Abstract
Background
A poorly understood protein‐losing enteropathy (PLE) disorder has been reported in Yorkshire Terrier dogs.
Objectives
To describe clinical features, intestinal histopathology, and outcome in Yorkshire Terrier dogs with PLE, and to identify variables predictive of outcome.
Animals
Thirty client‐owned Yorkshire Terrier dogs with PLE.
Methods
Retrospective study. Records of dogs with a diagnosis of PLE were reviewed. Intestinal histopathology was interpreted using the World Small Animal Veterinary Association gastrointestinal histopathology classification system. Discriminate analysis techniques were used to identify variables predictive of outcome.
Results
Females outnumbered males (20/30). Median age was 7 years (range 1–12). Common clinical signs were diarrhea (20/30), vomiting (11), ascites and abdominal distension (11), and respiratory difficulty (8). Histopathologic abnormalities included villous lymphatic dilatation, crypt lesions, villous stunting, and variable increases in cellularity of the lamina propria. All dogs were treated with glucocorticoids. Of 23 dogs with long‐term follow‐up, 9 had complete, and 3 had partial, resolution of signs, and 11 failed to respond to treatment. Median survival of responders was 44 months and of nonresponders was 12 months, with 4 dogs experiencing peracute death. Vomiting, monocytosis, severity of hypoalbuminemia, low blood urea nitrogen concentration, and villous blunting were predictive of survival <4 months.
Conclusions
In addition to classic GI signs, Yorkshire Terriers with PLE often show clinical signs associated with hypoalbuminemia and low oncotic pressure. Lymphatic dilatation, crypt lesions, and villous stunting are consistent histopathologic findings. Clinical outcomes are variable, but many dogs experience remission of clinical signs and prolonged survival.
Keywords: Endoscopic biopsy, Inflammatory bowel disease, Lymphangiectasia
Abbreviations
- CCECAI
canine chronic enteropathy clinical activity index
- GI
gastrointestinal
- ICU
Intensive Care Unit
- PLE
protein‐losing enteropathy
- VMC
Veterinary Medical Center
- WSAVA
World Small Animal Veterinary Association
A protein‐losing enteropathy (PLE) syndrome associated with lymphangiectasia has been reported in Yorkshire Terrier dogs.1, 2, 3, 4, 5, 6, 7 Clinical findings vary greatly and include diarrhea, vomiting, muscle tremors and seizure activity because of hypocalcemia and hypomagnesemia, severe panhypoproteinemia with subsequent transudative uni‐ or bicavitary effusion, hypocholesterolemia, lymphopenia, and low serum vitamin D concentration.1, 2, 4, 5, 6, 7, 8, 9 A unique sonographic feature described in some dogs with lacteal dilatation is mucosal speckling.10, 11 Lymphangiectasia and increased mucosal cellularity have been reported in many dogs with PLE, both Yorkshire Terriers and other breeds.1, 2, 3, 4, 5, 6, 7 Intestinal crypt lesions variously described as crypt abscesses, dilated crypts, or cystic crypts also have been reported in the Yorkshire Terrier.6, 8, 12, 13
Enteric protein loss can be measured directly by a commercially available fecal α1‐proteinase inhibitor assay.14 A working diagnosis of PLE in dogs with hypoalbuminemia or panhypoproteinemia, however, usually is made by excluding renal protein loss by urinalysis, urine protein to creatinine ratio, or both, and by excluding decreased hepatic albumin synthesis by normal pre‐ and postprandial serum bile acid concentrations or other liver function tests. Ultimately, intestinal biopsies are needed to identify GI histopathologic lesions that define an underlying etiology for PLE.4, 15
Substantial differences in the interpretation of intestinal histology by veterinary pathologists16 led to the convening of an expert panel with the financial sponsorship of the World Small Animal Veterinary Association (WSAVA). The group has published guidelines for the histopathologic interpretation of cellular and morphologic changes in intestinal mucosal biopsy specimens of dogs and cats.17, 18 In addition to characterizing the severity and type of mucosal inflammation, these guidelines place equal value on cytoarchitectural changes within the GI mucosa, such as epithelial injury, lymphatic dilatation, crypt changes, villous blunting, and fibrosis.17, 18 Wider application of these or other guidelines ultimately should provide a better standard for GI histopathologic interpretation, particularly in disease syndromes that result in more severe clinical presentations. The few reported cases of PLE in Yorkshire Terriers have been evaluated using nonstandardized histopathologic classification systems.1, 9, 10, 11, 12, 13
Because of an apparent breed association made in earlier reports, we performed a retrospective study to identify clinical features, histopathologic lesions, and outcome of PLE in the Yorkshire Terrier breed. The objectives of the present study were to (1) describe clinical findings in a group of Yorkshire Terriers with PLE, (2) interpret histopathologic findings in intestinal biopsies using the WSAVA standardization guidelines, and (3) determine whether any clinical or histopathologic features were predictive of long‐term survival. We hypothesized that severity of clinical signs, clinicopathologic abnormalities, and histopathologic findings would correlate with outcome and morbidity within 4 months of diagnosis.
