Abstract
Background
Many chemotherapy protocols have been reported for treatment of canine appendicular osteosarcoma (OSA), but outcome comparisons in a single population are lacking.
Objective
To evaluate the effects of protocol and dose intensity (DI) on treatment outcomes for carboplatin and doxorubicin‐based chemotherapy protocols.
Animals
Four hundred and seventy dogs with appendicular OSA.
Methods
A retrospective cohort study was performed comprising consecutive dogs treated (1997–2012) with amputation followed by 1 of 5 chemotherapy protocols: carboplatin 300 mg/m2 IV q21d for 4 or 6 cycles (CARBO6), doxorubicin 30 mg/m2 IV q14d or q21d for 5 cycles, and alternating carboplatin 300 mg/m2 IV and doxorubicin 30 mg/m2 IV q21d for 3 cycles. Adverse events (AE) and DI were evaluated. Kaplan–Meier survival curves and Cox proportional hazards regression were used to compare disease‐free interval (DFI) and survival time (ST) among protocols.
Results
The overall median DFI and ST were 291 days and 284 days, respectively. A lower proportion of dogs prescribed CARBO6 experienced AEs compared to other protocols (48.4% versus 60.8–75.8%; P = .001). DI was not associated with development of metastases or death. After adjustment for baseline characteristics and prognostic factors, none of the protocols provided a significant reduction in risk of development of metastases or death.
Conclusions and Clinical Importance
Although choice of protocol did not result in significant differences in DFI or ST, the CARBO6 protocol resulted in a lower proportion of dogs experiencing AEs, which could be advantageous in maintaining high quality of life during treatment. DI was not a prognostic indicator in this study.
Keywords: Canine, Limb amputation, Oncology, Primary bone tumor
Abbreviations
- 99mTc
technetium 99 m
- AE
adverse event
- ALP
serum alkaline phosphatase
- CARBO4
chemotherapy protocol with carboplatin 300 mg/m2 IV q21d for 4 doses
- CARBO6
chemotherapy protocol with carboplatin 300 mg/m2 IV q21d for 6 doses
- CARBODOX6
chemotherapy protocol with carboplatin 300 mg/m2 IV and doxorubicin 30 mg/m2 IV on an alternating schedule q21d for 3 cycles
- CBC
complete blood count
- CI
confidence interval
- DFI
disease‐free interval
- DI
dose intensity
- DOX5.2
chemotherapy protocol with doxorubicin 30 mg/m2 IV q14d for 5 doses
- DOX5.3
chemotherapy protocol with doxorubicin 30 mg/m2 IV q21d for 5 doses
- GI
gastrointestinal
- HR
hazard ratio
- OSA
osteosarcoma
- ST
survival time
- TD
treatment delay
Osteosarcoma (OSA) is a biologically aggressive primary bone tumor that has a very high rate of microscopic metastases at diagnosis in dogs.1 Curative‐intent treatments combine methods of local control such as limb amputation, limb‐sparing surgery, or radiation therapy, with chemotherapy for treatment of microscopic metastases.2 Amputation is a common and efficacious method of local control for appendicular OSA; the addition of chemotherapy postoperatively has been shown to improve survival times (ST).3, 4, 5, 6, 7 Many different chemotherapy protocols have been described resulting in a large range of median ST (235–540 days, with 1 study reporting up to 840 days).4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 Among these studies, single agent carboplatin protocols with 4 doses have been evaluated. Occasionally, this protocol is extended to 6 doses, but to date limited outcome information has been reported.8, 19, 20 Our clinical impression of using a 6‐dose single agent carboplatin protocol is that this protocol is well tolerated in dogs and results in comparatively long DFI and ST. Many studies have evaluated 1 chemotherapy protocol or compared the protocol to amputation alone, making comparisons of different chemotherapy protocols difficult given the diversity of baseline characteristics and prognostic factors among study populations.
The choice of chemotherapy protocol may be based on the perceived benefit versus risk of the available protocols with consideration of reported efficacy, toxicity, intensity of the protocol for the dog and owner, and cost. Many studies evaluating chemotherapy for OSA have reported efficacy, toxicity, and duration of the protocol, with few reporting a measure of chemotherapy protocol intensity.4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 Dose intensity (DI) is a measure of the intensity of the chemotherapy protocol and is defined as the quantity of drug administered to a dog per week of treatment (mg/m2/wk).21 The DI of chemotherapy protocols for canine appendicular OSA has rarely been reported, and to the authors' knowledge, DI has not been investigated as a prognostic factor in canine OSA.10 The importance of the DI of neo adjuvant chemotherapy protocols for treatment of human OSA is controversial, but has been associated with outcome in some studies.22, 23, 24, 25, 26
The primary purpose of this study was to evaluate the effects of protocol choice on treatment outcomes (adverse effects [AE], disease‐free interval [DFI], and ST) for 5 different chemotherapy protocols. The secondary purpose was to assess DI as a prognostic factor in the treatment of canine appendicular OSA. We hypothesized that 6 doses of single agent carboplatin chemotherapy would result in significantly longer DFI and ST compared to other protocols. We further hypothesized that DI would be associated with DFI and ST in dogs with appendicular OSA.
Materials and Methods
Case Selection and Medical Record Review
An electronic medical record search of an institutional primary bone tumor registry was performed to identify dogs diagnosed with appendicular OSA between January 1, 1997, and April 30, 2012 that received treatment consisting of limb amputation and 1 of 5 protocols of IV carboplatin or doxorubicin‐based chemotherapy or both. For inclusion in this retrospective cohort study, dogs must have had pre operative thoracic radiographs or computed tomography and histopathologic confirmation of the diagnosis. Furthermore, dogs must have been prescribed and received at least 1 treatment of 1 of 5 chemotherapy protocols postoperatively. Exclusion criteria were presence or suspicion of metastases at any site before amputation or prior treatment of appendicular OSA with radiation (palliative or curative‐intent) protocols, chemotherapy, or surgery.
