Abstract
Background
An excess of intra‐abdominal fat is observed frequently in dogs with hyperadrenocorticism (HAC). Adipokine dysregulation is a possible cause of complications related to visceral obesity, but little information is available on adipokine in dogs with naturally occurring HAC.
Objectives
To examine the differences in the circulating adipokines concentrations in overweight dogs with and without pituitary‐dependent HAC (PDH).
Animals
Thirty healthy dogs and 15 client‐owned dogs with PDH.
Methods
Case–controlled observational study, which enrolled 15 overweight dogs diagnosed with PDH and 30 otherwise healthy dogs of similar body condition score. Nine of 15 dogs with PDH were treated with low‐dose trilostane twice daily and reassessed after treatment.
Results
The serum leptin (P < .0001) and insulin (P < .0001) concentrations were significantly higher in the PDH group (leptin, 22.8 ± 8.8 [mean ± SD]; insulin, 9.1 ± 6.1) than the healthy group (leptin, 4.9 ± 3.7; insulin, 1.9 ± 0.9). However, there were no significant differences in the adiponectin, resistin, tumor necrosis factor (TNF)‐α, interleukin (IL)‐1β, IL‐6, IL‐10, and IL‐18 levels between the 2 groups. In the PDH group, the serum cortisol concentrations had a linear association with the leptin concentrations, and there were significant decreases in the leptin (P = .0039) and insulin (P = .0039) levels after trilostane treatment. However, the leptin and insulin levels remained higher after trilostane treatment than in healthy control dogs with similar body condition score.
Conclusions and Clinical Importance
Hypercortisolemia in dogs with PDH might upregulate the circulating leptin levels. However, a large population‐based study will be necessary to determine whether the upregulation of leptin is involved directly with the complications caused by HAC.
Keywords: Adiponectin, Canine, Cortisol, Cushing's disease, Leptin
Abbreviations
- ADH
adrenal‐dependent hyperadrenocorticism
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- BCS
body condition score
- CI
confidence interval
- DM
diabetes mellitus
- HAC
hyperadrenocorticism
- IL
interleukin
- LDDST
low‐dose dexamethasone suppression test
- PDH
pituitary‐dependent hyperadrenocorticism
- SD
standard deviation
- TNF
tumor necrosis factor
Hyperadrenocorticism (HAC) is a common endocrinopathy in middle‐aged and older dogs. Approximately 85% of dogs with HAC are estimated to have a pituitary‐dependent form, whereas 15% have an adrenal‐dependent form.1, 2 The clinical signs of HAC are caused by an excess of endogenous cortisol,3 which frequently include polyuria, polydipsia, polyphagia, dermatologic abnormalities, abdominal distension, and lethargy.4 The gradual development of visceral obesity is a classical sign of endogenous HAC.
Adipokines are considered to be biologically active substances produced in adipose tissue that are essential for normal physiological functioning.5 They are important for the regulation of many biological processes, including the energy balance, glucose and lipid metabolism, inflammation and immune function, hemostasis, vascular function, and angiogenesis.6 The most familiar adipokines are leptin and adiponectin, although others such as resistin and some proinflammatory cytokines have been studied in many species, including dogs and cats.6, 7
The increased visceral adiposity in humans with Cushing's syndrome is associated with changes in the production of adipokines.8 In dogs, it has been suggested that increased visceral adiposity and the dysregulation of adipokines are associated with endocrine disorders, including diabetes mellitus (DM) and hypothyroidism.9, 10 In human medicine, several studies have reported that patients with Cushing's syndrome had a higher prevalence of DM, hypertension, and cardiovascular diseases caused by adipokine dysregulation.11, 12, 13 In dogs, it is well known that the chronic hypercortisolemia caused by HAC is associated with complications, such as visceral obesity with insulin resistance, glucose intolerance, hypertension, cardiovascular disease, thromboembolism, and increased susceptibility to infection.14, 15, 16, 17 Circulating adiponectin concentrations are decreased in dogs with pituitary‐dependent HAC (PDH).18 However, there is still a lack of information available on the circulating adipokine levels in dogs with naturally occurring HAC. Therefore, the objective of this study was to examine whether there are differences in the circulating adipokine concentrations between dogs with naturally occurring PDH and healthy dogs, as well as to determine whether the adipokine concentrations in dogs with PDH are affected by trilostane treatment.
Materials and Methods
Case Selection
Forty‐three dogs with newly diagnosed, untreated PDH were enrolled in this case–controlled study. According to their body condition score (BCS; 9‐point scale), 26 overweight (BCS = 6 or 7) dogs were selected. Nine dogs among the 26 dogs were excluded owing to evidences of concurrent disease (3 dogs with chronic kidney disease and pancreatitis, 2 dogs with gallbladder mucocele, 2 dogs with myxomatous mitral valve disease, and 2 dogs with DM). Two intact bitches in diestrus phase and with recurrent bacterial cystitis were also excluded. Consequently, 15 dogs with PDH were included in this study. Thirty healthy client‐owned dogs with similar BCS (6 or 7) were included as controls. The healthy dogs were recruited from the same veterinary medical center from among the dogs that presented for health examination. Informed consent was obtained from the owners, and the University Ethics Committee approved all the animal studies.