Materials and Methods
The electronic medical, laboratory, and endoscopic records at the University of Minnesota Veterinary Medical Center (VMC) were searched for Yorkshire Terriers with intestinal biopsy submissions between 2002 and 2007. Records were reviewed if a diagnosis of PLE had been made on the basis of hypoalbuminemia and intestinal histopathology. Inclusion criteria included complete history and physical examination findings and results of a CBC and serum bio‐chemistry profile in the medical record. All dogs must have had a negative fecal flotation test within 1 month of presentation or been treated for intestinal parasites before presentation. All dogs had to have had hypoalbuminemia present, with or without hypoglobulinemia. In addition, a urinalysis must have been negative for protein on sulfasalicylic acid precipitation test, or a urine protein to creatinine ratio must have been <0.5. All included dogs also must have had normal pre‐ and postprandial serum bile acid concentrations. Our exclusion criteria were dogs that had evidence of protein‐losing nephropathy or impaired liver function, and dogs that had any glucocorticoid or other immunosuppressive within 2 weeks before intestinal biopsy.
A board‐certified radiologist (LC) reviewed all available survey radiographs and captured static images of abdominal ultrasound studies. Original gastric and intestinal histopathology reports were reviewed for the presence of intestinal lesions. Tissue blocks subsequently were retrieved from pathology archives, recut, and evaluated by a board‐certified pathologist (AW). Endoscopic biopsies were interpreted using the WSAVA GI standardization system for quality and diagnosis.17, 18 The pathologist was masked to the original histopathology results and to any clinical or outcome information.
Owners, referring veterinarians, or both were contacted if clinical outcomes were not documented in the medical record and asked whether the dog's presenting clinical signs had resolved, partially improved, or failed to improve. Follow‐up laboratory results were obtained when possible. Complete resolution was defined as resolution of all initial clinical signs and restoration of normal serum albumin concentration. Partial resolution was defined as resolution of some initial clinical signs according to the owner, veterinarian, or both; persistence of some degree of hypoalbuminemia; or both. No resolution was defined as no discernable improvement in clinical signs. Dates and circumstances of patient death or euthanasia were recorded, if applicable.
Statistical Analysis
Discriminant analysis techniques were used to classify animals into 2 groups (alive or dead at 4 months after discharge from the VMC). The a priori probabilities of an individual belonging to either group were set as equal for this discriminant analysis study. The method utilized was Wilks' lambda. Fisher's linear discriminant functions were utilized in a stepwise progression technique whereby the f test criteria for the variable to enter the equation and to exit the equation were P < .05 and P > .10, respectively. This method identifies the variables most reliable to predict future outcomes of animals with the same diagnosis (PLE). Some combinations of variables later were evaluated without applying the stepwise progression technique. Statistical significance for all analyses was defined as the probability of the null hypotheses (ie, no relationship or no difference) being true as less than 5.0% (P < .05).
Results
Medical records of 61 Yorkshire Terrier dogs suspected to have PLE based on clinical signs and hypoalbuminemia were reviewed. Nineteen were excluded because of missing or absent intestinal biopsies. Twelve were excluded because of absent or abnormal serum bile acid test results. Thirty dogs were included in the study.