A medical record review was performed to obtain information about baseline characteristics including age and body weight at diagnosis, breed, sex, and neuter status. Previously reported prognostic factors were assessed including presence or absence of proximal humeral OSA, and results of hematology and serum biochemistry performed at presentation (serum alkaline phosphatase [ALP], lymphocyte and monocyte counts).27, 28 If the dog received pre operative whole body scintigraphy (with technetium 99 m [99mTc]), the results of the test were recorded. Treatment information was obtained from the medical record including date of amputation, chemotherapy protocol prescribed, dose and agent(s) administered, and body weight at each treatment. Date of administration of the last dose of chemotherapy, total number of doses received, and whether or not the protocol was completed or terminated prematurely were recorded. If a dog failed to complete the prescribed chemotherapy protocol, the reason for premature termination was determined whenever possible. If chemotherapy treatments were delayed, the duration of the delay and reason were recorded. For this study, a clinically relevant treatment delay (TD) was defined as a delay in administration of chemotherapy for ≥7 days beyond the planned date of chemotherapy administration. The planned date of administration of the first dose of chemotherapy was 14 days after amputation. After the first dose of chemotherapy, the planned date of chemotherapy administration was estimated based on the prescribed interval (either 14 or 21 days depending on the protocol) from the previous dose of chemotherapy. The reason for TD was categorized as because of surgical complications, owner reasons (eg, schedule, travel, finances), toxicity, or for unknown or other specified reasons. Complete blood counts (CBC) from 7 to 21 days after administration of each chemotherapy dose were reviewed to determine, if hematologic AEs had occurred. The history given by the dog owner at each visit throughout the duration of the protocol allowed identification of gastrointestinal (GI) AEs. If present, these were retrospectively graded based on the Veterinary Co‐operative Oncology Group – Common Terminology Criteria for Adverse Events v1.1.29
Chemotherapy Protocols
Dogs receiving single agent carboplatin chemotherapy protocols were prescribed 300 mg/m2 of carboplatin administered IV q21d for either 4 (CARBO4) or 6 cycles (CARBO6). Dogs receiving single agent doxorubicin protocols were prescribed 30 mg/m2 of doxorubicin administered IV either q14d (DOX5.2) or q21d (DOX5.3) for a total of 5 cycles. Dogs prescribed an alternating carboplatin and doxorubicin protocol (CARBODOX6) were started with either agent at a dosage of 300 mg/m2 IV of carboplatin or 30 mg/m2 IV of doxorubicin; 1 agent was given q21d on an alternating schedule for 3 cycles (3 doses of each agent for a total of 6 doses).
Before the first dose of doxorubicin, screening with echocardiography was performed in some dogs at the clinician and owners' discretion. After the first dose, CBCs generally were performed at 7 or 14 days or both depending on clinician preference and the protocol prescribed. For all protocols, dogs had a CBC performed at each visit before administration of chemotherapy and a TD of variable length (dependent on clinician preference) was instituted, if the absolute neutrophil count was <2,000/μL or if the platelet count was <75,000/μL. If AEs occurred, dose reductions were performed at the clinicians' discretion.
Dose Intensity Evaluation
The target DI was calculated for each protocol by division of total amount of each drug planned to be administered in each protocol by the planned duration of the protocol and expressed as a total mg/m2/wk. The actual DI for each chemotherapy agent for each individual dog was calculated by the Hryniuk method as the total milligrams per body surface area of each drug received in the protocol divided by the actual duration of the protocol in weeks.21 This was calculated to account for any TDs or dose reductions that occurred. The body surface area at each dose was calculated using the standard equation (BSA = 10.1 × BW2/3/10,000). The duration of the protocol was defined as the time between the first dose of chemotherapy and 2 weeks for DOX5.2 or 3 weeks after the last dose of chemotherapy in all other protocols.21
To allow comparison of DI across protocols and for inclusion in the statistic analysis, the fractional DI was calculated to yield the summation DI for each protocol. The fractional DI was calculated as the actual DI for each agent divided by the target DI. If the dog received the full single agent chemotherapy protocol without any dose reduction or delay then the target fractional DI would be 1. For the dogs receiving CARBODOX6, the fractional DI was calculated for each agent separately as the actual DI of that agent divided by the target DI of the single agent protocol (100 mg/m2/wk for carboplatin and 10 mg/m2/wk for doxorubicin) resulting in target fractional DI of 0.6 for carboplatin and 0.6 for doxorubicin. The summation DI was calculated for the CARBODOX6 protocol as the sum of the fractional DI for the agents; carboplatin and doxorubicin were weighted equally based on approximately equivalent relative potencies of the single agents.10, 30 Summation DI of the single agent protocols is equivalent to the fractional DI. The mean ± standard deviation actual DI and summation DI were calculated as summary statistics for each protocol.
Follow‐up
Thoracic radiography was commonly recommended on the day of the last dose of chemotherapy or 1 month after this dose for all chemotherapy protocols. For the protocols with 6 prescribed doses (CARBO6 and CARBODOX6), thoracic radiography often was recommended before the 3rd or 4th dose of the protocol. Technectium‐99 m bone scans and other diagnostics tests were performed when indicated at clinician and client discretion. Diagnostic test reports were reviewed for the date of development of metastases or recurrence and site of first metastasis. After development of metastases or recurrence, additional treatments were used based on clinician and owner preference. Outcome information was prospectively obtained by recheck visits, telephone interview of the referring veterinarian and owner, or both as part of data collection for the institutional primary tumor registry. Information collected included the date of first detection of metastases or local recurrence, the first site of metastases detected, date of loss to follow‐up, death or euthanasia, and reason for death or euthanasia.
Statistic Analysis
Continuous data were assessed graphically for normality and described using mean and standard deviation if normally distributed, or median and interquartile range if nonnormally distributed. Frequencies and percentages were used to describe categoric data. Baseline characteristics at diagnosis were compared between groups of prescribed chemotherapy protocols using ANOVA for continuous variables and Chi‐square test for categoric variables. When the overall ANOVA F‐test result was statistically significant, Tukey's honest significant difference posthoc tests were applied to determine the presence of pairwise differences.
Kaplan–Meier methodology was used to generate survival curves and calculate median DFI and ST with 95% confidence intervals (CI). The DFI was calculated as the number of days from the date of amputation to the date of first detection of metastases or local recurrence, and the ST was calculated as the number of days from amputation to death attributable to any cause. Dogs were censored in the DFI analysis, if they did not have documented metastases at the time of last follow‐up or the time of death. Dogs were censored in the survival analysis, if they were alive at last follow‐up or were lost to follow‐up. The end point of death attributable to any cause was chosen over death caused by disease because of the aggressive nature of this disease. Many dogs will die of disease, but there is potential for underrecognition of death caused by disease because few may undergo necropsy and some may not have imaging before euthanasia and death. This in turn can result in longer STs than if death attributable to any cause is considered. The authors elected to utilize death attributable to any cause for this report so that the estimates of survival would be more conservative.