The dogs in the healthy group were considered to be healthy based on a physical examination, indirect measurement of their systolic blood pressure, examination of fecal specimens to determine the presence of parasites using a flotation technique, heartworm antigen testing, complete blood count analysis, serum biochemical analysis, urinalysis, adrenocorticotropic hormone (ACTH) response testing, and diagnostic imaging, including survey radiography and abdominal ultrasonography. The age range of the dogs in the healthy group was 8–15 years (mean ± SD = 10.77 ± 2.063 years), with body weight ranged between 4.12 and 14.25 kg (6.842 ± 2.564 kg), and 26 dogs (86%) were BCS 6/9 and 4 dogs were BCS 7/9. Sixteen of the 30 dogs were female and neutered, whereas 12 of the 14 male dogs were neutered. The breeds were Beagle (n = 2), Cocker Spaniel (n = 3), Maltese (n = 4), Miniature Pinscher (n = 2), Miniature Poodle (n = 2), Pekingese (n = 3), Pomeranian (n = 2), Spitz (n = 1), Yorkshire Terrier (n = 4), Shih Tzu (n = 5), and mixed (n = 2).
For the PDH group, a diagnosis of HAC was determined as described previously.1, 4, 19 A tentative diagnosis of HAC was based on the history, physical examination findings, hematological results, biochemical profiles, and urinalyses. The biochemical profiles were determined using an autoanalyzer2 and the electrolyte values were assayed using an electrolyte analyzer.3 The main signs noted by the owners were polyuria and polydipsia, which were defined as urine output >50 mL/kg/day and water intake >100 mL/kg/day, respectively. Each dog had at least 4 of the following clinicopathologic findings: high serum ALP activity, high serum alanine aminotransferase activity, hypercholesterolemia, hypertriglyceridemia, hyperglycemia, and urine‐specific gravity <1.020. In addition, each dog underwent ACTH stimulation testing and a low‐dose dexamethasone suppression test (LDDST). All the dogs with PDH were also examined by abdominal ultrasonography.4 PDH was differentiated from a functional adrenal tumor based on the results of the LDDST and abdominal ultrasonographic evaluations of the adrenal glands, as described previously.1, 4, 19
The dogs with PDH ranged in age from 8 to 16 years (mean ± SD, 11.6 ± 2.4 years), with body weights ranging from 3.8 to 15.5 kg (6.0 ± 3.3 kg), and 12 dogs (80%) were BCS 6/9 and 3 dogs were BCS 7/9. Of the 15 dogs, 9 were female and 3 were neutered, whereas 3 of the 6 male dogs were neutered. The breeds were as follows: Yorkshire Terrier (n = 4), Maltese (n = 4), Miniature Poodle (n = 2), Shih Tzu (n = 2), Pomeranian (n = 1), Cocker Spaniel (n = 1), and mixed (n = 1).
Adrenal Function Tests
The blood samples were collected for ACTH stimulation testing before and 1 hour after the intravenous administration of synthetic ACTH5 (0.25 mg/dog). The serum cortisol concentrations were analyzed using a chemiluminescent immunoassay‐based autoanalyzer.6 HAC was concluded based on an exaggerated increase (>22 μg/dL) in the ACTH‐stimulated serum cortisol concentration.19 Blood samples were collected for the LDDST before and at 4 and 8 hours after intravenous administration of dexamethasone7 (0.01 mg/kg). Suppression was defined as a serum cortisol concentration <1.5 μg/dL at 4 hours after dexamethasone administration or a serum cortisol concentration <50% of the baseline concentration at 4 or 8 hours after dexamethasone administration.1, 4
Assays
All the dogs were fasted for 12 hours before blood collection. Serum was separated from clotted whole blood by centrifugation at 1,200 × g for 10 minutes within 1 hour of blood collection and stored at −70°C until the assays. The following adipokines and hormones were analyzed: leptin, adiponectin, resistin, insulin, IL‐1β, IL‐6, IL‐10, IL‐18, and TNF‐α. Serum leptin and insulin were analyzed in duplicate according to the manufacturer's protocol using a Milliplex MAP Canine kit (Canine Gut Hormone MAGNETIC kit8), where the intra‐assay variabilities were 1% and the interassay variabilities were 8 and 7%, respectively, and the assay sensitivities of leptin and insulin were 0.0806 ng/mL and 1.266 μIU/mL, respectively. The serum IL‐6, IL‐10, IL‐18, and TNF‐α levels were analyzed in duplicate using a Milliplex MAP Canine kit (Canine Cytokine/Chemokine MAGNETIC kit).8 The intra‐ and interassay variabilities of all these assays were <5 and <15%, respectively, and the assay sensitivities for IL‐6, IL‐10, IL‐18, and TNF‐α were 3.7, 8.5, 5.8, and 6.1 pg/mL, respectively. The assays were quantified using a Luminex system.9
The serum adiponectin levels were analyzed using a canine‐specific ELISA kit,8 where the intra‐ and interassay variabilities were <5 and <3%, respectively, and the assay sensitivity was 0.03 ng/mL. Serum resistin was analyzed using a canine‐specific ELISA kit10 , where the intra‐ and interassay variabilities were <5 and <7%, respectively, according to the manufacturer's instructions. The serum IL‐1β levels were analyzed using a Canine ELISA kit,11 where the intra‐ and interassay variabilities were <10 and <12%, respectively, and the assay sensitivity was 6.4 pg/mL. All samples, standards, and controls were assayed in duplicate. The optical density was determined using an automated microplate reader12 at 450 nm.