Historical and Physical Examination Findings
Twenty dogs were spayed females, 8 were neutered males, and 2 were intact males. Median age at diagnosis was 7 years (range, 1–12 years).
Presenting complaints included small bowel diarrhea (20), vomiting (11), abdominal distention (11), lethargy (8), dyspnea or tachypnea (7), polyuria and polydipsia (7), anorexia (6), cough (3), weight loss (3), and melena (1). Median duration of clinical signs before presentation at the UMN‐VMC was 1 month (range, 1 day–36 months). Several different commercial foods had been fed before diagnosis. Previous medications included metronidazole (10), furosemide (4), tylosin (3), amoxicillin (3), metoclopramide (2), enalapril (2), and sulfasalazine, enrofloxacin, bismuth subsalicylate, loperamide, lactulose, human albumin transfusion, and probiotics (1 each). Two dogs had been diagnosed previously with untreated hyperadrenocorticism, 2 had skin allergies, and 2 had mammary tumors. One each had previous diagnoses of coccidiosis, giardiasis, and asymptomatic mitral valve disease; 21 dogs had no reported concurrent medical conditions.
Abdominal distension consistent with ascites was the most commonly noted physical examination abnormality (15), followed by dyspnea or tachypnea (8), muffled lung sounds (6), dental disease (5), dehydration (4), increased bronchovesicular sounds (3), muscle wasting (3), abdominal pain (2), poor hair coat (2), peripheral ventral edema (2), and borborygmus (1). Four dogs had unremarkable physical examinations.
Clinicopathologic Findings
Abnormal leukogram findings were mild mature neutrophilia (12), neutrophilia with left shift (2), mild monocytosis (9), and lymphopenia (3). Seven dogs had thrombocytosis, which in some cases was severe, with a median platelet count of 708,857/μL (range, 443,000–1,272,000/μL). Two of the dogs with thrombocytosis were the dogs previously diagnosed with untreated hyperadrenocorticism. Three dogs had mild nonregenerative anemia consistent with chronic disease. Four dogs had microcytic anemia; 1 had severe anemia associated with melena and moderate thrombocytopenia. Serum biochemistry abnormalities are summarized in Table 1. Although all dogs had low serum total calcium concentrations, only 4 had serum ionized calcium concentrations measured, 2 of which had concentrations below the reference range. Clinical signs of hypocalcemia, such as tremors, tetany, or seizures, were not reported in any of the study dogs. Three dogs had serum cobalamin and folate concentrations measured; 2 were hypocobalaminemic.
Table 1.
Summary of serum biochemistry abnormalities.
| Variable | No. of Dogs Abnormal/Measured (% Abnormal) | Median | Range | Reference Range |
|---|---|---|---|---|
| Albumin | 30/30 (100%) | 1.35 g/dL | 0.8–2.2 | 2.7–3.7 |
| Globulin | 14/30 (46%) | 1.67 g/dL | 1.0–2.8 | 1.7–3.5 |
| Calcium (total) | 30/30 (100%) | 6.66 mg/dL | 3.7–8.8 | 9.3–11.5 |
| Calcium (ionized) | 4/4 (100%) | 4.36 mg/dL | 2.90–5.32 | 4.48–5.82 |
| Magnesium | 12/30 (40%) | 1.46 mg/dL | 1.0–2.3 | 1.7–2.4 |
| Cholesterol | 24/26 (92%) | 95.75 mg/dL | 43–209 | 143–373 |
| Creatinine | 15/30 (50%) | 0.55 mg/dL | 0.2–1.5 | 0.6–1.6 |
| Blood urea nitrogen (BUN) | 4/30 (13%) | 15.5 mg/dL | 6–24 | 9–31 |
Imaging Results
Seventeen dogs had survey radiographs of the abdomen (5), thorax (2), or both (10) available for review. Peritoneal effusion was evident in 15 dogs and pleural effusion in 5. Bicavitary effusion was present in all dogs with pleural effusion. Two dogs were noted to have generalized osteopenia. Serum ionized calcium and magnesium concentrations were low in the 1 osteopenic dog in which they were measured.