Cox proportional hazards regression analysis was used to evaluate associations between baseline characteristics (canine age and body weight at diagnosis, breed [purebred versus mixed]), previously identified prognostic factors (proximal humeral tumor site, ALP, monocyte, and lymphocyte counts), chemotherapy treatment factors (protocol, grade 3 or 4 toxicity, number of TDs, or ≥2 TDs), and outcome measures (DFI and ST). Multivariable analysis was performed with Cox proportional hazards regression analysis to allow adjustment for other variables to assess protocol effectiveness on DFI and ST. Modified intent‐to‐treat and per‐protocol analyses were performed and reported to assess chemotherapy protocol effectiveness. The modified intent‐to‐treat analysis included all dogs prescribed the chemotherapy protocol regardless of the number of doses administered to allow assessment of treatment outcomes in both dogs that completed and those that did not complete the chemotherapy protocol to decrease the bias of exclusion of dogs experiencing rapid disease progression. The per‐protocol analysis included only dogs that completed the prescribed chemotherapy protocol. Given the susceptibility of this analysis to bias, the results from the per‐protocol analysis are presented in the supporting information.
A power analysis was performed with the PASS software package1 using a Cox proportional hazards model for comparisons of end points of DFI and ST among multiple groups according to methods described by Lachin and Foulkes.31, 32 Power calculations were performed to detect a hazard ratio of 0.75 (in 1 group) for 2 comparisons: a comparison of dogs receiving carboplatin (CARBO4 and CARBO6 [n = 200]) versus doxorubicin (DOX5.2 and DOX5.3 [n = 200]) single agent protocols and comparison of the 5 individual protocols (n = 100 in each group). The power for comparison of the single agent protocols was 0.73 and for the 5 protocols was 0.43.
Statistic significance was set at α = 0.05 and the statistic analysis was performed using software packages SAS2 and GraphPad Prism.3
Results
Patient Eligibility and Baseline Characteristics
Four hundred and ninety‐nine dogs were examined for eligibility after the medical record search; all dogs received treatment with amputation and were prescribed 1 of the 5 chemotherapy protocols described for treatment of appendicular OSA. Twenty‐nine dogs were excluded after medical record review: 18 for documented presence of metastatic disease (12 lymph node, 2 lung, and 4 bone), 8 for prior treatment with palliative radiation, 2 for prior treatment with chemotherapy, and 1 dog failed to receive any doses of chemotherapy after prescription of the protocol. Four hundred and seventy dogs were confirmed eligible and included in the statistic analysis. The baseline characteristics of these dogs are described in Table 1. Of the 470 dogs, pre operative whole body scintigraphy (with 99mTc) was performed in the majority of dogs (317, 68.5%). After surgery, CARBO4 and CARBO6 protocols were prescribed for 93 and 91 dogs, respectively, CARBODOX6 was prescribed for 97 dogs, and DOX5.2 and DOX5.3 were prescribed for 124 and 65 dogs, respectively. These dogs formed the basis of the modified intent‐to‐treat analysis presented. The temporal distribution of the prescription of the different protocols for these dogs (Fig 1) showed prescription of DOX5.2 and DOX5.3 protocols in the early‐ to mid‐study period (1997–2005) whereas CARBODOX6 was prescribed throughout most of the study period (1999–2009). CARBO4 protocol was prescribed during the mid‐ to late‐study period (2002–2012) and CARBO6 was prescribed more commonly in the late‐study period (2008–2012). Only 84 dogs (of 286 dogs; 29.4%) had echocardiography performed before receiving doxorubicin in the CARBODOX6, DOX5.2, and DOX5.3 protocols. The only difference in baseline characteristics among protocols was that dogs prescribed CARBO6 had a significantly lower mean body weight at diagnosis compared to dogs prescribed CARBO4, CARBODOX6, and DOX5.2 (35.2 kg versus 40.8 kg, 39.7 kg, and 39.7 kg, respectively; P < .005), but not compared to DOX5.3 (38.3 kg; P = .10).
Table 1.
Baseline characteristics of 470 dogs with appendicular osteosarcoma.
| Characteristic | |
| Mean age, ± SD (years) | 8.6 ± 2.5 |
| Mean body weight, ± SD (kg) | 38.9 ± 11.6 |
| Sex and neuter status (%) | |
| Female intact | 7 (1.5) |
| Female spayed | 209 (44.5) |
| Male castrated | 239 (50.9) |
| Male intact | 15 (3.2) |
| Breed (%) | |
| Mixed | 97 (20.6) |
| Purebred | 373 (79.4) |
| Anatomic site (%) | |
| Forelimb | 295 (62.8) |
| Hindlimb | 175 (37.2) |
| Proximal humeral tumor location | 127 (27.0) |
| Median (IQR) blood work results at presentation | |
| ALP (IU/dL) | 92 (100) |
| Monocyte (×106 cells) | 0.4 (0.4) |
| Lymphocyte (×106 cells) | 1.3 (0.8) |
SD, standard deviation; IQR, interquartile range; ALP, serum alkaline phosphatase.
Figure 1.
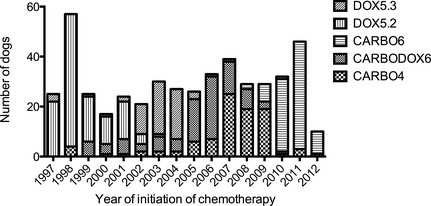
Temporal distribution of the prescribed chemotherapy protocols over the study period.
Chemotherapy Protocols and DI
More than 50% of dogs completed the prescribed chemotherapy protocols for all groups except CARBO6 in which only 45.1% of dogs completed the protocol (Table 2). The most common reason for early termination of the chemotherapy protocol was development of metastases in all protocols except CARBO6 in which owner reasons were the most common reason for termination (25.3% of CARBO6 dogs; Table 2). Inadequate finances and scheduling conflicts were examples of owner reasons for delay. The mean duration of chemotherapy was 9 days longer in dogs completing the CARBO6 protocol compared to those completing the CARBODOX6 protocol (138 versus 129 days). Comparing DI across protocols, the mean summation DI was closest to the target summation DI in dogs prescribed CARBO4 and DOX5.3 protocols (0.9 versus 1; Table 2).
Table 2.