Determination of the Time When the Circulating Adipokine Concentration Was Evaluated after Treatment with Trilostane
Of the 15 dogs diagnosed with PDH, 9 dogs received treatment with trilostane.19, 20 The initial trilostane1 dose was 0.5–1.0 mg/kg, which was administered PO every 12 hours. Subsequently, the dose was adjusted based on the clinical signs and the ACTH‐stimulated cortisol concentration. The second round of testing was conducted when the dogs were judged to be clinically well controlled. This judgment was based on an improvement or the disappearance of clinical signs, and the post‐ACTH cortisol concentration had to be within the target range (2–5.5 μg/dL).
The ACTH‐stimulated serum cortisol concentrations were within the target ranges in 6 dogs after 16 weeks and 3 dogs after 20 weeks of treatment, and the dogs were reassessed after a further 4 weeks to determine whether their adipokine concentrations had been affected by the treatment. Sixteen weeks after the initiation of treatment, the clinical signs, including polyuria, polydipsia, and polyphagia, had resolved, whereas the alopecia and abdominal distension improved in all the dogs.
Statistical Analyses
All the statistical analyses were carried out using a commercially available statistical program.13 Normality tests (D'Agostino & Pearson omnibus) were performed to determine whether the data were normally distributed. Unpaired t‐tests were used to compare the differences between the healthy group and PDH group, and the data are reported as mean ± standard deviation (SD). P values for two‐tailed tests and 95% confidence interval (CI) for differences between means are expressed. Univariable linear regression was used to test the association between adipokines and cortisol concentrations. Wilcoxon matched‐pairs signed rank tests were used to compare data within the PDH group before and after treatment with trilostane, and the data are reported as median (range) and 95% CI. P < .05 was considered significant. This study is reported in compliance with STROBE guidelines.
Results
Differences in the Adipokine and Hormone Profiles of Healthy Dogs and Dogs with PDH
In the PDH group, the serum leptin (difference between means = 17.90 ± 1.841; 95% CI = 14.2–21.6; P < .0001) and insulin (difference between means = 7.255 ± 1.121; 95% CI = 5.0–9.5; P < .0001) concentrations were significantly higher than those in the healthy group (Fig 1). However, the differences in the adiponectin (difference between means = −2.176 ± 1.121; 95% CI = −4.5 to 0.084; P = .0587) and resistin (difference between means = −1.391 ± 2.551; 95% CI = −1.4 to 8.9; P = .1484) concentrations were not significantly different between dogs with PDH and healthy dogs.
Figure 1.
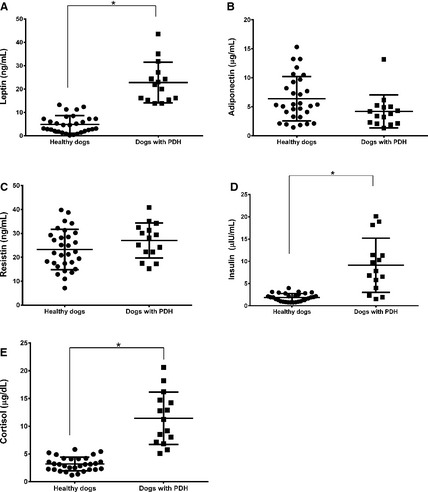
Scatter plots of the serum leptin, adiponectin, resistin, insulin, and cortisol concentrations in healthy dogs (n = 30) and dogs with pituitary‐dependent hyperadrenocorticism (n = 15). The horizontal bars indicate the mean ± SD. *The mean values were significantly (P < .05) different in the 2 groups (unpaired t‐test).