Abdominal ultrasonography was performed in 4 dogs. Review of still images identified small intestinal mucosal speckling in 2 dogs. Two dogs had cardiac ultrasonography performed during initial evaluation at the VMC. One of these dogs had a hyperechoic thrombus evident within the right atrium; no underlying heart disease was detected in this dog. No cardiac abnormalities were detected in the second dog.
Gastrointestinal Histopathology Results
Intestinal biopsy specimens were obtained from 28 dogs by intestinal endoscopy. Biopsies were obtained from 2 dogs at necropsy, and no biopsies were obtained by full‐thickness surgical biopsy. Duodenal histopathology findings are summarized in Table 2. Tissue quality from endoscopic sampling for a given dog ranged from inadequate to adequate. Lymphoplasmacytic inflammation was detected within the lamina propria in all 30 dogs. Twenty of 30 dogs had some degree of neutrophilic inflammation. Variable degrees of lacteal dilatation were present in 24/30 dogs (Fig 1). Endoscopic samples from 17/28 dogs were adequate for evaluation of intestinal crypts. Of those, 15/17 had crypt lesions (Fig 2). The 2 dogs with necropsy samples also had crypt lesions present. Mucosal fibrosis was present in 5/30. Villous blunting was present in 17/30.
Table 2.
Duodenal histopathology findings.
| WSAVA Score | Number of Dogs with Score | Median Score | |
|---|---|---|---|
| Biopsy quality (Endoscopic samples) | Adequate | 11 | N/A |
| Marginal | 6 | N/A | |
| Inadequate | 11 | N/A | |
| Lymphoplasmacytic infiltrate | 0 | 0 | 2 |
| 1 | 12a | ||
| 2 | 14 | ||
| 3 | 4 | ||
| Neutrophilic infiltrate | 0 | 10a | 1 |
| 1 | 16 | ||
| 2 | 3 | ||
| 3 | 1 | ||
| Crypt dilatation (Assessed in 19/30) | 0 | 2 | 2 |
| 1 | 5 | ||
| 2 | 11a | ||
| 3 | 1 | ||
| Lacteal dilatation | 0 | 6a | 1 |
| 1 | 12 | ||
| 2 | 11a | ||
| 3 | 1 | ||
| Villous blunting | 0 | 13a | 1 |
| 1 | 9 | ||
| 2 | 6 | ||
| 3 | 2 | ||
| Mucosal fibrosis | 0 | 25a | 1 |
| 1 | 5 | ||
| 2 | 0 | ||
| 3 | 0 |
WSAVA Score: 0 = Absent, 1 = Mild, 2 = Moderate, 3 = Severe.
Necropsy samples included.
Figure 1.

Duodenal endoscopic biopsy. Four to 5 villi are expanded by markedly dilated lacteals.
Figure 2.
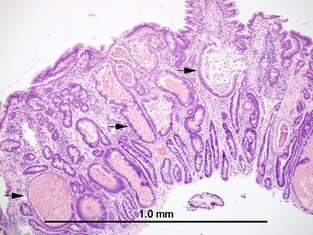
Duodenal endoscopic biopsy. Numerous crypts are markedly distended and contained amorphous material with cellular debris (“crypt abscesses”).
Biopsies of gastric mucosa in 28 cases also were reviewed using the WSAVA criteria. Variable mucosal inflammation was detected in all samples, with lymphoplasmacytic infiltration being most predominant (median score, 1). Ten dogs had low numbers of Helicobacter‐like organisms present in the gastric pits.
Treatment and Outcome
Seven dogs underwent thoracocentesis to alleviate signs of dyspnea. Three dogs underwent abdominocentesis. Fluid analysis in all cases was consistent with transudative effusion. Median serum albumin concentration for dogs with versus without cavitary effusions was 1.3 and 1.4 g/dL, respectively. Within a week of biopsies being performed, all dogs received immunosuppressive doses of prednisone with a dosage range of 1–2 mg/kg/day. Other initial therapies included metronidazole (23), famotidine (15), sucralfate (6), furosemide (5), low‐dose aspirin (3), and amoxicillin, rutin, and vitamin B‐12 injections (1 dog each). One dog had azathioprine added 4 weeks after diagnosis; this dog experienced complete resolution. Dietary recommendations varied widely and were categorized into either a limited antigen diet (20) or a low‐fat diet (7). Two dogs required hospitalization because of protracted vomiting and diarrhea. Both of these dogs received IV crystalloid and colloid fluid therapy.