Summary of chemotherapy treatment and dose intensity (DI) information for 470 dogs with appendicular osteosarcoma.
| CARBO4 (93 dogs) | CARBO6 (91 dogs) | CARBODOX6 (97 dogs) | DOX5.2 (124 dogs) | DOX5.3 (65 dogs) | |
|---|---|---|---|---|---|
| Doses received, median (IQR) | 4.0 (1.0) | 5.0 (2.0) | 6.0 (2.0) | 5.0 (0) | 5.0 (1.0) |
| Completion of protocol, dogs (%) | 57 (61.3) | 41 (45.1) | 59 (60.8) | 97 (78.2) | 37 (56.9) |
| Duration of chemotherapy (days), mean (±SD) | |||||
| All dogs | 79 (±29) | 111 (± 39) | 112 (±33) | 73 (±46) | 92 (±32) |
| Dog that completed protocol | 92 (±11) | 138 (±17) | 129 (±13) | 75 (±39) | 112 (±18) |
| Reasons for early termination (%) | |||||
| Metastases | 22 (23.7) | 20 (22.0) | 16 (16.5) | 18 (14.5) | 18 (27.7) |
| Owner reasons | 8 (8.6) | 23 (25.3) | 8 (8.3) | 0 (0.0) | 5 (7.7) |
| Death attributable to any cause | 1 (1.1) | 0 (0.0) | 1 (1.0) | 5 (4.0) | 1 (1.5) |
| Toxicity | 1 (1.1) | 0 (0.0) | 3 (3.3) | 2 (1.6) | 2 (3.1) |
| Other | 0 | 1a (1.1) | 2b (2.2) | 1c (1.6) | 2d (3.1) |
| Unknown | 3 (3.2) | 1 (1.1) | 4 (4.1) | 0 (0.0) | 1 (1.5) |
| Target DI, mg/m2/wk | 100.0 | 100.0 | 60.0 | 15.0 | 10.0 |
| Actual DI, mg/m2/wk, mean (±SD) | 85.3 (±14.6) | 84.7 (±11.1) |
C:49.4 (±13.9) D:4.1 (±1.3) |
12.6 (±2.7) | 8.9 (±1.2) |
| Target summation DI | 1.0 | 1.0 | 1.2 | 1.0 | 1.0 |
| Summation DI, mean (±SD) | 0.9 (±0.1) | 0.8 (±0.1) | 0.9 (±0.1) | 0.8 (±0.2) | 0.9 (±0.1) |
SD, standard deviation; IQR, interquartile range; C, carboplatin; D, doxorubicin.
The dog had a cervical myelopathy of unknown cause.
One dog had hindlimb mobility problems and 1 dog had recurrence at amputation site.
One dog had elbow luxation that had amputation of that limb.
One dog had poor HL mobility and 1 dog had worsening chronic renal disease.
Treatment Outcome
A total of 295 dogs experienced AE; a lower proportion of dogs prescribed CARBO6 experienced AE compared to all other protocols (48.4% versus 62.3% [CARBO4], 60.8% [CARBODOX6], 75.8% [DOX5.2], and 61.5% [DOX5.3]; P = .001; Table 3). Grade 1 AE were the most common highest‐grade AE reported in all protocols; the proportion of dogs experiencing grade 3 and 4 AE was not significantly different among protocols ranging from 4.1% to 9.6% (P = .62; Table 3). Gastrointestinal AE comprised the majority of all AE experienced in all protocols aside from CARBO6 where hematologic AE was the most common (P < .0001; Fig 2). More AE were reported after the first dose of chemotherapy in all protocols aside from CARBO6, and the number of AE generally tended to decrease as the number of administered doses increased with all protocols (Fig S1). A greater proportion of dogs (50.6%) had TD in the CARBO6 treatment protocol compared to other protocols (30.8–41.9%; Table S1). TD occurred throughout the chemotherapy protocols with no other consistent trends occurring across or within the protocols (Fig S2). Owner reasons and toxicity were the most common reasons for TD in all protocols (Table S1). Reasons for TD were considered unknown, if the medical records failed to provide sufficient information to determine the reason for delay.
Table 3.
The highest‐grade toxicity seen in 470 dogs with appendicular osteosarcoma treated with limb amputation and prescribed carboplatin and doxorubicin‐based chemotherapy protocols.
| Protocol | No Reported Toxicity (%) | Grade (%) | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| CARBO4 | 35 (37.6) | 37 (39.8) | 17 (18.3) | 1 (1.1) | 3 (3.2) | 0 (0.0) |
| CARBO6 | 47 (51.6) | 29 (31.9) | 9 (9.9) | 3 (3.3) | 3 (3.3) | 0 (0.0) |
| CARBODOX6 | 38 (39.2) | 37 (38.1) | 13 (13.4) | 4 (4.1) | 4 (4.1) | 1 (1.0) |
| DOX5.2 | 30 (24.2) | 48 (38.7) | 34 (27.4) | 7 (5.6) | 5 (4.0) | 0 (0.0) |
| DOX5.3 | 25 (38.5) | 20 (30.8) | 16 (24.6) | 4 (6.2) | 0 (0.0) | 0 (0.0) |
Figure 2.
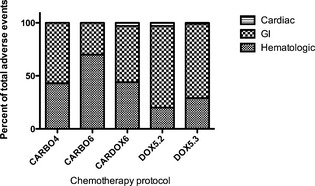
Distribution of type of adverse events by chemotherapy protocol.
The median DFI for all dogs prescribed any chemotherapy protocol (modified intent‐to‐treat analysis) was 291 days (95% CI: 253, 362 days) and the median ST was 284 days (95% CI: 248, 316 days). One hundred and seventy‐six dogs did not have documented metastatic disease at the time of last follow‐up or death. The distribution of metastatic disease, first site of metastasis, and whether or not the dog received medical treatment for metastatic disease are summarized by protocol in Table 4. Thirty‐one dogs were alive and 13 dogs were lost to follow‐up; these dogs were censored in the survival analysis. The median follow‐up time for censored dogs was 1,439 days (95% CI: 1,252, 3,372 days). Survival at 1, 2, and 3 years is summarized in Table 5.
Table 4.
Summary of distribution of first metastatic disease event and treatment for metastatic disease by chemotherapy protocol.
| CARBO 4 | CARBO6 | CARBODOX6 | DOX5.2 | DOX5.3 | |
|---|---|---|---|---|---|
| Number of dogs that developed metastases | 63 (67.7%) | 48 (52.8%) | 66 (68.0%) | 81 (65.3%) | 36 (55.4%) |
| First site of mets/recurrence | |||||
| Lungs | 43 | 35 | 49 | 60 | 30 |
| Bone | 13 | 12 | 5 | 14 | 2 |
| Lungs and bone | 2 | 0 | 7 | 2 | 0 |
| Recurrence | 0 | 0 | 1 | 0 | 0 |
| Othera | 5 | 1 | 4 | 5 | 4 |
| Treatment for metastasesb | |||||
| IV chemo | 9 | 3 | 2 | 1 | 0 |
| Metronomicsc | 7 | 8 | 7 | 0 | 7 |
| IV chemo followed by metronomicsc | 3 | 0 | 0 | 0 | 0 |
| Toceranibd ± metronomic cyclophosphamide | 2 | 8 | 1 | 0 | 0 |
Other organs including skin, lymph nodes, abdominal organs.