In the healthy group, the serum IL‐1β, IL‐6, and TNF‐α concentrations were below the detection limits in all dogs. The levels of IL‐10 and IL‐18 were detected in several dogs, ie, IL‐10 = 5/30 and IL‐18 = 9/30, and the median (range) concentrations were 9.3 pg/mL (8.9–23.0 pg/mL) and 21.0 pg/mL (12.0–123.0 pg/mL), respectively. In the PDH group, the serum IL‐1β, IL‐6, IL‐10, and IL‐18 concentrations were detected in several dogs, ie, IL‐1β = 4/15, IL‐6 = 7/15, IL‐10 = 6/15, and IL‐18 = 9/15, whereas the levels of TNF‐α were not detectable in any dogs.
Association between the Circulating Levels of Leptin and Cortisol
In the healthy dogs, the univariable linear regression did not identify an association between serum cortisol concentrations and any other of the measured hormones. In dogs with PDH, however, the linear regression detected associations between leptin and cortisol, indicating that an increase in the baseline cortisol concentration was associated with higher leptin concentrations in dogs with PDH (β = 1.292; 95% CI = 0.5088–2.075; P = .007) (Fig 2). There were no significant associations between cortisol and other hormones.
Figure 2.
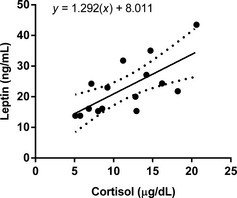
Linear association between the serum cortisol and leptin concentrations in dogs with pituitary‐dependent hyperadrenocorticism. The dotted line indicates the 95% confidence intervals.
Comparison before and after Treatment with Trilostane
In the dogs with PDH that were treated with trilostane, there were significant decreases in the serum leptin (95% CI = 10.1–21.7; P = .0039) and insulin (95% CI = 1.2–10.7; P = .0039) concentrations after treatment (Fig 3). However, there were no significant changes in serum adiponectin (95% CI = −1.5 to 0.2; P = .2031) and resistin (95% CI = −10.5 to 10.1; P = .8203) concentrations after treatment. There were significant decreases in the body weight (P = .0006) and BCS (P = .0060) after treatment.
Figure 3.
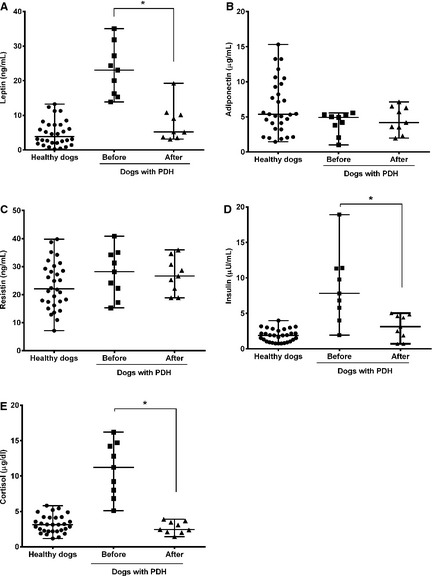
Scatter plots of the serum leptin, adiponectin, resistin, insulin, and cortisol concentrations in healthy dogs (n = 30) and dogs with pituitary‐dependent hyperadrenocorticism (PDH) (n = 9) before and after treatment with trilostane. The horizontal bars indicate the medians and ranges. *The values were significantly (P < .05) different within the PDH group (Wilcoxon matched‐pairs signed rank test).
Discussion
This study showed that the serum leptin concentrations of overweight dogs with PDH were higher than in similarly overweight dogs with normal adrenal function. This effect was supported by other data within this study, including a significant association between serum leptin and cortisol concentrations, and a decrease in leptin concentration after treatment with trilostane. In dogs with PDH, several factors might contribute to higher leptin concentrations than those in healthy overweight dogs. First, circulating leptin level is correlated with the body fat mass in dogs,21 so it is possible that an increased fat mass in the abdomen might lead to excessive hyperleptinemia. Second, in humans, it has been suggested that an increase in visceral fat has a relatively stronger effect on serum leptin in patients with Cushing's syndrome than it does in subjects with normal cortisolemia.22, 23, 24, 25 Therefore, it is also possible that an excess of endogenous cortisol promotes the production of leptin by adipose tissue without a change in fat mass.24 This theory is supported by the observation that the administration of glucocorticoids in dogs increased the leptin secretion in adipose tissue directly owing to the activation of leptin gene transcription.26, 27 Our findings suggest that the excessively increased circulating leptin levels in dogs with PDH might be associated with hypercortisolemia.
It is well known that chronic hypercortisolemia triggers insulin resistance in muscle, adipose tissue, and hepatocytes, which leads to high insulin concentrations in humans and rats.28, 29 Leptin might be involved in mediating insulin resistance in dogs.30 However, the relationship between leptin and insulin is not straightforward because hyperinsulinemia may cause hyperleptinemia by upregulating leptin mRNA expression in rat adipocytes.31 In human medicine, it has also been reported that insulin administration increases the blood leptin concentration.32 Thus, it is possible that hyperinsulinemia might contribute to hyperleptinemia.