Follow‐up information was available for 23 dogs. Twelve dogs were alive at the time of manuscript preparation and 11 were dead (Fig 3). Thirteen dogs had complete resolution of clinical signs and 3 had partial resolution. Time from diagnosis to complete or partial resolution ranged from 2 to 10 weeks. For these 16 dogs, median survival was 44 months (range, 3.5–80 months) calculated to the time of manuscript preparation. Reasons for death were available for 4 dogs with complete or partial resolution in which case all 4 dogs experienced a complete initial response to treatment, but experienced relapse of signs 3–20 months after diagnosis. Two of these dogs were euthanized because of relapse. Another 2 dogs died spontaneously at home after relapsing signs of disease. Seven dogs were classified as having no resolution; 5 of these dogs were euthanized because of their disease, 1 died spontaneously at home, and 1 experienced cardiopulmonary arrest in the VMC ICU shortly after diagnosis because of massive pulmonary thromboembolism (confirmed at necropsy). Median survival for the dogs with no resolution was 12 months (range, 1 day–24 months).
Figure 3.

Kaplan–Meier survival curve for 23/30 dogs that had follow‐up information available.
Discriminant statistical analysis identified several variables from the historical data, physical examination findings, CBC and serum biochemistry results, histopathologic findings, and treatments administered that were significant predictors of death within 4 months of diagnosis. These significant variables were vomiting (P = .015), monocytosis (P = .005), low BUN (P = .008), low serum albumin concentration (P = .027), and intestinal villous blunting (P = .033). Accuracy of individual variables in predicting outcome ranged from 78.3 to 94.1%. Accuracy of 100% in correctly predicting death within 4 months of diagnosis was reached when combining monocytosis, BUN, and serum albumin as variables in the stepwise progression technique. None of the pharmacologic treatments or dietary recommendations were statistically associated with, or predictive of, outcome.
Discussion
This study reports the clinical, clinicopathologic, and GI histopathologic findings in 30 Yorkshire Terriers with a diagnosis of PLE based on hypoalbuminemia, presumptively because of GI loss and associated histopathology. Yorkshire Terriers of any age can be affected and female dogs outnumbered males in this study. Ascites or pleural effusion, consistent with low oncotic pressure, were common historical complaints or physical examination findings, but were not negative prognostic indicators. Abdominal distension and dyspnea were noted peracutely by many owners. Only 2 dogs had evidence of peripheral ventral edema. A third of dogs in this study did not have a history of diarrhea. Therefore, PLE should not be excluded in Yorkshire Terriers that do not present with this sign. Vomiting was present in the history of approximately one‐third of dogs, and was a negative prognostic indicator.
Outcome and survival varied widely, with some dogs achieving prolonged survival and remission of clinical signs, whereas others failed to respond, with peracute death in a few. Thirteen of 23 dogs with long‐term follow‐up had a prolonged response to treatment with complete resolution of clinical signs. Three had partial clinical response and 7 were ultimately unresponsive to multiple therapeutic interventions. Complete and partial responders had a median survival of 44 months at the time of manuscript preparation. Prognosis for dogs with PLE in the current veterinary literature is guarded,4, 9 and there are few reports of survival data for dogs with PLE. The canine chronic enteropathy clinical activity index (CCECAI) has been proposed to identify risk factors for negative outcome.9 CCECAI ≥12 predicted refractoriness to treatment and euthanasia within 3 years with a sensitivity and specificity of 91 and 82%, respectively.9 In this study, predictors of death within 4 months of diagnosis were a history of vomiting, monocytosis, low BUN, degree of hypoalbuminemia, and intestinal villous blunting. These findings provide some support for our hypothesis that the severity of some clinical signs, serum biochemistry findings, and histopathologic findings should correlate with outcome. Of these variables, vomiting may be the most practical in identifying a dog with a less favorable prognosis. The presence or absence of vomiting is easy to identify with an accurate history, whereas the other variables require cutoff values or degrees of severity to be useful. Because all dogs received systemic glucocorticoid treatment, no analysis could be performed to evaluate the effect of this treatment on outcome. No effect was found for other treatments given.