Other than prednisolone or nonsteroidal anti‐inflammatory alone.
Metronomic cyclophosphamide and a nonsteroidal anti‐inflammatory.
Palladia, Zoetis, Florham Park, NJ.
Table 5.
Proportion of dogs surviving at 1, 2, and 3 years after amputation and different adjuvant chemotherapy protocols.
| Prescribed Chemotherapy Protocol | 1‐year Survival Proportion | 2‐year Survival Proportion | 3‐year Survival Proportion |
|---|---|---|---|
| CARBO4 | 0.35 | 0.19 | 0.09 |
| CARBO6 | 0.38 | 0.13 | 0.03 |
| CARBODOX6 | 0.44 | 0.20 | 0.10 |
| DOX5.2 | 0.42 | 0.15 | 0.04 |
| DOX5.3 | 0.29 | 0.14 | 0.06 |
For dogs that completed the chemotherapy protocol (per‐protocol analysis) the median DFI was 398 days (95% CI: 317, 492 days) and median ST was 367 days (95% CI: 325, 401 days). One hundred and twenty‐one dogs had no documented metastases at the time of last follow‐up or death. Twenty‐four dogs were censored in the survival analysis (20 were alive at last follow‐up and 4 dogs were lost to follow‐up). The median follow‐up of censored dogs was 3,372 days (95% CI: 1,256, 3,372 days).
Factors associated with DFI, ST, or both in the univariable analysis included age at diagnosis, body weight, purebred dog, proximal humeral tumor location, ALP, lymphocyte count, TD, treatment after development of metastatic disease, and summation DI (Table 6 and Table S2). Insufficient information was available for calculation of DI in 77 dogs. Summation DI was associated with an increased hazard of metastases or recurrence in all dogs (HR, 2.82; 1.05, 7.63; P = .04), but was not prognostic for survival in all dogs.
Table 6.
Factors associated with disease‐free interval (DFI), survival times, or both (ST) in univariable analysis for the modified intent‐to‐treat analysis.
| Variable | DFI | ST | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age at diagnosis (years) | 0.99 (0.95, 1.04) | .69 | 1.05 (1.01, 1.09) | .02 |
| Body weight (kg) | 1.02 (1.01, 1.03) | <.001 | 1.02 (1.01, 1.03) | <.001 |
| Pure breed dog | 1.40 (1.04, 1.87) | .027 | 1.35 (1.06, 1.72) | .016 |
| Proximal humeral tumor | 1.50 (1.16, 1.93) | .0017 | 1.49 (1.20, 1.85) | <.001 |
| ALP (continuous) | 1.001 (1.000, 1.001) | .0084 | 1.000 (1.000, 1.001) | .048 |
| Lymphocyte count (continuous) | 0.82 (0.66, 1.02) | .074 | 0.78 (0.65, 0.93) | .006 |
| Treatment delays (continuous) | 0.85 (0.76, 0.96) | .007 | 0.92 (0.83, 1.00) | .06 |
| Summation dose intensity | 2.82 (1.05, 7.63) | .04 | 1.84 (0.82, 4.14) | .14 |
| Treatment following development of metastatic disease | – | – | 1.89 (1.41, 2.54) | <.0001 |
HR, hazard ratio.
In the univariable analysis, dogs that received the CARBO6 protocol had a significantly lower risk of developing metastases (only dogs that completed the protocol) and of death attributable to any cause (in all dogs and dogs that completed the protocol) (Table 6 and Table S2). In the multivariable analysis after adjustment for age at diagnosis, body weight, purebred dog, proximal humeral tumor location, ALP, and treatment after development of metastatic disease (ST only), the risks of development of metastases and death attributable to any cause were not significantly associated with protocol choice (Table 7 and Table S3; Fig S5 and S6). The results were not adjusted for all baseline, prognostic, and chemotherapy factors because of concerns about collinearity. There was no difference in risk of development of metastases and death attributable to any cause between dogs receiving carboplatin single agent protocols (CARBO4 and CARBO6) versus doxorubicin single agent protocols (DOX5.2 and DOX5.3) (Table 7 and Table S3).
Table 7.
The effect of chemotherapy protocol on disease‐free interval (DFI) and survival times (STs) for the modified intent‐to‐treat analysis.
| Protocols | DFI | ST | ||||||
|---|---|---|---|---|---|---|---|---|
| Median DFI (days) | Crude HR | Adjusteda HR (95% CI) | P value | MST (days) | Crude HR | Adjustedb HR (95% CI) | P value | |
| CARBO4 | 282 | 1.02 | 1.13 (0.79, 1.60) | .50 | 299 | 0.88 | 0.83 (0.61, 1.13) | .23 |
| CARBO6 | 399 | 0.74 | 0.83 (0.57, 1.20) | .32 | 306 | 0.72c | 0.75 (0.54, 1.03) | .08 |
| CARBODOX6 | 302 | 0.93 | 0.99 (0.70, 1.40) | .97 | 314 | 0.80 | 0.85 (0.63, 1.13) | .26 |
| DOX5.2 | 269 | 1.0 | 1.0 (ref) | – | 252 | 1.0 | 1.0 (ref) | – |
| DOX5.3 | 302 | 0.90 | 1.08 (0.73, 1.62) | .69 | 241 | 0.91 | 1.00 (0.72, 1.39) | .99 |
| Carboplatin | 298 | 0.91 | 0.95 (0.73, 1.25) | .72 | 301 | 0.84 | 0.89 (0.71, 1.12) | .31 |
| Doxorubicin | 276 | 1.0 | 1.0 (ref) | – | 248 | 1.0 | 1.0 (ref) | – |
Adjusted for age at diagnosis, body weight, purebred dog, proximal humeral tumor location, and ALP.
Adjusted for age at diagnosis, body weight, purebred dog, proximal humeral tumor location, ALP, and treatment after development of metastatic disease.
