Abstract
Background
In the dog, the normal estrous cycle includes a prolonged luteal phase. Progesterone stimulates local canine mammary growth hormone (GH) production, which may act systemically and contribute to insulin resistance. Swedish Elkhounds are predisposed to progesterone‐related diabetes mellitus, and the relationship among insulin resistance, GH, and insulin‐like growth factor I (IGF‐I) is of particular interest.
Objective
To study insulin resistance in relation to GH and IGF‐I in nondiabetic Swedish Elkhounds during diestrus. We also assessed whether alterations in these hormones could predict diestrus‐linked diseases and all‐cause mortality.
Animals
Eighty‐four privately owned female intact Swedish Elkhounds >4 years of age.
Methods
Blood sampling and clinical examination during luteal phase, with a follow‐up questionnaire after 20 months. Insulin resistance was calculated by homeostasis model assessment (HOMA‐IR).
Results
In multivariable regression analysis, GH was positively associated with HOMA‐IR (P = .009). An increase in GH of 1 ng/mL was associated with a 12.7% increase in HOMA‐IR. Moreover, C‐peptide was positively associated with IGF‐I (P = .04), and an increase in C‐peptide of 0.1 ng/mL was associated with a 6.9% increase in IGF‐I. Structural equation modeling supported these results. Twenty‐three animals were found to have previously unrecognized mammary masses and had higher GH (P < .0001) and IGF‐I (P = .007) than dogs without mammary masses (n = 61). There was no association between high GH and IGF‐I concentrations at sampling and future mammary masses.
Conclusion
We showed that GH was strongly associated with insulin resistance in older Swedish Elkhounds during diestrus.
Keywords: Diestrus, GH, IGF‐I, Progesterone
Abbreviations
- BCS
body condition score
- DM
diabetes mellitus
- GH
growth hormone
- HOMA‐IR
homeostasis model assessment insulin resistance
- IGFBPs
insulin‐like growth factor–binding proteins
- IGF‐I
insulin‐like growth factor I
- IQR
interquartile range
- SEM
structural equation modeling
In contrast to other species, in the dog, progestagen‐induced growth hormone (GH) production by the mammary gland1 contributes substantially to circulating GH concentrations. Mammary‐derived GH thus may have systemic effects.1, 2 Circulating GH concentrations increase in response to endogenous progesterone production during diestrus3 and after exogenous progesterone administration to ovariohysterectomized animals.2 Growth hormone is a regulator of circulating insulin‐like growth factor I (IGF‐I), which is synthesized mainly by the liver and provides negative feedback on GH production by the pituitary gland. Growth hormone has a late anti‐insulin effect that leads to hyperglycemia and insulin resistance,4 which may contribute to the development of diabetes mellitus (DM).5
In addition to its direct effects on glucose homeostasis, GH acts indirectly through IGF‐I to promote cell growth and prevent apoptosis. In humans, increased blood concentrations of IGF‐I are associated with increased risk of prostate, breast and colorectal neoplasms.6 Local GH production may also play a role in the pathogenesis of mammary tumors in dogs.7 In 1 study, inflammatory mammary carcinomas in dogs were most commonly first palpated in diestrus.8 Intact female dogs with malignant mammary tumors have been shown to have higher GH and IGF‐I concentrations during anestrus than healthy controls.9
The incidence of DM in the adult Swedish dog population is 13 cases per 10,000 years at risk, with Swedish Elkhounds being particularly predisposed with an incidence of 45 cases per 10,000 years at risk.10 The increased incidence in Elkhounds in Sweden is likely a consequence of both genetic and environmental factors. Almost all cases of DM in Swedish Elkhounds occur in intact females during diestrus or pregnancy.10, 11 C‐peptide, which is cosecreted with insulin from the pancreas caused by cleavage of proinsulin, is higher in clinically healthy Elkhounds during diestrus than during anestrus,12 and those that develop DM have higher GH concentrations at diagnosis compared with healthy diestrus controls.11 Ovariohysterectomy is used in the management of the disease, with glucose concentrations normalizing after the procedure in 46% of cases.11
The aim of this study was to further clarify the relationship among GH, IGF‐I, and insulin resistance, as well as determinants of IGF‐I concentrations in healthy intact Swedish Elkhounds during diestrus. Because a number of animals were found to have previously unrecognized mammary masses, we also assessed the role of GH and IGF‐I as potential biomarkers of mammary masses as well as future diseases related to diestrus (mammary masses and pyometra) and mortality.
Materials and Methods
Study Design
All owners of female Swedish Elkhounds (“Jämthund”) registered at birth in the Swedish Kennel Club between 1997 and 2002 were invited by personal letters in 2008 and 2009 (n = 2,723). These dogs were recruited as controls for a larger project, the Swedish Canine Diabetes Mellitus Project.11, 12 Owners of healthy dogs willing to participate (n = 175) in the study were instructed to bring their dogs to their local veterinarians 3–8 weeks after the end of estral bleeding. Dogs were to be fasted for at least 12 hours before the visit. Owners filled in health questionnaires and provided written consent before blood sampling. The dogs underwent clinical examination following a specific protocol. Body condition score (BCS) was recorded by the veterinarian using a 3‐grade scale (underweight, normal, overweight). Dogs were included in this study if they were intact bitches, had been fasted appropriately, and were considered healthy. Some animals were found to have previously unrecognized mammary masses, and because of the relationship between the GH/IGF system and tumors, it was decided not to exclude these dogs and they were considered a separate group. Dogs were excluded if any abnormalities other than mammary masses were found during veterinary clinical examination, the dog was on any medication, had a known disease or pregnancy, abnormal clinical signs according to the owner, blood glucose concentration >126 mg/dL, fructosamine >375 μmol/L, or some combination of these findings (Fig 1). Because of low progesterone concentrations found in 2 dogs sampled at 8 weeks after the end of estral bleeding, only dogs sampled 3–7 weeks after the end of estral bleeding were included in this study. In 2010, a health questionnaire was sent out to the owners of enrolled dogs. The owners were asked about the health status of their dogs and were given written instructions on how to palpate the mammary glands.
