Abbreviations
- CBC
complete blood count
- CLIA
chemiluminescence immunoassay
- CT
computed tomography
- fT4
free thyroxine
- PAS
periodic acid‐Shiff
- RER
rough endoplasmatic reticulum
- TSH
thyroid‐stimulating hormone
- TT4
serum total
A 5‐year‐old, neutered male domestic shorthair cat was referred for evaluation of suspected hypothyroidism. In the previous 2 months, the owner reported mild lethargy, weight gain with decreased appetite, unkempt hair coat, and an episode of bilateral external otitis that temporarily improved after a 10‐day treatment with ear medications containing gentamicin, betamethasone, and clotrimazole1; treatment was completed 50 days before presentation. The cat had been slightly overweight for several months before the referring veterinarian was consulted, but more precise information was not available from the owner. The cat was started on a commercial diet to control obesity.2 One month before admission, the referring veterinarian performed a CBC, serum biochemical profile, and urinalysis, and the results were unremarkable. In addition, serum total thyroxine concentration (TT4) was within normal limits (1.1 μg/dL; reference range, 0.8–4.7) and free thyroxine concentration (fT4), measured by chemiluminescence immunoassay (CLIA), was low (<3.9 μg/dL; reference range, 9.0–33.5).
On admission, the cat was obese (body condition score, 8/93) with a body weight of 7.6 kg, and had an unkempt hair coat with diffuse scaling and hypotrichosis ventrally (Fig 1). On palpation of the thyroid region, bilateral symmetric nodules with a diameter of approximately 2–3 cm each were detected. Based on a dermatologic examination, widespread exfoliative dermatosis and bilateral ceruminous otitis externa were diagnosed. Fungal culture of plucked hairs and scraped scales identified Microsporum canis infection.
Figure 1.

The affected cat on admission; note the diffuse scaling with unkempt hair coat.
The thyroid tests were repeated at the same laboratory and results included a low TT4 (<0.7 μg/dL; reference range, 0.8–4.7), low fT4 measured by equilibrium dialysis (<0.4 ng/dL; reference range, 0.7–2.3), and high canine thyroid‐stimulating hormone (TSH) concentration (5.6 ng/mL; reference range, <0.5), leading to a diagnosis of primary hypothyroidism. The cat was started on levothyroxine4 at the dosage of 0.1 mg PO q24h. In addition, topical econazole, q3d and itraconazole, 5 mg/kg PO q24h, were prescribed for the dermatophytosis.
To investigate the thyroid gland and identify any ectopic thyroid tissue and to characterize the pituitary gland, computed tomography (CT) of the head, neck, and chest was scheduled 5 days later under general anesthesia, along with surgical excision of a thyroid nodule. Before induction of anesthesia, an electrocardiogram and echocardiography were performed that identified no abnormality. The CT was obtained with helical acquisition using a 4‐slice scanner5 with 1.25 mm slice thickness (acquisition parameters: 120 kV, 160 mAs, 1 pitch). After IV administration of 2 mL/kg iohexol,6 a dynamic study of the pituitary gland was obtained continuously scanning in sequence from the rostral to the caudal margin of the sella turcica until the contrast medium1 was washed out. Thereafter, a second dose of iohexol was given (1 mL/kg) and contrast medium‐enhanced images of the thyroid gland were acquired. The CT showed 2 symmetric masses originating from the thyroid lobes connected by a thin isthmus (left lobe size: 0.8 × 1.2 × 3.2 cm; right lobe size: 0.6 × 1.0 × 2.3 cm); (Fig. 2). The thyroid tissue was isoattenuating to the surrounding musculature (average precontrast Hounsfield Units 41.7). The pituitary gland, brain, and thorax were normal.
Figure 2.
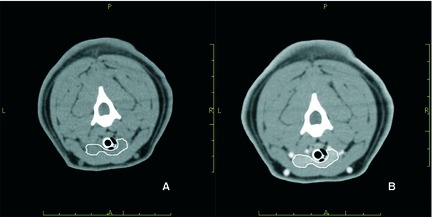
Transverse precontrast (A) and postcontrast (B) computed tomographic images of the neck. The thyroid tissue (outlined in white) is isoattenuating to the surrounding musculature in the precontrast image (A) and is hyperattenuating in the postcontrast image (B).