Significant decrease in hazard ratio (HR) in the univariable analysis. Carboplatin, single agent carboplatin protocols (CARBO4 CARBO6); doxorubicin, single agent doxorubicin protocols (DOX5.2, DOX5.3).
Discussion
The lowest risk of development of metastases and death attributable to any cause was seen in dogs prescribed or having completed the CARBO6 protocol, but after adjustment for baseline characteristics and prognostic factors, the result was not statistically significant. This protocol also resulted in the lowest proportion of dogs experiencing toxicity of any protocol.
The study population baseline characteristics and treatment outcomes reported were similar to previous reports.4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 Dogs prescribed CARBO6 had lower mean body weight than dogs prescribed other protocols. In this cohort and in other reports, body weight was identified as a prognostic factor, with heavier dogs having worse treatment outcomes.8, 33 This in part may explain changes in risk and lack of statistic significance for the CARBO6 protocol after adjustment for this and other factors in the multivariable Cox regression analysis. Another theoretical reason for longer survival in this group could be differences in treatment of metastatic disease with more dogs prescribed CARBO6 receiving toceranib,4 which may have resulted in delay of progression of metastatic disease and prolongation of survival.34 However, this was not the case in this study with increased hazard of death resulting in dogs that received treatment after development of metastatic disease. This finding could be because of selection bias resulting in owners or veterinarians being more likely to pursue or recommend treatment for metastatic disease in more biologically aggressive cases. The lack of detected difference among protocols could be a result of a lack of true difference between the evaluated protocols with the majority of the evaluated protocols being single agent protocols differing in agent, number of doses, or DI. In people, multi agent protocols are favored for treatment of OSA and are associated with 5‐year survival of >60%. A recent evaluation of a carboplatin and doxorubicin protocol in a developing country reported poorer survival outcomes in human patients.35, 36 Alternatively, it is possible that a difference existed, but was not detected because of the protocol group sample size resulting in relatively low power and higher risk of type 2 error.
Decreased toxicity was identified in dogs prescribed CARBO6, with the majority of the toxicity being hematologic rather than GI as in the other protocols. A smaller proportion of dogs may have experienced AE with this protocol because of lower DI of the protocol, meaning dogs were receiving lower doses weekly because of dose reductions or TD. This hypothesis is supported by a lower summation DI of 0.8 compared to the target summation DI of 1 for this protocol. In addition, the apparent lower toxicity could be the result of reporting. Adverse events may not have been reported by owners or healthcare professionals interviewing owners especially after the last doses of longer protocols because of habituation to milder GI AE. This could explain why the CARBO6 protocol had apparently lower toxicity than the CARBO4 protocol. Given that the majority of the AE in the CARBO6 group were hematologic, events may have been underappreciated because although CBCs were taken immediately before each dose, after the first dose interim CBCs may not have been performed unless toxicity was detected. Furthermore, there may be increased individual canine variation in timing of the neutrophil and platelet nadirs with carboplatin compared to doxorubicin. There also could be differences among dogs receiving multiple cycles of treatment with some dogs showing apparent worsening of nadirs with subsequent doses because of individual variation in bone marrow recovery time among individual dogs receiving carboplatin and doxorubicin. The use of different antiemetics (eg, maropitant5) during the study period also could have affected the rates of GI AE. The use of drugs to decrease AE was not recorded in this study, making it difficult to estimate the effect this had on the frequency of AE.
High summation DI was associated with an increased hazard of development of metastases and was not associated with survival in this study. This finding conflicted with the strong associations that have been identified between increased DI of methotrexate and doxorubicin and survival in humans with OSA.23, 25 To date, the effect of the DI of carboplatin on survival in humans with OSA is unknown. The observed effect of DI on DFI may have been affected by factors influencing DI including TD. TD in this study often were because of owner reasons rather than toxicity, which may have resulted in lower actual and summation DI. Alternatively, the association between summation DI and DFI could have resulted from type 1 error. This study does not provide evidence to suggest that decreasing DI will result in the same or improved survival, nor does it support recommendation of further dose intensification of existing protocols.
The CARBO6 protocol had the highest proportion of dogs experiencing TD and the lowest proportion of dogs that completed the protocol, with dogs more commonly being withdrawn for owner reasons rather than development of metastases. The high proportion of dogs experiencing TD could have been related to the greater proportion of hematologic AE experienced by dogs prescribed this protocol, resulting in a greater likelihood of TD compared to dogs experiencing GI signs. This conclusion is supported by the relatively high proportion of TD reported because of toxicity in dogs prescribed CARBO6 (30.4%). The most likely reason for low protocol completion could be that the owners were more likely to consider stopping after 4 doses of carboplatin given it was an established protocol and that staging at mid point of the protocol could have led to increased probability of detection of metastases and termination before completion.
The study population was assessed for prognostic factors, and common prognostic factors reported in other studies also were found in this study including body weight, proximal humeral tumor location, and ALP.8, 27, 33 Because of the observational nature of this study and lack of randomization, it was necessary to adjust for these factors across groups in the Cox proportional hazards regression analysis to assess the effect of chemotherapy protocol on DFI and ST. A limitation of this approach is that adjustment can only be performed for measured factors. Other unmeasured factors that could influence the treatment effect may have been present, but were not considered. Other study limitations include that re‐evaluation thoracic radiographs were recommended every 2–3 months, but were performed at the discretion of the owner. This may have resulted in lengthening of DFI caused by increased censorship, if radiographs were not taken and development of metastasis could not be documented or because of less frequent radiographs being performed resulting in detection of metastases at a later time point. Routine staging with whole body scintigraphy after amputation was not performed in dogs in this cohort, making it difficult to assess the effect of occult bone metastases on outcome in this study. Given the nonuniform collection of postchemotherapy CBC (7–21 days posttreatment), there may have been differences in the distribution of hematologic AE because of less appreciation of AE in individual dogs or relative under‐ or over appreciation of hematologic AE in some protocols because of different monitoring recommendations by clinicians and variable owner compliance. Some dogs did not have full data available for each chemotherapy dose (body weight, drug dose, date of chemotherapy) to allow calculation of DI, which resulted in a smaller dataset to evaluate summation DI as a prognostic factor. These dogs often received at least 1 chemotherapy dose at their local veterinarian often because of owner convenience and consequently were assumed to be missing at random (not introducing bias) but having the effect of decreasing available sample size, which could have decreased power and resulted increased probability of type 2 error.