In human medicine, hyperleptinemia in patients with Cushing's syndrome is related to a higher prevalence of cardiovascular diseases11 and it has been suggested that hyperleptinemia has proinflammatory, prothrombotic, and pro‐oxidant effects.33 The present findings suggest that the development and progression of complications related to HAC are probably associated with hyperleptinemia, although future longitudinal studies in dogs with HAC are required to support or refute this. Thus, this study analyzed proinflammatory cytokines, ie, TNF‐α, IL‐1β, IL‐6, and IL‐18, and an anti‐inflammatory cytokine, ie, IL‐10 to obtain further insights. The inflammatory cytokines were not detected in healthy overweight dogs, with the exception of IL‐18. Inflammatory cytokines were detected in several overweight dogs with PDH, although we did not examine the source of these cytokines so it is not clear if they were produced by adipose tissue (as is possible34), or whether there was infectious disease (to which dogs with HAC are prone). In human medicine, it has also been suggested that chronic hypercortisolemia in patients with Cushing's syndrome affects serum TNF‐α and IL‐6 levels, thereby contributing to the persistence of a low‐grade inflammatory state.11 However, further studies will be necessary to clarify the relationships between hyperleptinemia or inflammatory cytokines and the well‐known complications in dogs with HAC.
In this study, the administration of trilostane to dogs with PDH reduced serum leptin and insulin concentrations, body weight, and BCS once target post‐ACTH cortisol concentrations were attained. After the treatment, however, the mean leptin and insulin levels in dogs with PDH were still higher than those in healthy dogs. This might be attributable to the persistence of hypercortisolemia for parts of each day, and to persistently higher visceral fat mass after the treatment. In human studies, subjects with Cushing's syndrome have persistent central fat mass, higher leptin concentrations, and other abnormal adipokines, even after long‐term cure.35, 36 Therefore, it is possible that the maintenance of hyperleptinemia might have been the result of secretion of leptin by the persistent central fat mass in dogs with PDH. A quantitative evaluation of the visceral fat using appropriate methods, such as dual‐energy X‐ray absorptiometry scanning, would have helped to clarify whether the hyperleptinemia in dogs with PDH was related to the fat distribution in the abdomen relative to the total body fat, and this was an important limitation of this study. Thus, quantitative assessments of the visceral fat mass will be necessary in dogs with HAC.
This study found that the circulating adiponectin levels of overweight dogs with PDH were not significantly different from those of similarly overweight healthy dogs. In addition, there were no differences in the serum adiponectin concentrations of the 9 dogs after trilostane treatment. This might be the result of the small number of PDH dogs included in the study and resultant lack of statistical power. On the basis of power calculations using these data, we estimate that future studies should include at least 39 dogs in each group to have an 80% chance of finding a 50% difference in serum adiponectin concentrations in HAC dogs. The adiponectin gene is one of the most highly expressed genes in white adipose tissue depots in dogs.37 The best‐characterized effects of adiponectin include enhanced insulin sensitivity, anti‐inflammatory properties, and inhibition of the development of atherosclerosis.38 In contrast to leptin, it has been shown that an increase in the fat mass decreases the circulating adiponectin level, whereas weight loss increases the adiponectin concentrations in obese human, primates, and rodents.39, 40 Similarly, adiponectin might be inversely related to adiposity in obese dogs,41 although there is a controversy about the inverse relationship.30, 42 However, the effects of excess glucocorticoids on the blood adiponectin levels remain controversial. In human medicine, a study found that patients with Cushing's syndrome had lower adiponectin levels than the controls, which suggests that hypercortisolemia directly affected the adiponectin levels independent of body weight.43 However, another study reported that the serum adiponectin levels in patients with Cushing's syndrome did not differ from those in the control group and they were not changed significantly after hypophysectomy, despite a significant decrease in the body fat mass, insulin resistance, and cortisol.36, 44 In veterinary medicine, a recent study showed that the circulating adiponectin concentrations in 20 PDH dogs with blindness were significantly lower than those of 20 healthy dogs.18 Our study did not replicate this finding. The effect of HAC, like the effect of obesity, on serum adiponectin concentrations remains uncertain.
In this study, no difference was detected in the resistin concentrations of the dogs with PDH and healthy dogs. In addition, there were no changes in the serum resistin concentrations of 9 dogs administered trilostane after treatment. Resistin is a recently described adipokine, which was originally detected as a product of murine adipocytes.45, 46 In mice, resistin appears to be involved in mediating obesity‐induced insulin resistance, but this is not the case in humans. Little is known about the physiologic effects of resistin in dogs. In rodents, it has been shown that resistin secretion follows a similar pattern to leptin, where the circulating levels increase with fat mass.45 It has been suggested that the expression of resistin may be upregulated in humans with type I DM, type II DM, obesity, and inflammatory diseases.46 In veterinary medicine, however, there is only 1 report of increased serum resistin concentrations in dogs with diabetic ketoacidosis.7 Further study is required to fully understand the role of resistin in dogs with HAC, especially its associations with glucose dysregulation and the development of insulin resistance.