An excisional biopsy specimen of the right lobe of the thyroid gland, which grossly appeared dark red, was fixed in 10% neutral buffered formalin and embedded in paraffin; it was then sectioned at 4 μm and stained with hematoxylin and eosin and periodic acid‐Shiff (PAS). On light microscopy, the thyroid parenchyma was characterized by large lobules containing follicles (Fig 3). Follicles were variable in size, often very small, irregularly shaped, or collapsed multifocally, and lined by ≥1 layer of follicular cells. The follicular lumina generally were empty, but occasionally were filled with scant amorphous homogeneous PAS‐positive material, consistent with colloid. Some colloid vacuoles were evident. Follicular cells were increased in number and size. Cells were cuboidal with moderate, nonhomogeneous, slightly eosinophilic, sometimes vacuolated cytoplasm, and had round, centrally located nuclei with finely granular to marginated chromatin and small nucleoli. Anisocytosis and anisokaryosis were mild. Mitoses were absent. Finally, occasional mild hemorrhage was evident throughout the tissue. These findings were consistent with diffuse follicular hyperplasia of the thyroid gland. From the same thyroid sample, an aliquot was prepared for transmission electron microscopy. The tissue was divided into small segments 1 mm in length, fixed with 2% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4), and processed for ultrastructural analysis. Sections were stained with uranyl acetate and lead citrate and were examined with an electron microscope.7 The rough endoplasmatic reticulum (RER) of most thyroid cells was severely dilated, with distended cisternae. Mitochondria were sparse, decreased in number, and sometimes slightly swollen. Lysosomes, exocytic vesicles, and endocytic vesicles were few and scattered, and nuclei were centrally displaced or, in some cells, apically displaced (Fig. 4).
Figure 3.
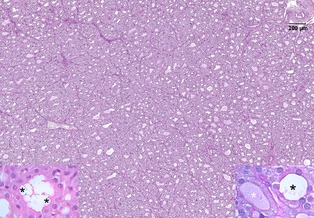
Thyroid gland of the affected cat. At low magnification, the architecture of the gland appears altered: the lobules are densely cellular, with variably sized or collapsed follicles. The follicular lumens are smaller than normal, irregularly shaped, and often empty. Bar = 200 μm, PAS stain. Left inset: high magnification (100×) of a follicle. Multiple small pseudovacuoles (asterisks) are visible in the lumen because of the intense endocytosis of colloid typical of diffuse hyperplasia. HE. Right inset: 2 adjacent follicles at high magnification (100×). The follicle on the left contains a small amount of colloid, weakly stained with PAS (white asterisk) in contrast to the follicle on the right, which has an empty lumen (black asterisk). PAS.
Figure 4.
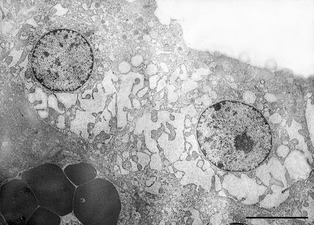
Transmission electron microscopic image of 2 follicular thyroid cells. The RER, predominantly at the base of the nucleus, is severely dilated, with distended cisternae. Lysosomes and secretory vesicles are not evident. The follicular lumen, in the right upper part of the image, is empty. Bar = 10 μm.
One month later, because the cat was still mildly lethargic and had diffuse scaling of the epidermis, and had no improvement in serum fT4 (<0.4 ng/dL) and TSH (9.8 ng/mL) concentrations, the levothyroxine dose was increased to 0.4 mg, PO q24h. After 4 additional weeks, the owner reported that the cat was again bright and alert, whereas skin lesions were not ameliorated; fT4 was measured and resulted above the upper limit of the reference range (4.6 ng/dL) and levothyroxine was thus lowered to 0.2 mg PO q24h. After another month, the cat was still bright and alert, skin lesions had improved, and antifungal treatment was discontinued. A month later, fT4 was normal (1.1 ng/dL), but TSH remained increased (>12 ng/mL). At the time of writing, 8 months after diagnosis, the cat still was in good health, without skin lesions, but with palpable goiter; the cat was continued on levothyroxine 0.2 mg PO q24h (Fig. 5).