Disorders of calcium metabolism manifesting as ionized hypocalcemia, hypomagnesemia, hypoparathyroidism, and hypovitaminosis D have been reported in dogs with PLE.1, 8, 19 Ionized hypocalcemia was documented in 2 dogs in this report, and 1 of these dogs had radiographic evidence of osteopenia. Another dog, which did not have ionized calcium concentrations measured, also had radiographic evidence of osteopenia. None of the dogs in this series had tremors or tetany‐like signs, which previously have been reported in Yorkshire Terriers with PLE‐related ionized hypocalcemia.1
Intestinal cytoarchitecture was evaluated on endoscopically obtained biopsies in almost all dogs in this study. Lacteal dilatation, villous blunting, mucosal fibrosis, and crypt dilatation (when assessment was possible) were the most consistent findings. Inflammatory changes were present in all cases, but were mild in some dogs and predominantly neutrophilic, rather than lymphocytic‐plasmacytic, in others.
Intestinal biopsy specimen quality is known to affect the sensitivity of endoscopy in identifying intestinal lesions.20 Endoscopic biopsy samples must be of good quality and include the entire thickness of the mucosa to evaluate intestinal crypts. Inadequate sample quality was a factor when discerning the prevalence of crypt lesions in 13/28 study dogs. Inadequate sample quality may have been a result of recutting the original archived tissue blocks or the original biopsy may have been inadequate in the first place and less stringent, nonstandardized histopathologic standards may have been applied to them. Lymphangiectasia previously has been identified as the most common intestinal lesion associated with PLE in Yorkshire Terriers.21 Twenty percent of dogs in this study lacked lymphatic dilatation, which suggests that lymphangiectasia in this breed may be secondary to other underlying pathology, possibly dilated crypts or inflammation. Sampling bias introduced by endoscopic biopsies and regional distribution of lymphangiectasia (ie, duodenum versus midileum) cannot be excluded. This study confirms that lymphangiectasia can be diagnosed by endoscopic biopsy of the duodenum.7 This finding is reassuring, given that low oncotic pressure, bicavitary effusion, and other sequelae of PLE make these dogs poor candidates for surgically obtained, full‐thickness biopsies. The overall degree of lacteal dilatation was moderate in dogs in this study, with a median score of 1 in the 24 dogs that showed this change.
Crypt lesions in the duodenum were a common finding (17/19 dogs) and previously have been reported in Yorkshire Terriers with PLE.12, 13 These lesions have been described as crypts dilated and filled with mucous, with or without inflammatory debris. Crypt lesions were more prevalent than reported in the original biopsy reports in the dogs in this study. It has been previously reported that crypt lesions do not seem to be associated with bacterial colonization in this breed.1 It has been speculated that these lesions represent a degenerative process in the intestine,12 and it is unknown if they are a direct cause of PLE or if they represent markers of other intestinal injury. There was no direct correlation with crypt dilatation and outcome in this study. However, crypt lesions are a repeatable finding in dogs with PLE, and the etiology remains unknown.
A hypercoagulable state of unknown pathogenesis has been confirmed in dogs with PLE.22 A prior study concluded that enteric loss of antithrombin III concurrently with albumin is not the only mechanism.22 Anemia, thrombocytosis, and a concurrent inflammatory state also may play important roles.22 Four dogs in this study died peracutely. Acute cardiopulmonary thromboembolism was confirmed in 1 dog and was strongly suspected in a second (in which a cardiac thrombus was identified during initial evaluation). Sudden death in another 2 dogs with well‐controlled clinical signs was consistent with thromboembolism. The results of this study support those of a previous study that found hypercoagulability as a cause of death in PLE dogs, even after clinical signs were well controlled.22 Prospective studies are needed to further assess mechanisms for hypercoagulability and assess thromboprophylaxis options.
