Abstract
Background
Canine T‐cell lymphoma (TCL) is conventionally considered an aggressive disease, but some forms are histologically and clinically indolent. CD4 TCL is reported to be the most common subtype of TCL. We assessed flow cytometric characteristics, histologic features when available, and clinical outcomes of CD4+ TCL to determine if flow cytometry can be used to subclassify this group of lymphomas.
Objective
To test the hypothesis that canine CD4+ T‐cell lymphoma (TCL) is a homogeneous group of lymphomas with an aggressive clinical course.
Animals
Sixty‐seven dogs diagnosed with CD4+ TCL by flow cytometry and treated at 1 of 3 oncology referral clinics.
Methods
Retrospective multivariable analysis of outcome in canine CD4+ TCL including patient characteristics, treatment, and flow cytometric features.
Results
The majority of CD4+ TCL were CD45+, expressed low class II MHC, and exhibited an aggressive clinical course independent of treatment regimen (median survival, 159 days). Histologically, CD4+ TCL were classified as lymphoblastic or peripheral T cell. Size of the neoplastic lymphocytes had a modest effect on both PFI and survival in this group. A small number of CD4+ TCL were CD45− and class II MHC high, and exhibited an apparently more indolent clinical course (median survival not yet reached).
Conclusions and Clinical Importance
Although the majority of CD4+ TCL in dogs had uniform clinical and flow cytometric features and an aggressive clinical course, a subset had a unique immunophenotype that predicts significantly longer survival. This finding strengthens the utility of flow cytometry to aid in the stratification of canine lymphoma.
Keywords: Canine, Immunophenotyping, Lymphoblastic, Peripheral T cell, Prognosis
Abbreviations
- LBT
lymphoblastic T‐cell lymphoma
- MFI
median fluorescence intensity
- OS
overall survival
- PFI
progression‐free interval
- PTCL‐NOS
peripheral T cell not otherwise specified
- TCL
T‐cell lymphoma
Lymphoma is the most common hematologic malignancy in dogs and has long been subdivided into T‐ and B‐cell phenotypes, with T‐cell subtypes typically considered to have a more aggressive clinical course. Further subclassification within the T‐cell phenotype has identified important subtypes that are associated with both aggressive disease (pleomorphic mixed/peripheral T cell, plasmacytoid, T‐cell lymphoblastic) and indolent disease (small clear cell/T zone lymphoma).1, 2 This distinction, important for both prognosis and rational selection of chemotherapy protocol, is currently made by histologic examination of tissue specimens.
Flow cytometry is a critical tool in human medicine for diagnosing and characterizing cases of lymphoma. Despite its expanding diagnostic role in veterinary medicine, there are few flow cytometric descriptions of canine lymphoma.3, 4, 5, 6, 7 In the authors' experience, CD4+ T cell lymphoma (TCL) is the most common T‐cell immunophenotype seen in aspirates from canine peripheral lymph nodes and this is consistent with 2 small case series of canine TCL.4, 7 In a very small number of cases with concurrent Kiel cytologic classification, CD4+ TCL were described as small clear cell/T zone, lymphoblastic, and pleomorphic mixed cell/peripheral T‐cell subtypes.3, 4 When lymphoma restricted to the Boxer breed was examined, >80% of cases were T‐cell origin and the majority were of the CD4+ phenotype.5, 8 In these studies and in a larger study of canine lymphoma, the CD4+ phenotype appeared to be associated with an aggressive clinical course,5, 8, 9 but to the authors' knowledge, no studies have described large numbers of cases of canine TCL based on the CD4+ immunophenotype. The purpose of this study was to further classify canine CD4+ TCL with peripheral lymph node involvement by examining clinical characteristics, flow cytometric parameters associated with clinical outcome, and histologic classification when available.
Materials and Methods
Case Selection
Cases submitted to the Clinical Immunology Laboratory at CSU between January 1, 2008 and February 1, 2012 comprised the population of patients for this study. Of these, 168 met the inclusion criteria, which were as follows: (1) cytologic or histologic diagnosis of lymphoma; and (2) >65% of the large cells or >80% of the entire population expressing CD4. Forty percent of these cases (67) were derived from 3 referral clinics, with the remainder coming from 57 other clinics. Because of the difficulty in obtaining consistent clinical follow‐up from multiple clinics, the 67 cases from 3 referral centers were chosen for further analysis. The 67 cases chosen for follow‐up were not significantly different from the remaining 101 cases with regard to age, breed distribution, sex, and the presence of selected clinical characteristics (Table 1). Data extracted from the medical records included patient age, sex, breed, date of diagnosis, date of treatment initiation, staging tests performed, the presence of hypercalcemia at diagnosis, clinical substage, treatment protocol, time to progression of disease, and date of death.