Figure 5.

The affected cat 7 months after treatment; hair coat is improved and scaling is not present.
In cats, the most common form of hypothyroidism is iatrogenic, caused by bilateral thyroidectomy,2 radioactive iodine treatment,3 or overdose of antithyroidal drugs.4 Recently, a case of secondary hypothyroidism after head trauma was reported in an 18‐month‐old cat.5 Spontaneous hypothyroidism is a rare condition in cats and is more common in kittens than in adults. In the former, all cases described were congenital, presumably with a genetic basis leading to either dyshormonogenesis (goiterous) or dysmorphogenesis (nongoiterous).6, 7, 8, 9 Congenital hypothyroidism is characterized by dwarfism. The first signs of abnormal growth become evident after 3 weeks and are most evident by 2 months of age. Kittens also may show hypotonia, macroglossia, distended abdomen, dry skin, delayed dental eruption, and central or peripheral nervous system abnormalities.10
Spontaneous adult‐onset hypothyroidism is extremely rare in cats, with only 2 documented cases11, 12; based on histopathology, in 1 cat it was secondary to lymphocytic thyroiditis and in the other cat, no identifiable thyroid tissue was found. The most common clinical signs of hypothyroidism in adult cats are lethargy, hypothermia, and obesity. Decreased appetite and weight gain, despite prescription of a calorie‐restricted diet, have been reported. Dermatologic abnormalities, including focal alopecia, hyperpigmentation, and seborrhea as well as pyoderma, demodicosis or dermatophytosis secondary to reduced cutaneous immunity, and myxedema, have been described.10 Similar to previous reports, the cat of this study was mildly lethargic, and was obese with decreased appetite. The dermatologic abnormalities also were characteristic for hypothyroidism.
With regard to serum biochemistry, cats with adult‐onset hypothyroidism usually are unremarkable; 1 reported cat had increased serum creatine kinase activity.5, 11 The mechanism for increased creatine kinase activity may be hypothyroid myopathy, as has been reported in dogs.10 The cat of the present study had normal blood test results, including serum creatine kinase activity and normal urinalysis.
Hypothyroidism can be diagnosed in adult cats by a combination of basal serum TT4, fT4, and endogenous TSH concentrations. As in hypothyroid dogs, affected cats are expected to have low serum TT4 and fT4 concentrations and high TSH concentration. With regard to the latter, the enzyme‐linked immunosorbent assay (ELISA) for canine TSH has been used to detect feline TSH.13 The assay may be helpful to detect cases of iatrogenic hypothyroidism, but it has never been evaluated in cases of spontaneous hypothyroidism in cats. Cats with primary hypothyroidism, either iatrogenic or spontaneous, are expected to have increased concentrations of TSH and those with secondary hypothyroidism are expected to have decreased concentrations.5, 13 In the cat of this report, low TT4 and fT4 concentrations were documented along with increased TSH, leading to a diagnosis of primary hypothyroidism. Of note, in older cats, low or low normal TT4 concentrations often are caused by nonthyroidal illness (ie, euthyroid sick syndrome) or can occur after administration of drugs, such as corticosteroids and barbiturates. Because fT4 measured by equilibrium dialysis may have limited specificity in cats, its use in this species in clinical practice should be combined with measurement of TT4 concentrations.14 Of note, along with decreased TT4 and fT4, the increased TSH documented in the cat of the present study makes the sick euthyroid syndrome very improbable.
Our cat had received ear medications containing betamethasone, but long before presentation and fT4 measurement, making an effect of corticosteroids on thyroid hormones unlikely. The cat's dermatologic disorder was secondary to hypothyroidism, as shown by full recovery after replacement treatment.