Although choice of carboplatin‐ or doxorubicin‐based chemotherapy protocol did not result in significant differences in DFI or ST in this study, the CARBO6 protocol resulted in a lower proportion of dogs experiencing AE, which could be an advantage in maintaining high quality of life in dogs during treatment for appendicular OSA. DI was not a prognostic indicator for dogs treated with amputation and adjuvant chemotherapy in this study.
Supporting information
Table S1. Summary of treatment delays (TDs) seen in 470 dogs with appendicular OSA treated with limb amputation and prescribed carboplatin and doxorubicin‐based chemotherapy protocols.
Table S2. Factors associated with disease‐free interval (DFI), survival time (ST), or both on univariable analysis (per‐protocol analysis).
Table S3. The effect of choice of chemotherapy protocol on disease‐free interval (DFI) and survival times (STs) for the per‐protocol analysis.
Figure S1. Trends in adverse events by chemotherapy protocol.
Figure S2. Trends in treatment delays by chemotherapy protocol.
Figure S3. Kaplan–Meyer curve for DFI for single agent carboplatin protocols (CARBO4 and CARBO6) compared to single agent doxorubicin protocols (DOX5.2 and DOX5.3) (modified intent‐to‐treat analysis).
Figure S4. Kaplan–Meyer curve for ST for single agent carboplatin protocols compared to single agent doxorubicin protocols (modified intent‐to‐treat analysis).
Figure S5. Kaplan–Meyer curve for DFI for all protocols (modified intent‐to‐treat analysis).
Figure S6. Kaplan–Meyer curve for ST for all protocols (modified intent‐to‐treat analysis).
Acknowledgment
This study did not utilize financial support or grants. The authors thank Anna Barón, PhD for assistance with the statistic analysis and Mary Lafferty, Vicki Jamieson, and Irene Mok for aid with data collection.
Conflict of Interest: Authors disclose no conflict of interest.
Selected preliminary results were presented in abstract form at the Veterinary Cancer Society Meeting, Las Vegas, NV on October 20,, 2012
Footnotes
PASS 12, NCSS, LLC, Kaysville, UT
SAS software, Version 9.3 of the SAS System for PC. Copyright © 2012 SAS Institute Inc SAS and all other SAS Institute Inc product or service names are registered trademarks or trademarks of SAS Institute Inc, Cary, NC
GraphPad Prism for Windows, Version 6, San Diego, CA
Palladia, Zoetis, Florham Park, NJ
Cerenia, Zoetis
References
- 1. Straw RC, Withrow SJ, Powers BE. Management of canine appendicular osteosarcoma. Vet Clin North Am Small Animal Pract 1990;20:1141–1161. [DOI] [PubMed] [Google Scholar]
- 2. Ehrhart NP, Ryan SD, Fan TM. Tumors of the skeletal system In: Withrow SJ, Vail DM, Page RL, eds. Withrow & MacEwen's Small Animal Clinical Oncology, 5th ed St. Louis, MO: Elsevier; 2013:463–503. [Google Scholar]
- 3. Berg J, Weinstein MJ, Schelling SH, et al. Treatment of dogs with osteosarcoma by administration of cisplatin after amputation or limb‐sparing surgery: 22 cases (1987‐1990). J Am Vet Med Assoc 1992;200:2005–2008. [PubMed] [Google Scholar]
- 4. Berg J, Gebhardt MC, Rand WM. Effect of timing of postoperative chemotherapy on survival of dogs with osteosarcoma. Cancer 1997;79:1343–1350. [PubMed] [Google Scholar]
- 5. Straw RC, Withrow SJ, Richter SL, et al. Amputation and cisplatin for treatment of canine osteosarcoma. J Vet Intern Med 1991;5:205–210. [DOI] [PubMed] [Google Scholar]
- 6. Thompson JP, Fugent MJ. Evaluation of survival times after limb amputation, with and without subsequent administration of cisplatin, for treatment of appendicular osteosarcoma in dogs: 30 cases (1979‐1990). J Am Vet Med Assoc 1992;200:531–533. [PubMed] [Google Scholar]
- 7. Mauldin GN, Matus RE, Withrow SJ, et al. Canine osteosarcoma. Treatment by amputation versus amputation and adjuvant chemotherapy using doxorubicin and cisplatin. J Vet Intern Med 1988;2:177–180. [DOI] [PubMed] [Google Scholar]
- 8. Bergman PJ, MacEwen EG, Kurzman ID, et al. Amputation and carboplatin for treatment of dogs with osteosarcoma: 48 cases (1991 to 1993). J Vet Intern Med 1996;10:76–81. [DOI] [PubMed] [Google Scholar]
- 9. Bacon NJ, Ehrhart NP, Dernell WS, et al. Use of alternating administration of carboplatin and doxorubicin in dogs with microscopic metastases after amputation for appendicular osteosarcoma: 50 cases (1999‐2006). J Am Vet Med Assoc 2008;232:1504–1510. [DOI] [PubMed] [Google Scholar]
- 10. Bailey D, Erb H, Williams L, et al. Carboplatin and doxorubicin combination chemotherapy for the treatment of appendicular osteosarcoma in the dog. J Vet Intern Med 2003;17:199–205. [DOI] [PubMed] [Google Scholar]
- 11. Berg J, Weinstein MJ, Springfield DS, et al. Results of surgery and doxorubicin chemotherapy in dogs with osteosarcoma. J Am Vet Med Assoc 1995;206:1555–1560. [PubMed] [Google Scholar]
- 12. Chun R, Kurzman ID, Couto CG, et al. Cisplatin and doxorubicin combination chemotherapy for the treatment of canine osteosarcoma: A pilot study. J Vet Intern Med 2000;14:495–498. [DOI] [PubMed] [Google Scholar]
- 13. Chun R, Garrett LD, Henry C, et al. Toxicity and efficacy of cisplatin and doxorubicin combination chemotherapy for the treatment of canine osteosarcoma. J Am Anim Hosp Assoc 2005;41:382–387. [DOI] [PubMed] [Google Scholar]
- 14. Liptak JM, Dernell WS, Straw RC, et al. Proximal radial and distal humeral osteosarcoma in 12 dogs. J Am Anim Hosp Assoc 2004;40:461–467. [DOI] [PubMed] [Google Scholar]
- 15. McMahon M, Mathie T, Stingle N, et al. Adjuvant carboplatin and gemcitabine combination chemotherapy postamputation in canine appendicular osteosarcoma. J Vet Intern Med 2011;25:511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moore AS, Dernell WS, Ogilvie GK, et al. Doxorubicin and BAY 12‐9566 for the treatment of osteosarcoma in dogs: A randomized, double‐blind, placebo‐controlled study. J Vet Intern Med 2007;21:783–790. [DOI] [PubMed] [Google Scholar]