Figure 1.
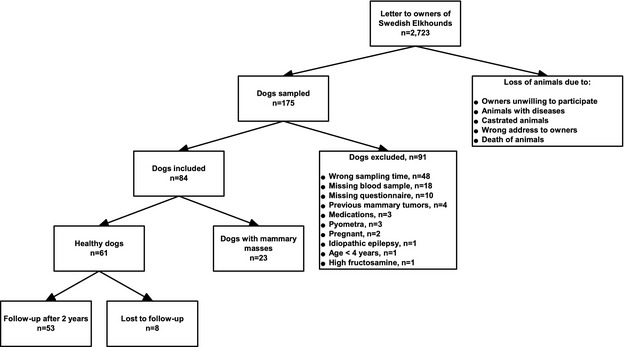
Flowchart of study design.
The study was approved by the Swedish Animal Ethical Committee (no. C267/5) and the Swedish Animal Welfare Agency (no. 2005‐2038).
Sample Handling
Blood samples were drawn from the cephalic vein into tubes without anticoagulant. Within an hour after sampling, serum was separated and sent at ambient temperature to the Clinical Pathology Laboratory, University Animal Hospital at the Swedish University of Agricultural Sciences. Upon arrival at the laboratory, samples were analyzed for glucose and fructosamine and then stored at −80°C until further analysis. Samples analyzed at a second laboratory (Clinical Pathology Laboratory, Utrecht University) were sent on dry ice from the primary laboratory.
Analytical Methods
Growth hormone was analyzed singly at the Clinical Pathology Laboratory, Utrecht University, using a commercially available RIA for porcine and canine GH1 as described by Beijerink et al13 with an interassay coefficient of variation (CV) of 7.6%. Intra‐assay CV was 5%. Insulin‐like growth factor I was analyzed in duplicate using a commercially available ELISA2 using excess IGF‐II for preventing interference of IGF‐binding proteins (IGFBPs). For the validation of this assay, canine serum pools were diluted and spiked with recombinant human IGFBP‐1,3 ‐24 and 35 at total concentrations of 8 and 30 mg/L. For comparison, the serum pools, as well as a sample from an acromegalic dog, were diluted without any additives. The recovery with added IGFBPs was 99–109 and 54–61%, respectively. Diluted serum pools and serum from an acromegalic dog without any additives showed a recovery of 95–113 and 100–106%, respectively. The recovery after adding recombinant human IGF‐I to a canine serum sample was 103%. We also performed acid gel chromatography in a healthy dog to separate IGFBPs from IGF‐I according to the protocol of Mohan and Baylink14 except that serum was mixed with 1 M acetic acid and incubated for 30 minutes before its application to the column. Serum after removal of IGFBPs by acid gel chromatography was compared with nonchromatographed serum. Serum without IGFBPs showed a recovery of 98% compared with serum with IGFBPs when run in the IGF‐I ELISA. Samples with high, medium, and low concentrations showed intra‐ and interassay CV of 2.1–9.9 and 3.4–8.5%, respectively. There was no cross‐reactivity with canine C‐peptide6 and purified porcine insulin,7 which is identical to canine insulin.15
C‐peptide was measured using a commercially available species‐specific radioimmunoassay for canine C‐peptide.8 According to the manufacturer, recovery after dilution and addition of canine C‐peptide was 95–101 and 95–111%, respectively. Intra‐assay CV was 4.1–7.6% and interassay CV was 7%. The instructions given for the kit were followed, except that the protease inhibitor aprotinin9 was added just before analysis instead of immediately after sampling as described earlier by Fall et al.16 In a stability study comparing 3 samples stored with and without aprotinin at room temperature for up to 48 h, 2 samples without aprotinin were stable after 24 h and the concentration of the third had decreased by 24%. After 48 h, the concentration of 1 sample had decreased by 23%; 1 sample was stable; and the concentration of the third sample had increased by 17%.17 Samples with concentrations under the detection limit (0.17 ng/mL) were assigned a concentration of 0.085 ng/mL.
Serum progesterone was analyzed by a chemiluminescent immunoassay previously validated in the dog.10 , 18 Intra‐ and interassay CV were 3.4–7.5 and 4.2–7.4%, respectively. Serum glucose concentrations were determined by a glucose hexokinase method.11 Fructosamine was measured by a colorimetric assay, based on the ability of ketoamines to reduce nitrotetrazolium blue formazans in an alkaline medium.12 Intra‐ and interassay CV were 0.6 and 3%, respectively. Recovery upon dilution of samples down to 1 : 8 was 99–118%.
Statistical Methods
Descriptive Statistics
Continuous variables were checked for normality by examining normal probability plots. Descriptive statistics are presented as mean ± standard deviation (SD) and median and interquartile range. Time from when the owner noticed the last bleeding until sampling is given in days. Minitab 16 Statistical Software,13 STATA 12,14 and SAS 9.315 were used for statistical calculations.
The dogs were categorized into 2 groups: healthy dogs and dogs with mammary masses at baseline sampling. The nonparametrical Mann‐Whitney U‐test was used for comparing these groups when considering continuous variables.
Causal Web
A causal web with hypothesized associations in diestrous dogs was built based on published studies (Fig 2). Because of the number of variables and sample size in the study, the number of causal relationships had to be restricted and thus only the relationships considered to be strongest, based on published knowledge, were included in the causal web. Insulin resistance was estimated using homeostasis model assessment (HOMA‐IR) by the nonlinear method developed by Levy et al19 using C‐peptide and glucose. This approach has been used previously in dogs.12
Figure 2.