With regard to diagnostic imaging, in humans the normal thyroid gland is hyperattenuating to surrounding tissues in CT acquired without contrast medium.15 The high attenuation is directly related to thyroid iodine content. Hypothyroidism leads to isoattenuating or hypoattenuating thyroid glands compared with adjacent muscle in humans.16 This occurs if the thyroid contains less iodine, and if more follicular cells or interstitial tissue is present. The normal feline thyroid gland is hyperattenuating to surrounding tissues without contrast medium.16 In our cat, the thyroid tissue was isoattenuating as observed in hypothyroid people, possibly because of less iodine content or diffuse follicular hyperplasia. In addition, brain and thorax scans were normal, excluding pituitary lesions and ectopic thyroid tissue.
The cat of the present study had a palpable thyroid gland. Unilateral or bilateral lobe enlargement is detectable in cats with thyroid nodules, as often happens in hyperthyroidism, and the thyroid gland was palpable in several cats with congenital hypothyroidism,6, 12 but an enlarged thyroid gland has never been recognized in cats with adult‐onset hypothyroidism. Goiter refers to an enlarged thyroid gland and in mammals, goiter can be associated with euthyroidism, hypothyroidism, or hyperthyroidism. Goitrogenic mechanisms associated with hypothyroidism include dietary iodine deficiency or excess, ingestion of goitrogenic compounds, and genetic defects.17 These causes lead to inadequate thyroxine synthesis or secretion and low thyroid hormone concentrations, causing a compensatory increase in TSH. In turn, a prolonged increase in TSH results in hypertrophic and hyperplastic follicular cells. The thyroid lobes are enlarged and dark red because of the presence of an extensive parenchymal capillary network, as was the case in the cat of the present study. Microscopically, 2 patterns are observed, diffuse hyperplastic goiter and colloid goiter. The first is characterized by diffuse follicular cell hyperplasia, with ≥1 layer of tall, columnar cells, and irregularly sized and shaped, or collapsed follicles17, 18; colloid in the lumina usually is scarce or absent. These features were similar to those observed in our cat. Colloid goiter is the involutionary phase of diffuse hyperplastic goiter and occurs when the need for thyroid hormone is diminished or a sufficient amount of iodine is added to the diet. After any increase in serum thyroid hormone concentrations, TSH decreases, and the hyperplastic follicular cells continue to produce colloid, but endocytosis of colloid from the lumen is decreased. As a consequence, the follicles are distended by a large amount of colloid and the epithelium is flattened.17, 18 With regard to transmission electron microscopy, major lesions were observed in the rough endoplasmatic reticulum (RER) of the cat of the present study. The RER was severely dilated. RER dilatation is a nonspecific degenerative change in the thyroid gland, secondary to different cellular stressors or sublethal injuries and its presence does not differentiate between congenital and acquired disease. Swollen mitochondria and a decreased number of mitochondria, scattered lysosomes and vesicles, as observed in our cat, also can be observed in both forms of the disease. Although these lesions have not been described previously in cats, they have been documented in rats, mice, and humans affected by congenital and acquired thyroid dysfunction.19, 20, 21
In humans and animals, goitrogenic compounds include drugs and synthetic or natural chemicals as sulfonamides, benzodiazepines, plants of the genus Brassica, and other xenobiotics.18 Our cat lived indoor in a large, multiple cat household and was the only animal showing clinical signs of hypothyroidism. Therefore, the chance of exposure to goitrogenic compounds seems very low, even though blood analyses were not performed in the remaining cats to exclude other cases of hypothyroidism. In humans, besides goitrogenic substances, acquired adult‐onset primary hypothyroidism with goiter may be secondary to iodine deficiency22 or, although less commonly, to iodine excess.23 Whether the cat had dietary iodine imbalance cannot be excluded. Indeed, all cats were fed the same food except for the present one, which was fed a calorie‐restricted diet. However, based on listed ingredients, the new diet contained an adequate amount of iodine (2.5 mg iodine/kg dry diet), fulfilling recent nutritional recommendations for cats.24 Thus, a role of the new diet in the development of hypothyroidism seems implausible. Of note, it cannot be excluded that the cat was chronically exposed to low doses of thyrotoxic substances, leading to hypothyroidism and in turn causing progressive increase in serum TSH concentration, as documented during follow‐up evaluation. However, it is difficult to support this hypothesis because, in the large, multiple cat household, the cat described here was the only one with overt hypothyroidism. Increased TSH concentration, despite replacement treatment, has been described in a Toy Fox Terrier with congenital goiter.25 As assumed in that dog, it is possible that the dose or frequency of thyroxine given to our cat was inadequate to decrease TSH. Alternatively, it cannot be excluded that absorption of thyroxine from the gastrointestinal tract decreased over time or that peripheral tissue resistance to thyroid hormone developed. The pathogenesis of increased TSH in our cat remains unclear, especially considering the substantial clinical improvement observed.