- 17. Shapiro W, Fossum TW, Kitchell BE, et al. Use of cisplatin for treatment of appendicular osteosarcoma in dogs. J Am Vet Med Assoc 1988;192:507–511. [PubMed] [Google Scholar]
- 18. Kraegel SA, Madewell BR, Simonson E, et al. Osteogenic sarcoma and cisplatin chemotherapy in dogs: 16 cases (1986‐1989). J Am Vet Med Assoc 1991;199:1057–1059. [PubMed] [Google Scholar]
- 19. Phillips B, Powers BE, Dernell WS, et al. Use of single‐agent carboplatin as adjuvant or neoadjuvant therapy in conjunction with amputation for appendicular osteosarcoma in dogs. J Am Anim Hosp Assoc 2009;45:33–38. [DOI] [PubMed] [Google Scholar]
- 20. Saam DE, Liptak JM, Stalker MJ, et al. Predictors of outcome in dogs treated with adjuvant carboplatin for appendicular osteosarcoma: 65 cases (1996‐2006). J Am Vet Med Assoc 2011;238:195–206. [DOI] [PubMed] [Google Scholar]
- 21. Longo DL, Duffey PL, DeVita VT, Jr. , et al. The calculation of actual or received dose intensity: A comparison of published methods. J Clinical Oncol 1991;9:2042–2051. [DOI] [PubMed] [Google Scholar]
- 22. Bacci G, Ferrari S, Longhi A, et al. Relationship between dose‐intensity of treatment and outcome for patients with osteosarcoma of the extremity treated with neoadjuvant chemotherapy. Oncol Rep 2001;8:883–888. [DOI] [PubMed] [Google Scholar]
- 23. Delepine N, Delepine G, Bacci G, et al. Influence of methotrexate dose intensity on outcome of patients with high grade osteogenic osteosarcoma. Analysis of the literature. Cancer 1996;78:2127–2135. [PubMed] [Google Scholar]
- 24. Eselgrim M, Grunert H, Kuhne T, et al. Dose intensity of chemotherapy for osteosarcoma and outcome in the Cooperative Osteosarcoma Study Group (COSS) trials. Pediatr Blood Cancer 2006;47:42–50. [DOI] [PubMed] [Google Scholar]
- 25. Kawai A, Sugihara S, Kunisada T, et al. The importance of doxorubicin and methotrexate dose intensity in the chemotherapy of osteosarcoma. Arch Orthop Trauma Surg 1996;115:68–70. [DOI] [PubMed] [Google Scholar]
- 26. Lewis IJ, Weeden S, Machin D, et al. Received dose and dose‐intensity of chemotherapy and outcome in nonmetastatic extremity osteosarcoma. European Osteosarcoma Intergroup. J Clinical Oncol 2000;18:4028–4037. [DOI] [PubMed] [Google Scholar]
- 27. Boerman I, Selvarajah GT, Nielen M, et al. Prognostic factors in canine appendicular osteosarcoma – A meta‐analysis. BMC Vet Res 2012;8:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sottnik JL, Rao S, Lafferty MH, et al. Association of blood monocyte and lymphocyte count and disease‐free interval in dogs with osteosarcoma. J Vet Intern Med 2010;24:1439–1444. [DOI] [PubMed] [Google Scholar]
- 29. Veterinary cooperative oncology group – Common terminology criteria for adverse events (VCOG‐CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.1. Vet Comp Oncol 2011:1–30. doi:10.1111/j.1476‐5829.2011.00283.x. [DOI] [PubMed] [Google Scholar]
- 30. Frei E, 3rd , Elias A, Wheeler C, et al. The relationship between high‐dose treatment and combination chemotherapy: The concept of summation dose intensity. Clin Cancer Res 1998;4:2027–2037. [PubMed] [Google Scholar]
- 31. Lachin JM, Foulkes MA. Evaluation of sample size and power for analyses of survival with allowance for nonuniform patient entry, losses to follow‐up, noncompliance, and stratification. Biometrics 1986;42:507–519. [PubMed] [Google Scholar]
- 32. Lachin JM. Sample size and power for a logrank test and Cox proportional hazards model with multiple groups and strata, or a quantitative covariate with multiple strata. Stat Med 2013; doi:10.1002/sim.5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lascelles BD, Dernell WS, Correa MT, et al. Improved survival associated with postoperative wound infection in dogs treated with limb‐salvage surgery for osteosarcoma. Ann Surg Oncol 2005;12:1073–1083. [DOI] [PubMed] [Google Scholar]
- 34. London C, Mathie T, Stingle N, et al. Preliminary evidence for biologic activity of toceranib phosphate (Palladia® in solid tumours. Vet Comp Oncol 2012;10:194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hagleitner MM, de BE, Te Loo DM. Survival trends and long‐term toxicity in pediatric patients with osteosarcoma. Sarcoma 2012;2012; Article ID 636405, 5 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Choeyprasert W, Natesirinilkul R, Charoenkwan P, et al. Carboplatin and doxorubicin in treatment of pediatric osteosarcoma: A 9‐year single institute experience in the northern region of Thailand. Asian Pac J Cancer Prev 2013;14:1101–1106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Summary of treatment delays (TDs) seen in 470 dogs with appendicular OSA treated with limb amputation and prescribed carboplatin and doxorubicin‐based chemotherapy protocols.
Table S2. Factors associated with disease‐free interval (DFI), survival time (ST), or both on univariable analysis (per‐protocol analysis).
Table S3. The effect of choice of chemotherapy protocol on disease‐free interval (DFI) and survival times (STs) for the per‐protocol analysis.
Figure S1. Trends in adverse events by chemotherapy protocol.
Figure S2. Trends in treatment delays by chemotherapy protocol.
Figure S3. Kaplan–Meyer curve for DFI for single agent carboplatin protocols (CARBO4 and CARBO6) compared to single agent doxorubicin protocols (DOX5.2 and DOX5.3) (modified intent‐to‐treat analysis).
Figure S4. Kaplan–Meyer curve for ST for single agent carboplatin protocols compared to single agent doxorubicin protocols (modified intent‐to‐treat analysis).
Figure S5. Kaplan–Meyer curve for DFI for all protocols (modified intent‐to‐treat analysis).
Figure S6. Kaplan–Meyer curve for ST for all protocols (modified intent‐to‐treat analysis).