This study was originally designed as a pilot study to identify fruitful areas of research, and includes several limitations. One limitation was the small number of dogs with HAC included, which limits the reliance that can be placed on negative findings (such as the nonsignificant difference in adiponectin concentrations). However, this limitation does not reduce the validity of positive findings, such as the significantly higher insulin and leptin concentrations in dogs with HAC. Another limitation was that although the control dogs were chosen to have similar body condition score to the dogs with HAC, they were not matched on other criteria such as neuter status. The dogs with HAC included several intact females, whereas the control females were neutered, so some of the differences might be attributable to this rather than to the presence or absence of HAC.42 Future studies should avoid this limitation by strictly matching controls with cases.
Overall, our study suggests that higher circulating leptin concentrations are associated with hypercortisolemia in dogs with PDH. This might explain the development of complications in dogs with HAC, but further studies will be necessary to confirm this result and to elucidate the effects of chronic hypercortisolemia on the expressions of other adipokines.
Acknowledgments
The authors thank all the owners of dogs included in the study. We especially thank Dr Kurt Verkest of VetWrite (vetwrite@gmail.com) for helpful discussions in the revision of our manuscript. We also thank Professor Tae‐Young Heo (Department of Information and Statistics, Chungbuk National University, Republic of Korea) for statistical consultation and analyses.
Grant support: This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (2013R1A1A1011113).
Conflicts of Interest: The authors disclose no conflict of interest.
An abstract that includes results from this study was presented in part at the 2013 ACVIM Forum, Seattle, WA
Footnotes
Vetoryl, Arnolds Veterinary Products, Shrewsbury, UK
Hitachi 7020, Hitachi High‐Technologies Co., Tokyo, Japan
Humalyte, Human GmbH, Wiesbaden, Germany
Alpha 5, Aloka Co., Tokyo, Japan
Synacthen, Novartis, Basel, Switzerland
Immulite 1000 analyzer, Diagnostic Products Co, Los Angeles, CA
Dexamethasone, Je Il Pharm Co., Daegu, Korea
Millipore Co, Billerica, MA
Luminex®200™, Luminex Co, Billerica, MA
Canine Resistin ELISA kit, TSZ ELISA, Framingham, MA
Canine ELISA kit, USCN Life Sciences Co Ltd, Wuhan, China
ELx 808, BioTek instruments Inc, Winooski, VT
Prism 6, GraphPad Software Inc, La Jolla, CA
References
- 1. Feldman EC. Distinguishing dogs with functioning adrenocortical tumors from dogs with pituitary‐dependent hyperadrenocorticism. J Am Vet Med Assoc 1983;183:195–200. [PubMed] [Google Scholar]
- 2. Reusch CE, Feldman EC. Canine hyperadrenocorticism due to adrenocortical neoplasia. Pretreatment evaluation of 41 dogs. J Vet Intern Med 1991;5:3–10. [DOI] [PubMed] [Google Scholar]
- 3. Guptill L, Scott‐Moncrieff JC, Widmer WR. Diagnosis of canine hyperadrenocorticism. Vet Clin North Am Small Anim Pract 1997;27:215–235. [DOI] [PubMed] [Google Scholar]
- 4. Feldman EC, Nelson RW, Feldman MS. Use of low‐ and high‐dose dexamethasone tests for distinguishing pituitary‐dependent from adrenal tumor hyperadrenocorticism in dogs. J Am Vet Med Assoc 1996;209:772–775. [PubMed] [Google Scholar]
- 5. Pan W, Kastin AJ. Adipokines and the blood brain barrier. Peptides 2007;28:1317–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Radin MJ, Sharkey LC, Holycross BJ. Adipokines: A review of biological and analytical principles and an update in dogs, cats, and horses. Vet Clin Pathol 2009;38:136–156. [DOI] [PubMed] [Google Scholar]
- 7. O'Neill S, Drobatz K, Satyaraj E, Hess R. Evaluation of cytokines and hormones in dogs before and after treatment of diabetic ketoacidosis and in uncomplicated diabetes mellitus. Vet Immunol Immunopathol 2012;148:276–283. [DOI] [PubMed] [Google Scholar]