The retrospective nature of this study was a limitation. Diagnostic evaluation differed widely among dogs. A surprisingly low number of the dogs in this study underwent abdominal ultrasound examination during the diagnostic evaluation. It is unknown why this modality was omitted as part of the evaluation in most of these cases. Numerous clinicians evaluated these cases over a period of 5 years. We can only speculate that diagnostic testing was performed in order of priority, within the confines of client resources, to eliminate alternative differential diagnoses based on index of suspicion for the most likely underlying cause of disease. A few dogs had serum ionized calcium concentrations measured, and fewer still had assessments of coagulation parameters. It is not known whether abnormalities in these tests would better predict response to treatment or help elucidate the cause of PLE in Yorkshire Terriers. We could not definitively exclude primary liver disease in any of the dogs, but we believe liver disease was unlikely because serum bile acid concentrations were normal, and no dog was diagnosed with liver disease during the follow‐up period. Treatments were not standardized and therefore it was not possible to determine which therapies or diets were most useful. Systemic glucocorticoids (prednisone or budesonide) with cyclosporine and a low‐fat diet are commonly used in Yorkshire Terriers with PLE, but this study was unable to provide support for any of these treatments.
In conclusion, Yorkshire Terrier dogs develop a mild to severe form of PLE characterized clinically by uni‐ or bicavitary effusion and histologically by lymphangiectasia, crypt lesions, and inflammatory infiltrates in the intestinal mucosa. Females may be overrepresented. Clinical outcomes of PLE are variable with approximately half of the treated dogs having complete or partial control of clinical signs and prolonged survival, and half failing to respond and surviving a median of 12 months. Vomiting may be a useful clinical finding, which may be predictive of morbidity within 4 months of diagnosis, especially when combined with monocytosis, degree of hypoalbuminemia, low BUN, and villous blunting.
Acknowledgments
No external funding was used to support this research.
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Work performed at the University of Minnesota, College of Veterinary Medicine, Veterinary Medical Center, Veterinary Diagnostic Laboratory, and Department of Veterinary Clinical Sciences, St. Paul, Minnesota. This research was presented, in part, as an abstract at the 2009 American College of Veterinary Internal Medicine Forum and Canadian Veterinary Medical Association Convention, Montreal, Quebec, Canada
Footnote
Craven, M, Duhamel GE, Sutter NB, et al. Absence of bacterial association in Yorkshire Terriers with protein‐losing enteropathy and cystic intestinal crypts. J Vet Intern Med 2009;23:757 (abstract)
References
- 1. Kimmel SE, Waddell LS, Michel KE. Hypomagnesemia and hypocalcemia associated with protein losing enteropathy in Yorkshire Terriers: 5 cases (1992‐1998). J Am Vet Med Assoc 2000;217:703–706. [DOI] [PubMed] [Google Scholar]
- 2. Kull PA, Hess RS, Craig LE, et al. Clinical, clinicopathologic, radiographic, and ultrasonographic characteristics of intestinal lymphangiectasia in dogs: 17 cases (1996‐1998). J Am Vet Med Assoc 2001;219:197–202. [DOI] [PubMed] [Google Scholar]
- 3. Van Kruiningen HS, Lees GE, Hayden DW, et al. Lipogranulomatous lymphangitis in canine intestinal lymphangiectasia. Vet Pathol 1984;21:377–383. [DOI] [PubMed] [Google Scholar]
- 4. Dossin O, Lavoue R. Protein‐losing enteropathies in dogs. Vet Clin North Am Small Anim Pract 2011;41:399–418. [DOI] [PubMed] [Google Scholar]