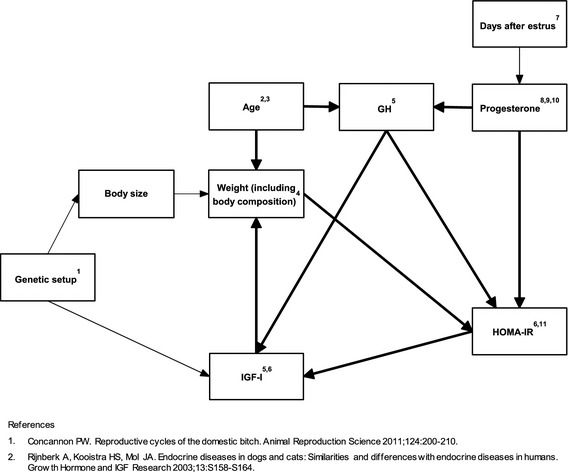
Hypothetical causal pathways in GH axis and insulin resistance in Swedish Elkhounds. The bold paths shown in the web were included in structural equation modeling.
Multivariable Linear Regression
Only healthy dogs without mammary masses were used for regression analysis. We fitted a linear regression model of the effect of GH on insulin resistance. Potential confounders of the effect of GH on insulin resistance included progesterone and weight (Fig 2). Because progesterone was only available in 69% of the samples, a multiple imputation procedure20 based on days after estrus was used for the missing values using the “mi impute” option in STATA12 and 100 imputations. Regression modeling was done using the “mi estimate” option in STATA12. Estimation of the variance explained in the models was done with the “mibeta” command.
Insulin‐like growth factor I is often used as a proxy for GH measurement in the clinical setting and it is important to know what factors could confound the association of GH with IGF‐I. On the basis of references used for the causal web (Fig 2), we assessed C‐peptide as a potential confounder of this association.
The distributions of HOMA‐IR and IGF‐I were highly skewed: preliminary models showed non‐normality of the residuals and the dependent variables were therefore transformed to the natural logarithmic scale before multivariable modeling. Regression models were built including all potential confounders in the models. The cutoff for statistical significance was set to P < .05. We considered a covariate as a significant confounder if the elimination of the covariate in the model changed the beta coefficient of GH by >20%.
The underlying assumptions of homoscedasticity were evaluated by examining plots of standardized residuals against predicted values. Normality of residuals was examined by plotting QQ‐plots and histograms. Linearity of predictor‐outcome was examined by plotting standardized residuals predictor over values of each predictor.
Structural Equation Modeling
Structural equation modeling (SEM) was used to further investigate all relationships among variables in the causal web (Fig 2). The pathway analysis was performed using the CALIS procedure in SAS 9.3 and was developed by excluding variables in a stepwise elimination procedure by considering their level of significance, adjusted goodness of fit, and Akaike information criterion. The level of significance for excluding a path was set to .20. Only healthy dogs without mammary masses were used for analysis. The distribution of IGF‐I, C‐peptide, progesterone, and GH was highly skewed and was therefore transformed to the natural logarithmic scale before modeling.
Prediction of Development of Diestrous Diseases and Mortality
Serum concentrations of GH and IGF‐I, weight, and age were compared with the Mann‐Whitney U‐test between dogs that had developed (1) mammary masses, (2) pyometra, and (3) died at follow‐up and those that had not developed any disease or died. For this analysis, dogs with mammary masses at baseline sampling were excluded (n = 23). Animals that developed ≥2 of the outcomes could contribute to more than 1 group. Time of sampling after the end of estral bleeding was compared among groups using Kruskall‐Wallis Test.
Results
Dogs
Figure 1 illustrates the recruitment process. Of the 175 dogs initially participating in the study, 84 met the final inclusion and exclusion criteria. Two dogs had missing glucose concentrations but normal fructosamine concentration and no clinical signs of diseases and were considered healthy and were included. Six dogs were scored as overweight, 76 as having normal weight, and 2 had missing information regarding BCS. No dog was scored as underweight.
Sixty‐one dogs were considered healthy based on veterinary examination and owner health questionnaire. Twenty‐three dogs were found to have newly palpable mammary masses and no other clinical signs. Eighty‐seven percent of the dogs were considered healthy at baseline sampling and their owners responded to the follow‐up questionnaire (n = 53). Mean (±SD) follow‐up time was 20 (8.9) months.
Descriptive Statistics
Descriptive statistics for measured biomarkers, age, weight, and time after estrus stratified on healthy dogs and dogs with mammary masses are shown in Table 1. Growth hormone (P < .0001) and IGF‐I (P = .007) concentrations were higher in dogs with mammary masses than in healthy dogs. There were no differences in serum concentrations of glucose or C‐peptide between the 2 groups.
Table 1.
Descriptive data and comparison of groups in 84 Swedish Elkhounds sampled during diestrus. Univariate analysis was performed by Mann‐Whitney U‐test.