- 8. Rockall AG, Sohaib SA, Evans D, et al. Computed tomography assessment of fat distribution in male and female patients with Cushing's syndrome. Eur J Endocrinol 2003;149:561–567. [DOI] [PubMed] [Google Scholar]
- 9. Nishii N, Yamasaki M, Takasu M, et al. Plasma leptin concentration in dogs with diabetes mellitus. J Vet Med Sci 2010;72:809–811. [DOI] [PubMed] [Google Scholar]
- 10. Mazaki‐Tovi M, Feuermann Y, Segev G, et al. Increased serum leptin and insulin concentrations in canine hypothyroidism. Vet J 2010;183:109–114. [DOI] [PubMed] [Google Scholar]
- 11. Valassi E, Biller BM, Klibanski A, Misra M. Adipokines and cardiovascular risk in Cushing's syndrome. Neuroendocrinology 2012;95:187–206. [DOI] [PubMed] [Google Scholar]
- 12. Pivonello R, Faggiano A, Lombardi G, Colao A. The metabolic syndrome and cardiovascular risk in Cushing's syndrome. Endocrinol Metab Clin North Am 2005;34:327–339. [DOI] [PubMed] [Google Scholar]
- 13. Iwasaki Y, Takayasu S, Nishiyama M, et al. Is the metabolic syndrome an intracellular Cushing state? Effects of multiple humoral factors on the transcriptional activity of the hepatic glucocorticoid‐activating enzyme (11beta‐hydroxysteroid dehydrogenase type 1) gene. Mol Cell Endocrinol 2008;285:10–18. [DOI] [PubMed] [Google Scholar]
- 14. Peterson ME, Altszuler N, Nichols CE. Decreased insulin sensitivity and glucose tolerance in spontaneous canine hyperadrenocorticism. Res Vet Sci 1984;36:177–182. [PubMed] [Google Scholar]
- 15. Ortega TM, Feldman EC, Nelson RW, et al. Systemic arterial blood pressure and urine protein/creatinine ratio in dogs with hyperadrenocorticism. J Am Vet Med Assoc 1996;209:1724–1729. [PubMed] [Google Scholar]
- 16. LaRue MJ, Murtaugh RJ. Pulmonary thromboembolism in dogs: 47 cases (1986–1987). J Am Vet Med Assoc 1990;197:1368–1372. [PubMed] [Google Scholar]
- 17. Forrester SD, Troy GC, Dalton MN, et al. Retrospective evaluation of urinary tract infection in 42 dogs with hyperadrenocorticism or diabetes mellitus or both. J Vet Intern Med 1999;13:557–560. [DOI] [PubMed] [Google Scholar]
- 18. Cabrera Blatter MF, del Prado B, Miceli DD, et al. Interleukin‐6 and insulin increase and nitric oxide and adiponectin decrease in blind dogs with pituitary‐dependent hyperadrenocorticism. Res Vet Sci 2012;93:1195–1202. [DOI] [PubMed] [Google Scholar]
- 19. Feldman EC. Evaluation of twice‐daily lower‐dose trilostane treatment administered orally in dogs with naturally occurring hyperadrenocorticism. J Am Vet Med Assoc 2011;238: 1441–1451. [DOI] [PubMed] [Google Scholar]
- 20. Cho KD, Kang JH, Chang D, et al. Efficacy of low‐ and high‐dose trilostane treatment in dogs (<5 kg) with pituitary‐dependent hyperadrenocorticism. J Vet Intern Med 2013;27:91–98. [DOI] [PubMed] [Google Scholar]
- 21. Ishioka K, Soliman MM, Sagawa M, et al. Experimental and clinical studies on plasma leptin in obese dogs. J Vet Med Sci 2002;64:349–353. [DOI] [PubMed] [Google Scholar]
- 22. Schafroth U, Godang K, Ueland T, et al. Leptin levels in relation to body composition and insulin concentration in patients with endogenous Cushing's syndrome compared to controls matched for body mass index. J Endocrinol Invest 2000;23:349–355. [DOI] [PubMed] [Google Scholar]
- 23. Robaczyk M, Krzyzanowiska‐Swiniarska B, Andrysiak‐Mamos E, et al. Plasma leptin levels in relation to body composition and body fat distribution in patients with Cushing's syndrome. Pol Arch Med Wewn 2003;110:1299–1308. [PubMed] [Google Scholar]
- 24. Masuzaki H, Ogawa Y, Hosoda K, et al. Glucocorticoid regulation of leptin synthesis and secretion in humans: Elevated plasma leptin levels in Cushing's syndrome. J Clin Endocrinol Metab 1997;82:2542–2547. [DOI] [PubMed] [Google Scholar]
- 25. Leal‐Cerro A, Considine RV, Peino R, et al. Serum immnoreactive‐leptin levels are increased in patients with Cushing's syndrome. Horm Metab Res 1996;28:711–713. [DOI] [PubMed] [Google Scholar]
- 26. Nishii N, Takasu M, Ohba Y, et al. Effects of administration of glucocorticoids and feeding status on plasma leptin concentrations in dogs. Am J Vet Res 2006;67:266–270. [DOI] [PubMed] [Google Scholar]