- 5. Peterson PB, Willard MD. Protein‐losing enteropathies. Vet Clin North Am Small Anim Pract 2003;33:1061–1082. [DOI] [PubMed] [Google Scholar]
- 6. Lecoindre P, Chevallier M, Guerret S. Protein losing enteropathy of non‐neoplastic origin in the dog: A retrospective study of 34 cases. Schweiz Arch Tiereilkd 2010;152:141–146. [DOI] [PubMed] [Google Scholar]
- 7. Larson RN, Ginn JA, Bell CM, et al. Duodenal endoscopic findings and histopathologic confirmation of intestinal lymphangiectasia in dogs. J Vet Intern Med 2012;26:1087–1092. [DOI] [PubMed] [Google Scholar]
- 8. Mellanby RJ, Mellor PJ, Roulois A, et al. Hypocalcemia associated with low serum vitamin D metabolite concentrations in two dogs with protein losing enteropathies. J Small Anim Pract 2005;46:345–351. [DOI] [PubMed] [Google Scholar]
- 9. Allenspach K, Wieland B, Grone A, et al. Chronic enteropathies in dogs: Evaluation of risk factors for negative outcome. J Vet Intern Med 2007;21:700–708. [DOI] [PubMed] [Google Scholar]
- 10. Sutherland‐Smith J, Penninck DG, Keating JH, Webster CR. Ultrasonographic intestinal hyperechoic mucosal striations in dogs are associated with lacteal dilation. Vet Radiol Ultrasound. 2007;48:51–57. [DOI] [PubMed] [Google Scholar]
- 11. Gaschen L, Kircher P, Stussi A, et al. Comparison of ultrasonographic findings with clinical activity index (CIBDAI) and diagnosis in dogs with chronic enteropathies. Vet Radiol Ultrasound 2008;49:56–64. [DOI] [PubMed] [Google Scholar]
- 12. Willard MD, Helman G, Fradkin JM, et al. Intestinal crypt lesions associated with protein losing enteropathy in the dog. J Vet Intern Med 2000;14:298–307. [DOI] [PubMed] [Google Scholar]
- 13. Willard MD, Zenger E, Mansell JL. Protein losing enteropathy associated with cystic mucoid changes in the intestinal crypts of two dogs. J Am Anim Hosp Assoc 2003;39:187–191. [DOI] [PubMed] [Google Scholar]
- 14. Melgarejo T, Williams DA, Asem EK. Enzyme‐linked immunosorbent assay for canine α1‐protease inhibitor. Am J Vet Res 1998;59:127–130. [PubMed] [Google Scholar]
- 15. Willard MD. Diagnosis, Protein‐losing enteropathy In: Washabau RJ, Day MJ, eds. Canine and Feline Gastroenterology. St Louis, MO: Saunders; 2013:681–682. [Google Scholar]
- 16. Willard MD, Jergens AE, Duncan RB, et al. Interobserver variation among histopathologic evaluations of intestinal tissue from dogs and cats. J Am Vet Med Assoc 2002;220:1177–1182. [DOI] [PubMed] [Google Scholar]
- 17. Day MJ, Bilzer T, Mansell J, et al. Histopathologic standards for the diagnosis of gastrointestinal inflammation in endoscopic biopsies from the dog and cat: A report from the World Small Animal Veterinary Association Gastrointestinal Standardization Group. J Comp Pathol 2008;138:S1–S43. [DOI] [PubMed] [Google Scholar]
- 18. Washabau RJ, Day MJ, Willard MD, et al. Endoscopic biopsy and histopathologic guidelines for evaluation of gastrointestinal inflammation in companion animals. J Vet Intern Med 2010;24:377–383. [DOI] [PubMed] [Google Scholar]
- 19. Bush WW, Kimmel SE, Wosar MA, et al. Secondary hypoparathyroidism attributed to hypomagnesemia in a dog with protein losing enteropathy. J Am Vet Med Assoc 2001;219:1732–1734. [DOI] [PubMed] [Google Scholar]
- 20. Willard M, Mansell J, Fostgate G, et al. Effect of sample quality on sensitivity of endoscopic biopsy for detecting gastric and duodenal lesions in dogs and cats. J Vet Intern Med 2008;22:1084–1089. [DOI] [PubMed] [Google Scholar]
- 21. Hall EJ, German AG. Diseases of the small intestine In: Ettinger S, Feldman E, eds. Textbook of Veterinary Internal Medicine 7th edition. St Louis, MO: Saynders Elsevier; 2010:1566–1567. [Google Scholar]
- 22. Goodwin LV, Goggs R, Chan DL, Allenspach K. Hypercoagulability in dogs with protein losing enteropathy. J Vet Intern Med 2011;25:273–277. [DOI] [PubMed] [Google Scholar]