| Healthy (n = 61) | Mammary Masses (n = 23) | Mann‐Whitney U‐Test | |||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | N | Mean (SD) | Median (IQR) | N | P value | |
| Glucose (mg/dL) | 90 (10.3) | 92 (83–99) | 59 | 91 (10.3) | 90 (83–94) | 23 | .76 |
| C‐peptide (ng/mL) | 0.37 (0.28) | 0.29 (0.20–0.50) | 44 | 0.55 (0.48) | 0.35 (0.21–0.78) | 21 | .21 |
| HOMA‐IR | 0.8 (0.6) | 0.6 (0.4–1.0) | 43 | 1.2 (1.1) | 0.74 (0.45–1.74) | 21 | .17 |
| Fructosamine (μmol/L) | 304 (29.7) | 300 (285–329) | 61 | 311 (29.9) | 312 (286–340) | 23 | .33 |
| GH (ng/mL) | 5.8 (2.5) | 5.2 (4.2–6.6) | 53 | 11.8 (11.1) | 8.6 (6.7–13.0) | 22 | <.0001 |
| IGF‐I (ng/mL) | 387 (199) | 355 (247–471) | 61 | 615 (353) | 521 (325–923) | 23 | .007 |
| Progesterone (nmol/L) | 26.5 (15.7) | 22.9 (15.9–37.7) | 38 | 33.6 (24.6) | 19.0 (13.8–58.2) | 9 | .79 |
| Time after estrus (days) | 35 (6.5) | 34 (30–41) | 61 | 33 (6.7) | 31 (28–38) | 23 | .25 |
| Weight (kg) | 25.6 (3.5) | 25.0 (23.0–28.0) | 56 | 26.9 (3.3) | 26.1 (24.0–29.6) | 22 | .23 |
| Age (years) | 7.5 (1.4) | 7.6 (6.6–8.2) | 61 | 8.2 (1.2) | 8.0 (7.1–9.0) | 23 | .06 |
Multivariable Linear Regression
The results from the multivariable regressions with HOMA‐IR and IGF‐I as outcome are shown in Table 2. Growth hormone was positively associated with HOMA‐IR (P = .009). Progesterone and weight had no significant association with HOMA‐IR and did not confound the relationship between GH and HOMA‐IR. An increase in GH of 1 ng/mL was associated with an increase in HOMA‐IR of 12.7%. C‐peptide (P = .04) was associated with IGF‐I. Growth hormone was a confounder for the association between IGF‐I and C‐peptide, but C‐peptide was still significant when GH was kept in the model. An increase of 0.1 ng/mL in C‐peptide was associated with an estimated increase in IGF‐I of 6.9%. For the models with outcome HOMA‐IR and IGF‐I, the variance explained by the models (adj. R 2) was 15.3 and 21.9%, respectively. The assumptions of homoscedasticity, normality, and linearity were fulfilled. No interactions were seen between significant variables.
Table 2.
Final linear multivariable regression models for associations with insulin‐like growth factor I (lnIGF‐I) and insulin resistance (lnHOMA‐IR) in Swedish Elkhounds.
| Model | Predictor | ß | eß a | 95% CI | P | R 2 adj (%) |
|---|---|---|---|---|---|---|
| lnHOMA‐IR (n = 38) | GH | 0.12 | 1.13 | 0.31; 0.21 | .009 | 15.3 |
| Progesterone | 0.007 | 1.01 | −0.12; 0.027 | .4 | ||
| Weight | 0.006 | 1.01 | −0.07; 0.08 | .9 | ||
| lnIGF‐I (n = 43) | C‐peptide | 0.67 | 1.94 | 0.05; 1.28 | .04 | 21.9 |
| GH | 0.038 | 1.04 | −0.03; 0.1 | .2 |
eß = change in IGF‐I on multiplicative scale for 1 unit change in the independent variable.
Structural Equation Modeling
The results of the structural equation modeling are shown in Figure 3. Using the cutoff for significance of P < .2, the model showed a positive causal effect of GH on HOMA‐IR (P = .05). Also, a positive effect of HOMA‐IR was seen on IGF‐I (P = .04), and IGF‐I had a positive effect on weight (P = .17).
Figure 3.
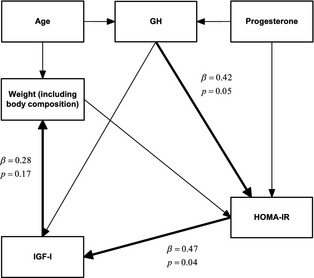
Results of structural equation modeling in Swedish Elkhounds (n = 37) based on the causal web depicted in Fig 2. Significance was set to P < .2. Significant paths are bold.
Prediction of Development of Diestrous Diseases and Mortality
Of the 61 questionnaires sent to owners of healthy dogs, 8 were not returned (Fig 1). At follow‐up, according to the questionnaire, 9 dogs had developed new mammary masses (4 diagnosed by a veterinarian and 5 by palpation by the owner); 6 dogs had developed pyometra; and 6 had died. These animals were compared with those that were healthy at sampling and follow‐up. As shown in Table 3, there was a tendency for dogs that had died by follow‐up to be older at the time of sampling and for GH to be lower in dogs that had mammary masses at follow‐up. Insulin‐like growth factor I concentrations at sampling did not differ between any of the groups. There was no significant difference between sampling time after the end of estral bleeding among groups. Only 1 dog developed DM.
Table 3.
Follow‐up of healthy Swedish Elkhounds. Mean follow‐up time was 20 months after baseline sampling. Using the values obtained at baseline sampling, Mann‐Whitney U‐test was used to compare groups (developed mammary masses, developed pyometra, dead at follow‐up) with “Healthy at sampling and follow‐up” as control group. Of 61 questionnaires sent out to owners, 8 were not returned.
| Healthy at Follow‐up | N | Developed Mammary Masses | N | P Value | Developed Pyometra | N | P Value | Dead at Follow‐up | N | P Value | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | ||||||||
| IGF‐I (ng/mL) | 392 (245, 477) | 34 | 355 (259, 533) | 9 | .94 | 264 (202, 517) | 6 | .38 | 372 (284, 515) | 6 | .98 |
| GH (ng/mL) | 5.5 (4.1, 6.7) | 29 | 4.2 (2.9, 5.1) | 8 | .03 | 5.4 (3.1, 7.2) | 4 | .84 | 5.6 (3.6, 8.8) | 5 | .92 |
| Weight (kg) | 25.0 (23.3, 28.0) | 33 | 27.4 (25.4–28.6) | 6 | .34 | 26.0 (19.5, 29.0) | 5 | .77 | 23.0 (20.0, 30.0) | 5 | .27 |
| Age (years) | 7.1 (6.4, 7.9) | 34 | 8.1 (7.0–8.8) | 9 | .06 | 8.0 (7.0–9.4) | 6 | .15 | 8.5 (7.3, 10.1) | 6 | .05 |
Discussion
The main findings of this study in nondiabetic, middle‐aged, and older, intact diestrous Swedish Elkhounds are 2‐fold: (1) the independent positive effect of serum GH on fasting measures of insulin resistance; and (2) a positive GH‐independent effect of insulin measured as C‐peptide on IGF‐I, supporting previous observations that there is a relationship between IGF‐I and insulin concentrations.21 The homogenous population studied and the high response rate on the follow‐up questionnaire are considered strengths of this study.
