.002) | .031 | −0.02 (−0.04 to −0.001) | .005 |
HR, heart rate; VVTI, vasovagal tone index; CI, confidence interval; CKCS, Cavalier King Charles Spaniel; NS, nonsignificant; NT‐proBNP, N‐terminal probrain natriuretic peptide; FS, fractional shortening. *Shows the reference category for the dichotomous variables.
For the assessment of the progression of HR, VVTI20, and VVTI60 over time, 102 dogs met the inclusion criteria: 28 dogs in Group 1, 40 dogs in Group 2, and 34 dogs in Group 3 (Table 1). Repeated measures linear models showed that HR was different between the 3 study groups (P < .001) but there was no overall difference between the visits (P = .091). When age and breed were included in the model, only group class (P < .001) and being a CKCS (P = .016) were independently associated with a higher HR. Posthoc analysis showed that the HR for the Group 3 was significantly lower than the other 2 groups at each visit and that Group 1 had higher HR at visit ‐1 than at visit ‐3 (P = .046) (Table 4).
Table 4.
Estimated marginal mean ± SD for heart rate for each group and visit. A significant difference was found between the groups and visits with different letters.
| Visit ‐3 | Visit ‐2 | Visit ‐1 | Overall | |
|---|---|---|---|---|
| Group 1 | 135 ± 29.76 | 136 ± 21.70 | 143 ± 24.09 |
138 ± 25.30 A |
| Group 2 | 127 ± 23.17 | 128 ± 26.82 | 129 ± 28.30 |
128 ± 26.02 A |
| Group 3 | 113 ± 22.73 | 111 ± 22.48 | 115 ± 22.60 |
113 ± 21.63 B |
| Overall |
125 ± 24.94 a |
125 ± 24.74 a |
129 ± 24.94 b |
Repeated measure models showed that VVTI20 was different between the 3 study groups (P < .001), but no overall difference was found between the 3 visits (P = .672). When age and breed were included into the model, only group class (P < .001) and being a CKCS (P = .002) were independently associated with a lower VVTI20. Posthoc analysis showed that the VVTI20 for Group 3 was significantly higher than the other 2 study groups at each visit, but the other 2 groups were not different at any visit (Table 5).
Table 5.
Estimated marginal mean ± SD for VVTI20 for each group and visit. A significant difference was found between the groups with different letters.
| Visit ‐3 | Visit ‐2 | Visit ‐1 | Overall | |
|---|---|---|---|---|
| Group 1 | 7.16 ± 1.75 | 6.76 ± 1.46 | 7.07 ± 1.11 |
7.00 ± 1.46 A |
| Group 2 | 7.42 ± 1.34 | 7.38 ± 1.77 | 7.33 ± 1.78 |
7.37 ± 1.63 A |
| Group 3 | 8.24 ± 1.58 | 8.69 ± 1.58 | 8.07 ± 1.59 |
8.33 ± 1.22 B |
| Overall |
7.60 ± 1.63 a |
7.62 ± 1.59 a |
7.47 ± 1.64 a |
VVTI, vasovagal tone index.
Similarly, VVTI60 was different between groups (P < .001), but no overall difference was found between visits (P = .656). When age and breed were included into the model, only group class (P < .001) and being a CKCS (P < .001) were independently associated with lower VVTI60. Posthoc analysis showed that all 3 groups had significantly different VVTI60, with the Group 3 having the highest VVTI60 and the Group 1 having the lowest (Table 6). Furthermore, dogs suffering cardiac death had significantly lower VVTI60 than dogs with noncardiac death only on visit ‐2 (P = .027) but not on visits ‐3 or ‐1, but there were no differences between visits for any group.
Table 6.
Estimated marginal mean ± SD for VVTI60 for each group and visit. A significant difference was found between the groups with different letters.
| Visit ‐3 | Visit ‐2 | Visit ‐1 | Overall | |
|---|---|---|---|---|
| Group 1 | 7.39 ± 1.47 | 6.84 ± 1.52 | 7.06 ± 0.93 |
6.97 ± 1.54 A |
| Group 2 | 7.64 ± 1.20 | 7.65 ± 1.35 | 7.53 ± 1.50 |
7.61 ± 1.34 B |
| Group 3 | 8.58 ± 1.44 | 8.88 ± 1.44 | 8.42 ± 1.46 |
8.63 ± 1.38 C |
| Overall |
7.76 ± 1.48 a |
7.79 ± 1.45 a |
7.65 ± 1.48 a |
VVTI, vasovagal tone index.