Hypothyroidism and goiter are observed with different inborn errors of the thyroid hormone biosynthetic pathway (dyshormonogenesis18, 21, 26). In our cat, a congenital form seems less likely because clinical signs developed in adulthood, and dwarfism, which has been identified in all congenital cases reported in cats, was not present. In humans, however, some forms of congenital hypothyroidism can remain subclinical for long periods of time. An increase in TSH secretion can maintain TT4 within normal limits and only few human patients develop clinically overt hypothyroidism during childhood or later. Hence, it cannot be excluded that the present cat had a subclinical congenital form of hypothyroidism that became clinically evident only during adulthood. Persistence of a palpable goiter during treatment, despite adequate response to supplementation, also may indicate a subclinical congenital defect.
In conclusion, a cat affected by adult‐onset primary hypothyroidism and goiter is presented, in which a dietary iodine imbalance or chronic exposure to thyrotoxic substances, and a congenital form of hypothyroidism with late clinical development may have played a causative role.
Acknowledgments
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Footnotes
Surolan, Merial, Milan, Italy
Obesity Management (Feline), dry, Royal Canin, Milan, Italy
Nine‐point body condition scale for cats, Nestlé Purina, St. Louis, MI
Leventa, Intervet Italia Spa, Milan, Italy
Light Speed; GE Medical System, Bergamo, Italy
Visipaque, 305mgI/mL, Nycomed Inc, Princeton, NJ
Philips CM10 TEM, Eindhoven, Germany
References
- 1. Van der Vlugt‐Meijer RH, Meij BP, Voorhout G. Dynamic helical computed tomography of the pituitary gland in healthy dogs. Vet Radiol Ultrasound 2007;48:118–124. [DOI] [PubMed] [Google Scholar]
- 2. Birchard SJ. Thyroidectomy in the cat. Clin Tech Small Anim Pract 2006;21:29–33. [DOI] [PubMed] [Google Scholar]
- 3. Peterson ME. Radioiodine treatment of hyperthyroidism. Clin Tech Small Anim Pract 2006;21:34–39. [DOI] [PubMed] [Google Scholar]
- 4. Williams TL, Elliott J, Syme HM. Association of iatrogenic hypothyroidism with azotemia and reduced survival time in cats treated for hyperthyroidism. J Vet Intern Med 2010;24:1086–1092. [DOI] [PubMed] [Google Scholar]
- 5. Mellanby RJ, Jeffery ND, Gopal MS, Herrtage ME. Secondary hypothyroidism following head trauma in a cat. J Feline Med Surg 2005;7:135–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jones BR, Gruffydd‐Jones TJ, Sparkes AH, Lucke VM. Preliminary studies on congenital hypothyroidism in a family of Abyssinian cats. Vet Rec 1992;131:145–148. [DOI] [PubMed] [Google Scholar]
- 7. Crowe A. Congenital hypothyroidism in a cat. Can Vet J 2004;45:168–170. [PMC free article] [PubMed] [Google Scholar]
- 8. Traas AM, Abbott BL, French A, Giger U. Congenital thyroid hypoplasia and seizures in 2 littermate kittens. J Vet Intern Med 2008;22:1427–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Quante S, Fracassi F, Gorgas D, et al. Congenital hypothyroidism in a kitten resulting in decreased IGF‐I concentration and abnormal liver function test. J Feline Med Surg 2010;12:487–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Greco DS. Diagnosis of congenital and adult‐onset hypothyroidism in cats. Clin Tech Small Anim Pract 2006;21:40–44. [DOI] [PubMed] [Google Scholar]