Growth Hormone, Progesterone, and Insulin Resistance
In this study, we found evidence that GH had a positive effect on HOMA‐IR. We did not find evidence of an association between progesterone and HOMA‐IR, which was somewhat surprising because both GH and progesterone are known to increase insulin resistance.22, 23 Progesterone‐induced GH excess is associated with glucose intolerance in dogs,3 but to our knowledge, the independent contribution of mammary GH and progesterone on insulin resistance in diestrous dogs has not previously been evaluated using either SEM or multivariable models. In 1 study, glucose homeostasis was studied in dogs given estradiol and progesterone mimicking hormone concentrations during the 3rd trimester, and the authors concluded that progesterone and estradiol exposure caused insulin resistance.24 Unfortunately, GH was not measured in that study, and therefore it cannot be determined if the insulin resistance was mediated by an increase in GH or not. In 1981, Eigenmann et al25 found that insulin concentrations, but not GH concentrations, were significantly increased after 2 medroxyprogesterone acetate (MPA) injections supporting an effect of progesterone on insulin resistance. Because those dogs were given high doses of MPA, it is possible we would have seen an association between progesterone and HOMA‐IR if animals in our study had been sampled earlier in the luteal phase, when progesterone concentrations are higher.
Increased GH during the first half of the luteal phase may cause insulin resistance.26 Our results support this hypothesis and suggest that GH affects insulin sensitivity even at physiological concentrations. A previous smaller study in Swedish Elkhounds failed to show differences in GH concentrations between anestrus and diestrus, even though progesterone concentrations were significantly different.12
IGF‐I, C‐peptide, and Insulin Resistance
Using multivariable regression analysis, we found a positive association between C‐peptide and IGF‐I, independent of GH. Growth hormone was considered a confounder because it lowered the estimate, but the association was still significant after adjusting for GH. To further investigate this association, we performed SEM analysis by HOMA‐IR, and confirmed a positive effect of HOMA‐IR on IGF‐I.
An association between IGF‐I and insulin concentrations has been described previously in pancreatectomized dogs given exogenous insulin21 and during postprandial hyperinsulinemia in obese Beagle dogs,27 but to our knowledge, no studies have evaluated this relationship in healthy diestrous dogs, taking other parameters into account. Insulin resistance in humans with normal beta cell function is accompanied by an increase in insulin concentrations, which can suppress insulin‐like growth factor–binding protein‐1 and lead to high concentrations of free IGF‐I.28 Insulin also stimulates biosynthesis of GH receptors in the liver,29, 30 increasing hepatic GH sensitivity and therefore IGF‐I production.31 In the normal state, IGF‐I provides negative feedback on pituitary GH synthesis, and thus total IGF‐I production will decrease. In diestrous dogs with autonomous mammary GH production,2 this feedback mechanism may be lacking and we speculate that this leads to a strong positive relationship between insulin resistance and IGF‐I.
A homeostasis model assessment using insulin concentrations has been validated in dogs for the assessment of insulin sensitivity.32 For this study, C‐peptide concentrations were available and we used these results for HOMA‐IR calculations based on the nonlinear method developed by Levy et al.19 We analyzed C‐peptide rather than insulin. Both are cosecreted from the pancreas caused by cleavage of proinsulin, but C‐peptide is reported to be a more reliable marker attributable to low hepatic extraction and longer circulating half‐life.33 A limitation of the C‐peptide measurement was that aprotinin was added to the samples just before analysis and not at sampling. In the absence of aprotinin, the variability was ±20% and the results should be interpreted with caution.
Additional studies on the association between insulin resistance and IGF‐I, along with studies of IGFBPs that have a role in regulating tissue‐specific IGF‐I action,28 will be important to understand the pathogenesis of diseases linked to IGF‐I. The association between insulin and IGF‐I is considered a possible link between insulin resistance and cancer, and is currently under debate as reviewed by Arcidiacono et al.34 The association between IGF‐I and C‐peptide could also be of importance when using IGF‐I for diagnosing growth hormone disorders in dogs with altered glucose homeostasis, but additional studies are needed to clarify this effect.
IGF‐I and GH
Growth hormone is known to increase IGF‐I, which provides negative feedback on pituitary GH, and studies on dogs have shown a positive relationship between these 2 variables.23, 35 The lack of a significant positive causal effect of GH on IGF‐I in this study is likely because of the fact that too few animals were sampled. Another possible reason for the lack of association is that only 1 sample was collected from the animal. Because GH is secreted in a pulsatile pattern, it is possible that multiple sampling may have yielded a different result.
Weight and IGF‐I
We found a positive effect of IGF‐I on body weight when using SEM. A study in dogs has reported a connection between genetic variation in the IGF1 gene and body size, which may explain our results.36 We did not include weight as a predictor of IGF‐I in the multivariable regression analysis, because we hypothesized the effect of weight on IGF‐I to be mediated by insulin resistance. Canine obesity has been correlated with either higher or no effect on IGF‐I concentrations in previous studies.37, 38 In 1 study comparing overweight and lean dogs, both IGF‐I and insulin were increased in obese animals, making it impossible to conclude an independent direct effect of obesity on IGF‐I.37 Because only 6 dogs were classified as overweight, we could not determine the effect of obesity, as opposed to body size, on IGF‐I.