The assessment of the accuracy of these 3 parameters to predict cardiac mortality and all‐cause mortality was assessed as the AUC for the ROC curves (Table 7). The variable with the highest AUC is VVTI60 at visit ‐2, with a sensitivity = 0.63 and specificity = 0.75 for the VVTI60 = 7.72. With this cut‐off value, the negative predictive value of the test for cardiac mortality on visit ‐2 is 0.95.
Table 7.
Area under the curve for the receiver operator characteristic curve at the different visits for HR, VVTI20, and VVTI60.
| All‐Cause Mortality | Cardiac Mortality | |
|---|---|---|
| HR | ||
| Visit ‐3 | AUC = 0.693 (0.587–0.799), P = .002 | AUC = 0.639 (0.514–0.765), P = .034 |
| Visit ‐2 | AUC = 0.728 (0.621–0.834), P < .001 | AUC = 0.683 (0.572–0.795), P = .004 |
| Visit ‐1 | AUC = 0.716 (0.612–0.820), P < .001 | AUC = 0.725 (0.619–0.830), P = .001 |
| VVTI20 | ||
| Visit ‐3 | AUC = 0.693 (0.587–0.799), P = .002 | AUC = 0.598 (0.467–0.730), P = .140 |
| Visit ‐2 | AUC = 0.741 (0.642–0.839), P < .001 | AUC = 0.700 (0.591–0.808), P = .002 |
| Visit ‐1 | AUC = 0.638 (0.519–0.757), P = .028 | AUC = 0.629 (0.512–0.746), P = .053 |
| VVTI60 | ||
| Visit ‐3 | AUC = 0.702 (0.593–0.811), P = .001 | AUC = 0.660 (0.536–0.784), P = .016 |
| Visit ‐2 | AUC = 0.772 (0.675–0.869), P < .001 | AUC = 0.736 (0.630–0.842), P < .001 |
| Visit ‐1 | AUC = 0.731 (0.628–0.834), P < .001 | AUC = 0.691 (0.581–0.802), P = .004 |
HR, heart rate; VVTI, vasovagal tone index; AUC, area under the curve.
Discussion
The main hypotheses of this longitudinal study of HR and HRV in dogs with DMVD were confirmed. HR increases and HRV decreases in dogs before cardiac death, at least 18 months before this occurs. In addition, and contrary to our original hypothesis, dogs dying for other reasons also have increased HR and reduced VVTI.
The longitudinal evaluation of dogs with DMVD for which we knew the subsequent outcome allowed us to construct repeated measures models (as a form of linear mixed effects model). This model controls for the influence of the variability caused by the random effects (or the individual variability in our case), letting us evaluate more precisely the effect of the fixed effect variables. This model showed that there are several variables that covary with HR and VVTI in a linear fashion (Table 2). Some of these variables are known markers of DMVD severity in dogs (ie, LVEDDN, LA : Ao ratio, NT‐proBNP). This means that VVTI might also be useful as a relatively easy and inexpensive biomarker of DMVD severity if other more specialized techniques were not available.
The multivariable model for factors affecting VVTI showed that CKCS have lower VVTI than non‐CKCS once age, weight, and other parameters were controlled for. In addition, only group and breed independently affected the progression of HR and VVTI over time. A related finding has already been shown by Rasmussen et al17 who showed that CKCS have lower HRV. In addition, a recent cross sectional study evaluating urinary catecholamines in healthy dogs showed breed differences in blood pressure, HR, and also in both epinephrine to creatinine ratio and norepinephrine to creatinine ratio,18 implying breed variation in autonomic tone.
The other 3 factors derived from our multivariable model that affect VVTI independently (NT‐proBNP, E : A ratio and FS) covaried with the left ventricular diastolic dimension, which has been demonstrated in the past to be an independent predictor of mortality in dogs with DMVD.14, 16 To prove this point, the model was constructed forcing in LVEDDN, which resulted in the other 3 variables no longer remaining in the model; however, the variance of the residuals of the model was larger for this simpler model including only CKCS and LVEDDN (data not shown).