- 11. Rand JS, Levine J, Best SJ, Parker W. Spontaneous adult‐onset hypothyroidism in a cat. J Vet Intern Med 1993;7:272–276. [DOI] [PubMed] [Google Scholar]
- 12. Blois SL, Abrams‐Ogg ACG, Mitchell C, et al. Use of thyroid scintigraphy and pituitary immunohistochemistry in the diagnosis of spontaneous hypothyroidism in a mature cat. J Feline Med Surg 2010;12:156–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Graham PA, Refsal KR, Nachreiner RF, et al. Measurement of feline thyrotropin (TSH) using a commercial canine immunoradiometric assay. J Vet Intern Med 2000;14:342. [Google Scholar]
- 14. Mooney CT, Little CJ, Macrae AW. Effect of illness not associated with the thyroid gland on serum total and free thyroxine concentrations in cats. J Am Vet Med Assoc 1996; 208:2004–2008. [PubMed] [Google Scholar]
- 15. Drost WT, Mattoon JS, Samii VF, et al. Computed tomographic densitometry of normal feline thyroid glands. Vet Radiol Ultrasound 2004;45:112–116. [DOI] [PubMed] [Google Scholar]
- 16. Silverman PM, Newman GE, Korobkin M, et al. Computed tomography in the evaluation of thyroid disease. Am J Roentgenol 1984;142:897–902. [DOI] [PubMed] [Google Scholar]
- 17. Capen CC. Endocrine glands. In Jubb, Kennedy, Palmer's Pathology of Domestic Animals, vol. 3, 5th ed. Philadelphia, PA: Saunders Elsevier; 2007:389–396. [Google Scholar]
- 18. Kiupel M, Capen C, Miller M, Smedley R. Histological Classification of Tumors of the Endocrine System of Domestic Animals, second series, vol. XII. Washington DC: Armed Forces Institute of Pathology; 2008:33–35. [Google Scholar]
- 19. Ketelbant‐Balasse P, Glinoer D, Neve P. Ultrastructural aspects of the thyroid in a case of human congenital goitre with cretinism. Pathol Eur 1975;10:155–165. [PubMed] [Google Scholar]
- 20. Krupp PP, Lee KP. The effects of dietary iodine on thyroid ultrastructure. Tissue Cell 1988;20:79–88. [DOI] [PubMed] [Google Scholar]
- 21. Medeiros‐Neto G, Kim PS, Yoo SE, et al. Congenital hypothyroid goiter with deficient thyroglobulin identification of an endoplasmic reticulum storage disease with induction of molecular chaperones. J Clin Invest 1996;98:2838–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zimmermann MB. The role of iodine in human growth and development. Semin Cell Dev Biol 2011;22:645–652. [DOI] [PubMed] [Google Scholar]
- 23. Wolff J. Iodide goiter and the pharmacologic effects of excess iodide. Am J Med 1969;47:101–124. [DOI] [PubMed] [Google Scholar]
- 24. Wedekind KJ, Blumer ME, Huntington CE, et al. The feline iodine requirement is lower than the 2006 NRC recommended allowance. J Anim Physiol Anim Nutr (Berl) 2010;94:527–539. [DOI] [PubMed] [Google Scholar]
- 25. Fyfe JC, Kampschmidt K, Dang V, et al. Congenital hypothyroidism with goiter in Toy Fox Terriers. J Vet Intern Med 2003;17:50–57. [DOI] [PubMed] [Google Scholar]
- 26. Park SM, Chatterjee VKK. Genetics of congenital hypothyroidism. J Med Genet 2005;42:379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]