As do all models of causal associations, SEM heavily relies on the a priori assumptions of possible causal relationships. False assumptions will cause false causal inference. A large benefit of SEM is the possibility of including feedback loops. There was a considerable number of missing values in the sampled dogs, which affects power and how many relationships can be incorporated in the SEM analysis. Thus, we considered the special features of diestrous dogs and only modeled relationships based on knowledge of dogs. Because mammary GH production is considered to be autonomous,2 we did not include the negative feedback of IGF‐I on GH. An association between age and IGF‐I has been reported in dogs.39 However, in this study, we regarded this association as a consequence of the relationship between age and GH.40 Insulin‐like growth factor I is considered to improve insulin sensitivity in humans. However, as reviewed by Clemmons41 increased concentrations of IGF‐I decreases GH concentrations because of negative feedback, and it is difficult to establish how much of the improved insulin sensitivity is attributable to the lower GH concentration. It is also difficult to conclude that this effect occurs at physiological concentrations. Thus, we decided not to include this mechanism in our models. Because we did not include all potential relationships in our models, a hidden confounder cannot be excluded.
IGF‐I and Mammary Tumors
Because GH/IGF‐I may have a particular role in the pathogenesis of mammary tumors,7 we investigated concentrations of these hormones in dogs with mammary masses diagnosed at sampling. Mammary tumors can produce GH42 and indeed, we found that GH and IGF‐I were significantly higher in dogs with mammary masses at sampling compared with healthy dogs during diestrus. Queiroga et al9 also showed that female dogs with mammary tumors sampled in anestrus had higher GH concentrations than control dogs. However, in another study, no difference was seen when estrous cycle phase and exogenous progestins were taken into consideration.43 In that study, only few animals were sampled during diestrus. Queiroga et al9 reported higher IGF‐I in dogs with malignant tumors compared with healthy dogs, but serum IGF‐I concentration was not higher in animals with benign masses. A weakness of this study was that no histology was performed. In a study of Swedish dogs with spontaneous mammary masses diagnosed by veterinarians, only 2% were of non‐neoplastic origin.44 Thus, although it is likely that most of the mammary masses were mammary tumors, the proportion of malignant tumors is not known.
Prediction of Future Disease
High serum concentrations of IGF‐I or GH in healthy dogs did not predict which animals developed mammary masses. Indeed, contrary to what we expected, GH tended to be lower in animals that developed mammary masses, and was not explained by the time elapsed from the end of bleeding until sampling. We have no explanation of this finding. However, multiple testing was used, which increases the risk of type I error. When correcting for multiple testing, using the Bonferroni correction, GH was not significant (data not shown).
It has been suggested that animals with a history of malignant mammary tumors should be excluded from prospective studies.45 In our prospective study, we excluded all animals with a history of mammary tumors and this is considered a strength. However, development of mammary masses was based on a questionnaire, and owners were also instructed to palpate their dogs' mammary glands themselves. This might have led to under‐ or overdiagnosis of mammary masses. This misclassification could have affected the estimates, but we expect the misclassification ratio to be equal in all groups and therefore not to cause bias. The frequency of pyometra and DM in this study is likely to be reliable because diagnosis during follow‐up was made by local veterinarians.
Although the results of this study suggest that GH and IGF‐I are not useful predictive markers for development of mammary masses, pyometra, or survival, the power of the analysis was low because of limited sample size and lack of diagnosis date. The power to find a 20% difference in IGF‐I or GH between dogs with mammary masses at follow‐up and healthy dogs at follow‐up was estimated to be 26 and 72% percent, respectively, assuming log‐normal distributions and an alpha of .05. A follow‐up of 20 months is relatively short when considering the development of mammary masses, and future studies should include longer periods. Because the exact date of death was not available, it was not possible to do a survival analysis, which would have increased the power. The external validity of our findings is hard to evaluate, and the initial response rate may be considered low. However, the Swedish Kennel Club's registry is not rigorously updated regarding death of animals, diseases in the animals, and change in addresses. Thus, we deemed the initial participation rate to be acceptable. At follow‐up, a response rate of 87% of the questionnaire is considered high.
Conclusions
In this study, we demonstrate a strong relationship between GH and insulin resistance in older Swedish Elkhounds during diestrus. This relationship may partly contribute to DM development. Furthermore, we showed a positive association between C‐peptide and IGF‐I in diestrous Swedish Elkhounds. To our knowledge, this is the first time that this association has been shown in a homogenous group of diestrous dogs. Insulin‐like growth factor I reference values in veterinary medicine may benefit from adjustment for insulin or C‐peptide concentrations, and this should be considered when diagnosing growth hormone disorders in dogs with altered glucose homeostasis.
Acknowledgments
The authors acknowledge Professor Ian Dohoo and Dr David Morrison for advice on statistical methods. The study was supported by the Agria and Swedish Kennel Club Research Foundation.
Conflict of Interest: Tove Fall and Emma Strage received payment from MSD (Merck) for lecturing.