Another interesting observation is that dogs in Group 2 also had a significant increase in HR and reduction in VVTI before they died. One could argue that these dogs represent an older population; however (as shown in Table 1), these dogs are at similar stages of DMVD than Group 3 dogs and, when age was controlled for in the model, there was still a significant difference between Group 2 and Group 3. This implies that dogs dying of noncardiac reasons also suffer a modulation of their autonomic nervous system independent of their DMVD severity. As shown in previous literature, this finding reinforces that HR and VVTI are not specific markers of cardiac disease but rather increase in response to various diseases that may result in death.16
This study also shows that dogs with DMVD have a low frequency of arrhythmias compared to other cardiac conditions, with 19% of dogs showing some form of ectopic activity but only 1.6% of dogs developing atrial fibrillation in the course of their disease. Previous reports describe arrhythmia frequencies between 2%19 and 26%,20 and similarly, very few of these were symptomatic. In this study, in all cases with atrial fibrillation, the arrhythmia occurred shortly before cardiac death, and only 1 of these 4 dogs was seen more than once after the onset of atrial fibrillation before dying; as dogs were followed up at 6 months intervals, this means that only 1 dog in atrial fibrillation survived for longer than 6 months. This confirms the general suspicion that DMVD is a disease with a low prevalence of arrhythmia occurrence and supports the assumption that atrial fibrillation may be regarded as a poor prognostic indicator, or at least be suggestive of more advanced stages.13
The additional hypothesis that VVTI calculated over a longer period would help with a better discrimination was also demonstrated in this study. We evaluated VVTI measured over 20 and 60 heart beats. Both measurements are highly correlated (r = 0.92). However, inspection of Figures 2 and 3 suggests that VVTI60 discriminates more effectively between the 3 study groups than VVTI20, and further analysis shows that this is indeed the case (Tables 5 and 6). Moreover, the variance of the residuals as a measure of the goodness of fit for the two multivariable models shows that the model is slightly improved with the assessment of VVTI over a longer period (data not shown). The implication is that VVTI60 allowed us to observe a significant difference between groups at visit ‐2 for Group 1 compared to Group 2 (P = .026). Moreover, VVTI60 at visit ‐2 was the test that possessed the greatest AUC for cardiac mortality. This was caused by a low sensitivity and higher specificity, which coincides with previous observations about this test being influenced by other sources of activation of sympathetic nervous system (e.g., fear, stress). This translated to a high negative predictive value, which implies that higher VVTI60 values can be interpreted as an indicator of a good prognosis.
This study accounts for several limitations. Development of a comorbiditiy during the study period was not an exclusion criterion and this, certainly influenced the results, most likely reducing differences between groups. It is common that elderly dogs suffer several diseases at a time, and many of these can affect HR and VVTI, as it is reflected by the changes in Group 2. However, to reflect the normal situation in practice and to avoid selection bias, we deemed it necessary to keep these dogs in the analysis. Another limitation is that dogs were included in the study from detection of the murmur; this meant that dogs did not present all at the same stage of DMVD and therefore their follow‐up was not the same for all dogs. Also, the cause of death was obtained from the clinical notes of the practices and description of the owners; in general, a clear reason of death was given, but misclassification could have occurred and, finally, this could influence the results.
In summary, longitudinal assessment of VVTI suggested that vagal withdrawal occurs in small breed dogs at least a year before they die, followed by a further significant increase in their HR 6 months later. This may have implications on the use of VVTI as a biomarker because of its high negative predictive value.
Acknowledgments
This manuscript complies with the Royal Veterinary College's Good Research Practice Policy on Publications (manuscript number CSS_00578).
Conflict of Interest Declaration: The authors disclose no conflict of interest.