- 27. Yilmaz Z, Ilcol YO, Golcu E. Serum leptin and ghrelin levels in response to methylprednisolone injection in healthy dogs. Res Vet Sci 2007;82:187–194. [DOI] [PubMed] [Google Scholar]
- 28. Andrews RC, Walker BR. Glucocorticoids and insulin resistance: Old hormones, new targets. Clin Sci 1999;96:513–523. [DOI] [PubMed] [Google Scholar]
- 29. Ruzzin J, Wagman AS, Jensen J. Glucocorticoid‐induced insulin resistance in skeletal muscle: Defects in insulin signalling and the effects of a selective glycogen synthase kinase‐3 inhibitor. Diabetologia 2005;48:2119–2130. [DOI] [PubMed] [Google Scholar]
- 30. Verkest KR, Fleeman LM, Morton JM, et al. Compensation for obesity‐induced insulin resistance in dogs: Assessment of the effects of leptin, adiponectin, and glucagon‐like peptide‐1 using path analysis. Domest Anim Endocrinol 2011;41:24–34. [DOI] [PubMed] [Google Scholar]
- 31. Cusin I, Sainsbury A, Doyle P, et al. The ob gene and insulin. A relationship leading to clues to the understanding of obesity. Diabetes 1995;44:1467–1470. [DOI] [PubMed] [Google Scholar]
- 32. Laferrere B, Caixas A, Fried SK, et al. A pulse of insulin and dexamethasone stimulates serum leptin in fasting human subjects. Eur J Endocrinol 2002;146:839–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Katagiri H, Yamada T, Oka Y. Adiposity and cardiovascular disorder: Disturbance of the regulatory system consisting of humoral and neuronal signals. Circ Res 2007;101:27–39. [DOI] [PubMed] [Google Scholar]
- 34. Fischer‐Posovszky P, Wabitsch M, Hochberg Z. Endocrinology of adipose tissue – an update. Horm Metab Res 2007;39:314–321. [DOI] [PubMed] [Google Scholar]
- 35. Barahona MJ, Sucunza N, Resmini E, et al. Persistent body fat mass and inflammatory marker increases after long‐term cure of Cushing's syndrome. J Clin Endocrinol Metab 2009;94:3365–3371. [DOI] [PubMed] [Google Scholar]
- 36. Krsek M, Silha JV, Jezkova J, et al. Adipokine levels in Cushing's syndrome; Elevated resistin levels in female patients with Cushing's syndrome. Clin Endocrinol (Oxf) 2004;60:350–357. [DOI] [PubMed] [Google Scholar]
- 37. Brunson BL, Zhong Q, Clarke KJ, et al. Serum concentrations of adiponectin and characterization of adiponectin protein complexes in dogs. Am J Vet Res 2007;68:57–62. [DOI] [PubMed] [Google Scholar]
- 38. Hopkins TA, Ouchi N, Shibata R, Walsh K. Adiponectin actions in the cardiovascular system. Cardiovasc Res 2007;74:11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose‐specific gene dysregulated in obesity. J Biol Chem 1996;271:10697–10703. [DOI] [PubMed] [Google Scholar]
- 40. Hotta K, Funahashi T, Bodkin NL, et al. Circulating concentrations of the adipocyte protein adiponectin are decreased in parallel with reduced insulin sensitivity during the progression to type 2 diabetes in rhesus monkeys. Diabetes 2001;50:1126–1133. [DOI] [PubMed] [Google Scholar]
- 41. Ishioka K, Omachi A, Sagawa M, et al. Canine adiponectin: cDNA structure, mRNA expression in adipose tissues and reduced plasma levels in obesity. Res Vet Sci 2006;80:127–132. [DOI] [PubMed] [Google Scholar]
- 42. Verkest KR, Rose FJ, Fleeman LM, et al. Adiposity and adiponectin in dogs: Investigation of causes of discrepant results between two studies. Domest Anim Endocrinol 2011;41:35–41. [DOI] [PubMed] [Google Scholar]
- 43. Fallo F, Scarda A, Sonino N, et al. Effect of glucocorticoids on adiponectin: A study in healthy subjects and in Cushing's syndrome. Eur J Endocrinol 2004;150:339–344. [DOI] [PubMed] [Google Scholar]
- 44. Libe R, Morpurgo PS, Cappiello V, et al. Ghrelin and adiponectin in patients with Cushing's disease before and after successful transsphenoidal surgery. Clin Endocrinol (Oxf) 2005;62:30–36. [DOI] [PubMed] [Google Scholar]
- 45. Lazar MA. Resistin‐ and obesity‐associated metabolic diseases. Horm Metab Res 2007;39:710–716. [DOI] [PubMed] [Google Scholar]
- 46. Schwartz DR, Lazar MA. Human resistin: Found in translation from mouse to man. Trends Endocrinol Metab 2011;22:259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]