The study was supported by the Agria and Swedish Kennel Club Research Foundation. Some of these results have been summarized in a student degree project (in Swedish with an English summary). The validation of an ELISA for measuring canine IGF‐I has been presented as an abstract at the 13th Congress of the European Society of Veterinary Clinical Pathology in Dublin 2011
Footnotes
Porcine and Canine GH radioimmunoassay, PGH‐46HK, Linco Research, St. Charles, MO
Mediagnost E20 IGF‐I ELISA, Mediagnost, Reutlingen, Germany
IGFBP‐1, 871‐B1, R&D Systems Inc, McKinley Place NE, Minneapolis, MN
IGFBP‐2, 674‐B2, R&D Systems Inc
IGFBP‐3, 10‐663‐45149, GenWay Biotech, Inc, Nancy Ridge Drive, San Diego, CA
Standard, Canine C‐peptide radioimmunoassay, Linco Research
Insulin porcine, 11‐663‐45797, GenWay Biotech, Inc
Canine C‐peptide radioimmunoassay, Linco Research
Trasylol, Bayer, Göteborg, Sweden
Immulite, Siemens AG, Erlangen, Germany
Glucose, Thermo Clinical Labsystems Oy, Vantaa, Finland
ABX Pentra Fructosamine, ABX Diagnostics, Montpellier, France
Minitab Inc, State College, PA
Stata Corp. 2012, College Station, TX
SAS Institute Inc, Cary, NC
References
- 1. Selman PJ, Mol JA, Rutteman GR, et al. Progestin‐induced growth hormone excess in the dog originates in the mammary gland. Endocrinology 1994;134:287–292. [DOI] [PubMed] [Google Scholar]
- 2. Selman PJ, Mol JA, Rutteman GR, Rijnberk A. Progestin treatment in the dog. I. Effects on growth hormone, insulin‐like growth factor I and glucose homeostasis. Eur J Endocrinol 1994;131:413–421. [DOI] [PubMed] [Google Scholar]
- 3. Eigenmann JE, Eigenmann RY, Rijnberk A, et al. Progesterone‐controlled growth hormone overproduction and naturally occurring canine diabetes and acromegaly. Acta Endocrinol 1983;104:167–176. [DOI] [PubMed] [Google Scholar]
- 4. Kopchick JJ. Growth and maturation In: Melmed S, ed. Endocrinology. Philadelphia, PA: W.B. Saunders; 2001:389–404. [Google Scholar]
- 5. Pierluissi J, Campbell J. Metasomatotrophic diabetes and its induction: Basal insulin secretion and insulin release responses to glucose, glucagon, arginine and meals. Diabetologia 1980;18:223–228. [DOI] [PubMed] [Google Scholar]
- 6. Clayton PE, Banerjee I, Murray PG, Renehan AG. Growth hormone, the insulin‐like growth factor axis, insulin and cancer risk. Nat Rev Endocrinol 2011;7:11–24. [DOI] [PubMed] [Google Scholar]
- 7. Queiroga FL, Perez‐Alenza MD, Silvan G, et al. Crosstalk between GH/IGF‐I axis and steroid hormones (progesterone, 17beta‐estradiol) in canine mammary tumours. J Steroid Biochem Mol Biol 2008;110:76–82. [DOI] [PubMed] [Google Scholar]
- 8. Pérez Alenza MD, Tabanera E, Peña L. Inflammatory mammary carcinoma in dogs: 33 cases (1995‐1999). J Am Vet Med Assoc 2001;219:1110–1114. [DOI] [PubMed] [Google Scholar]
- 9. Queiroga FL, Perez‐Alenza D, Silvan G, et al. Serum and intratumoural GH and IGF‐I concentrations: Prognostic factors in the outcome of canine mammary cancer. Res Vet Sci 2010;89:396–403. [DOI] [PubMed] [Google Scholar]
- 10. Fall T, Hamlin HH, Hedhammar A, et al. Diabetes mellitus in a population of 180,000 insured dogs: Incidence, survival, and breed distribution. J Vet Intern Med 2007;21:1209–1216. [DOI] [PubMed] [Google Scholar]
- 11. Fall T, Hedhammar A, Wallberg A, et al. Diabetes mellitus in Elkhounds is associated with diestrus and pregnancy. J Vet Intern Med 2010;24:1322–1328. [DOI] [PubMed] [Google Scholar]
- 12. Mared M, Catchpole B, Kampe O, Fall T. Evaluation of circulating concentrations of glucose homeostasis biomarkers, progesterone, and growth hormone in healthy Elkhounds during anestrus and diestrus. Am J Vet Res 2012;73:242–247. [DOI] [PubMed] [Google Scholar]
- 13. Beijerink NJ, Bhatti SFM, Okkens AC, et al. Adenohypophyseal function in bitches treated with medroxyprogesterone acetate. Domest Anim Endocrinol 2007;32:63–78. [DOI] [PubMed] [Google Scholar]
- 14. Mohan S, Baylink DJ. Development of a simple valid method for the complete removal of insulin‐like growth factor (IGF)‐binding proteins from IGFs in human serum and other biological fluids: Comparison with acid‐ethanol treatment and C18 Sep‐Pak separation. J Clin Endocrinol Metab 1995;80:637–647. [DOI] [PubMed] [Google Scholar]
- 15. Smith LF. Species variation in the amino acid sequence of insulin. Am J Med 1966;40:662–666. [DOI] [PubMed] [Google Scholar]
- 16. Fall T, Holm B, Karlsson A, et al. Glucagon stimulation test for estimating endogenous insulin secretion in dogs. Vet Rec 2008;163:266–270. [DOI] [PubMed] [Google Scholar]
- 17. Fall T. Characterisation of Diabetes Mellitus in Dogs. Doctoral Thesis. Uppsala: Swedish University of Agricultural Sciences; 2009. [Google Scholar]
- 18. Kutzler MA, Mohammed HO, Lamb SV, et al. Accuracy of canine parturition date prediction from the initial rise in preovulatory progesterone concentration. Theriogenology 2003;60:1187–1196. [DOI] [PubMed] [Google Scholar]
- 19. Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care 1998;21:2191–2192. [DOI] [PubMed] [Google Scholar]
- 20. Royston P, White IR. Multiple imputation by chained equations (MICE): Implementation in Stata. J Stat Softw 2011;45:1–20. [Google Scholar]