Footnotes
Microsoft Office Excel 2007
IBM SPSS Statistics 20, Armonk, NY
GraphPad Prism 6, San Diego, CA
References
- 1. Häggström J, Höglund K, Borgarelli M. An update on treatment and prognostic indicators in canine myxomatous mitral valve disease. J Small Anim Pract 2009;50(Suppl 1):25–33. [DOI] [PubMed] [Google Scholar]
- 2. Buchanan JW. Chronic valvular disease (endocardiosis) in dogs. Adv Vet Sci Comp Med 1977;21:75–106. [PubMed] [Google Scholar]
- 3. Parker HG, Kilroy‐Glynn P. Myxomatous mitral valve disease in dogs: Does size matter? J Vet Cardiol 2012;14:19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Borgarelli M, Häggström J. Canine degenerative myxomatous mitral valve disease: Natural history, clinical presentation and therapy. Vet Clin North Am Small Anim Pract 2010;40:651–663. [DOI] [PubMed] [Google Scholar]
- 5. Opie LH. Heart physiology. In: From Cell to Circulation, 4th ed Baltimore, MD: Lippincott Williams & Wilkins; 2004. [Google Scholar]
- 6. Oliveira MS, Muzzi RA, Araújo RB, et al. Heart rate variability parameters of myxomatous mitral valve disease in dogs with and without heart failure obtained using 24‐hour Holter electrocardiography. Vet Rec 2012;170:622. [DOI] [PubMed] [Google Scholar]
- 7. Tallaj J, Wei CC, Hankes GH, et al. Beta1‐adrenergic receptor blockade attenuates angiotensin II‐mediated catecholamine release into the cardiac interstitium in mitral regurgitation. Circulation 2003;108:225–230. [DOI] [PubMed] [Google Scholar]
- 8. Akselrod S, Gordon D, Ubel FA, et al. Power spectrum analysis of heart rate fluctuation: A quantitative probe of beat‐to‐beat cardiovascular control. Science 1981;213:220–222. [DOI] [PubMed] [Google Scholar]
- 9. Berntson GG, Bigger JT Jr, Eckberg DL, et al. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology 1997;34:623–648. [DOI] [PubMed] [Google Scholar]
- 10. Häggström J, Hamlin RL, Hansson K, et al. Heart rate variability in relation to severity of mitral regurgitation in Cavalier King Charles Spaniels. J Small Anim Pract 1996;37:69–75. [DOI] [PubMed] [Google Scholar]
- 11. Doxey S, Boswood A. Differences between breeds of dog in a measure of heart rate variability. Vet Rec 2004;154:713–717. [DOI] [PubMed] [Google Scholar]
- 12. Boswood A, Murphy A. The effect of heart disease, heart failure and diuresis on selected laboratory and electrocardiographic parameters in dogs. J Vet Cardiol 2006;8:1–9. [DOI] [PubMed] [Google Scholar]
- 13. Rasmussen CE, Falk T, Zois NE, et al. Heart rate, heart rate variability, and arrhythmias in dogs with myxomatous mitral valve disease. J Vet Intern Med 2012;26:76–84. [DOI] [PubMed] [Google Scholar]
- 14. Moonarmart W, Boswood A, Luis Fuentes V, et al. N‐terminal pro B‐type natriuretic peptide and left ventricular diameter independently predict mortality in dogs with mitral valve disease. J Small Anim Pract 2010;51:84–96. [DOI] [PubMed] [Google Scholar]
- 15. Martinez Pereira Y, Woolley R, Culshaw G, et al. The vasovagal tonus index as a prognostic indicator in dogs with dilated cardiomyopathy. J Small Anim Pract 2008;49:587–592. [DOI] [PubMed] [Google Scholar]
- 16. Hezzell MJ, Boswood A, Chang YM, et al. The combined prognostic potential of serum high‐sensitivity cardiac troponin I and N‐terminal pro‐B‐type natriuretic peptide concentrations in dogs with degenerative mitral valve disease. J Vet Intern Med 2012;26:302–311. [DOI] [PubMed] [Google Scholar]
- 17. Rasmussen CE, Vesterholm S, Ludvigsen TP, et al. Holter monitoring in clinically healthy Cavalier King Charles Spaniels, Wire‐haired Dachshunds, and Cairn Terriers. J Vet Intern Med 2011;25:460–468. [DOI] [PubMed] [Google Scholar]
- 18. Höglund K, Hanås S, Carnabuci C, et al. Blood pressure, heart rate, and urinary catecholamines in healthy dogs subjected to different clinical settings. J Vet Intern Med 2012;26:1300–1308. [DOI] [PubMed] [Google Scholar]
- 19. Borgarelli M, Savarino P, Crosara S, et al. Survival characteristics and prognostic variables of dogs with mitral regurgitation attributable to myxomatous valve disease. J Vet Intern Med 2008;22:120–128. [DOI] [PubMed] [Google Scholar]
- 20. Häggström J, Boswood A, O'Grady M, et al. Effect of pimobendan or benazepril hydrochloride on survival times in dogs with congestive heart failure caused by naturally occurring myxomatous mitral valve disease: The QUEST study. J Vet Intern Med 2008;22:1124–1135. [DOI] [PubMed] [Google Scholar]