- 21. Eigenmann JE, Becker M, Kammermann B, et al. Decrease of non‐suppressible insulin‐like activity after pancreatectomy and normalization by insulin therapy. Acta Endocrinol 1977;85:818–822. [DOI] [PubMed] [Google Scholar]
- 22. Wada T, Hori S, Sugiyama M, et al. Progesterone inhibits glucose uptake by affecting diverse steps of insulin signaling in 3T3‐L1 adipocytes. Am J Physiol Endocrinol Metab 2010;298:E881–E888. [DOI] [PubMed] [Google Scholar]
- 23. Prahalada S, Stabinski LG, Chen HY, et al. Pharmacological and toxicological effects of chronic porcine growth hormone administration in dogs. Toxicol Pathol 1998;26:185–200. [DOI] [PubMed] [Google Scholar]
- 24. Batista MR, Smith MS, Snead WL, et al. Chronic estradiol and progesterone treatment in conscious dogs: Effects on insulin sensitivity and response to hypoglycemia. Am J Physiol Regul Integr Comp Physiol 2005;289:R1064–R1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Eigenmann JE, Rijnberk A. Influence of medroxyprogesterone acetate (Provera) on plasma growth hormone levels and on carbohydrate metabolism. I. Studies in the ovariohysterectomised bitch. Acta Endocrinol 1981;98:599–602. [DOI] [PubMed] [Google Scholar]
- 26. Kooistra HS, Okkens AC. Secretion of prolactin and growth hormone in relation to ovarian activity in the dog. Reprod Domest Anim 2001;36:115–119. [PubMed] [Google Scholar]
- 27. Gayet C, Bailhache E, Dumon H, et al. Insulin resistance and changes in plasma concentration of TNFalpha, IGF1, and NEFA in dogs during weight gain and obesity. J Anim Physiol Anim Nutr (Berl) 2004;88:157–165. [DOI] [PubMed] [Google Scholar]
- 28. Renehan AG, Frystyk J, Flyvbjerg A. Obesity and cancer risk: The role of the insulin–IGF axis. Trends Endocrinol Metab 2006;17:328–336. [DOI] [PubMed] [Google Scholar]
- 29. Baxter RC, Bryson JM, Turtle JR. Somatogenic receptors of rat liver: Regulation by insulin. Endocrinology 1980;107:1176–1181. [DOI] [PubMed] [Google Scholar]
- 30. Leung KC, Doyle N, Ballesteros M, et al. Insulin regulation of human hepatic growth hormone receptors: Divergent effects on biosynthesis and surface translocation. J Clin Endocrinol Metab 2000;85:4712–4720. [DOI] [PubMed] [Google Scholar]
- 31. Calle EE, Kaaks R. Overweight, obesity and cancer: Epidemiological evidence and proposed mechanisms. Nat Rev Cancer 2004;4:579–591. [DOI] [PubMed] [Google Scholar]
- 32. Verkest KR, Fleeman LM, Rand JS, et al. Basal measures of insulin sensitivity and insulin secretion and simplified glucose tolerance tests in dogs. Domest Anim Endocrinol 2010;39:194–204. [DOI] [PubMed] [Google Scholar]
- 33. Polonsky KS, Pugh W, Jaspan JB, et al. C‐peptide and insulin secretion. Relationship between peripheral concentrations of C‐peptide and insulin and their secretion rates in the dog. J Clin Investig 1984;74:1821–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Arcidiacono B, Iiritano S, Nocera A, et al. Insulin resistance and cancer risk: An overview of the pathogenetic mechanisms. Exp Diabetes Res 2012;2012:789174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Eigenmann JE, Patterson DF, Zapf J, Froesch ER. Insulin‐like growth factor I in the dog: A study in different dog breeds and in dogs with growth hormone elevation. Acta Endocrinol 1984;105:294–301. [DOI] [PubMed] [Google Scholar]
- 36. Sutter NB, Bustamante CD, Chase K, et al. A single IGF1 allele is a major determinant of small size in dogs. Science (New York, NY) 2007;316:112–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yamka RM, Friesen KG, Frantz NZ. Identification of canine markers related to obesity and the effects of weight loss on the markers of interest. Intern J Appl Res Vet Med 2006;4:282–292. [Google Scholar]
- 38. Martin LJ, Siliart B, Dumon HJ, Nguyen PG. Hormonal disturbances associated with obesity in dogs. J Anim Physiol Anim Nutr (Berl) 2006;90:355–360. [DOI] [PubMed] [Google Scholar]
- 39. Greer KA, Hughes LM, Masternak MM. Connecting serum IGF‐1, body size, and age in the domestic dog. Age 2011;33:475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Corpas E, Harman SM, Blackman MR. Human growth hormone and human aging. Endocr Rev 1993;14:20–39. [DOI] [PubMed] [Google Scholar]
- 41. Clemmons DR. Metabolic actions of insulin‐like growth factor‐I in normal physiology and diabetes. Endocrinol Metab Clin North Am 2012;41:425–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mol JA, Van Garderen E, Selman PJ, et al. Growth hormone mRNA in mammary gland tumors of dogs and cats. J Clin Invest 1995;95:2028–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rutteman GR, Misdorp W, Van den Brom WE, Rijnberk A. Anterior pituitary function in female dogs with spontaneous mammary tumors: I. Growth hormone. Anticancer Res 1989;9:235–239. [PubMed] [Google Scholar]
- 44. Hellmén E, Bergström R, Holmberg L, et al. Prognostic factors in canine mammary tumors: A multivariate study of 202 consecutive cases. Vet Pathol 1993;30:20–27. [DOI] [PubMed] [Google Scholar]
- 45. Matos AJF, Baptista CS, Gärtner MF, Rutteman GR. Prognostic studies of canine and feline mammary tumours: The need for standardized procedures. Vet J 2012;193:24–31. [DOI] [PubMed] [Google Scholar]