Table 1.
Characteristics of the dogs in this study, and comparison of selected features with dogs not included in the study
| Parameter | Study Population (n = 67) | Dogs Not in Study (n = 101)a | P Valueb |
|---|---|---|---|
| Age (years) | |||
| Mean ± SD | 7.2 ± 2.1 | 7.2 ± 2.5 | .26 |
| Sex | |||
| Male | 2 (3%) | 8 (8%) | .053 |
| Neutered male | 34 (51%) | 52 (52%) | |
| Female | 6 (9%) | 1 (1%) | |
| Spayed female | 25 (37%) | 36 (36%) | |
| Weight (kg) | |||
| Mean ± SD | 29.6 ± 12.9 | N/A | N/A |
| Breed | |||
| Mixed breed | 17 (25%) | 14 (14%) | .29 |
| Boxer | 13 (19%) | 26 (26%) | |
| Golden Retriever | 10 (15%) | 16 (16%) | |
| Other | 27 (40%) | 45 (45%) | |
| Substage | |||
| a | 54 (81%) | N/A | N/A |
| b | 13 (19%) | N/A | |
| Calcium status | |||
| Elevated | 28 (50%) | 23 (68%) | .13 |
| Normal | 27 (50%) | 11 (32%) | |
| Mediastinal mass | |||
| Present | 15 (43%) | 3 (30%) | .2 |
| Absent | 20 (57%) | 12 (70%) | |
Numbers will not add up to the total cases because of missing information for some patients. For example, information about sex was only available for 97 of the 101 patients.
Calculated by chi‐square analysis.
Flow Cytometry
Samples were submitted based on palpable peripheral lymphadenopathy. Multiple fine needle aspirates from the lymph node were placed in isotonic saline with 10% canine serum and submitted immediately or shipped overnight on ice. Lymph node samples were prepared as described previously.10 The lymph node suspension was centrifuged and resuspended in 1 mL of lysis buffer (0.15 M NH4CL, 1 M KHO3, 0.1 mM Na2 EDTA, 1 N HCL at a pH of 7.2–7.4) for 5 minutes at room temperature. Samples were subsequently centrifuged, lysed a second time, and resuspended in 200 μL of phosphate‐buffered saline (PBS)‐2% fetal bovine serum (FBS). A 96‐well plate was used in which 25 μL of cell suspension was added to individual wells plus 25 μL of one of the combinations of antibodies. Antibody combinations were (1) None, (2) M IgG1‐FITC/CD45‐PE, (3) CD18‐FITC/M IgG1‐PE, (4) CD4‐FITC/CD8‐PE, (5) CD5‐FITC/CD21‐PE, (6) CD3‐FITC/CD45‐PE, (7) CD4‐FITC/CD14‐PE, and (8) Class II MHC‐FITC/CD34‐PE.1 Samples were incubated for 15 minutes at room temperature and then washed twice. Samples then were resuspended in PBS‐2% FBS with 10 μg/mL of propidium iodide (PI) for dead cell exclusion, and analyzed within 1 hour. Samples with >50% PI‐positive cells were excluded from further analysis. Samples were acquired on a Coulter XL flow cytometer. All data analysis was carried out by Kaluza software.2
Immunohistochemistry and Histologic Classification
For immunophenotyping of tissue samples, 5‐μm sections from formalin‐fixed, paraffin‐embedded tissues were cut and immunostained utilizing antibodies directed against the CD3 antigen to stain T cells (clone LN103 ) or either Pax5 or CD79a antigen to stain B cells (clone DAK‐Pax5 and HM574). Deparaffinization, antigen retrieval, immunohistochemical (IHC) staining, and counterstaining were performed on the Bond maX Automated Staining System by the Bond Polymer Detection System.3 Antigen retrieval was accomplished on line using Bond Epitope Retrieval Solution 2 (EDTA based, pH 9.0 solution) using a 30‐minute incubation.
Biopsies were available for 15 cases. H&E, as well as IHC from these 15 cases, and an additional 13 cases of T‐cell lymphoma with a different immunophenotype (companion paper, Seelig et al5) were reviewed by 2 board‐certified veterinary pathologists (D.M.S. and E.J.E.) and classified according to WHO criteria.11 At the time of review, the pathologists were blinded to the results of flow cytometry and clinical data. To make their diagnosis, the pathologists used previously described histologic and immunohistologic criteria.11
Data Analyses
Progression‐free interval (PFI) and overall survival (OS) were calculated in days from the time of treatment initiation until progressive disease or death, respectively. Dogs still alive at the time of data analysis or lost to follow‐up were censored at the last date reported to be alive. Dogs that had died or were euthanized were considered to be dead either secondary to their treatment or disease. Dogs treated with prednisone only were excluded from PFI analysis, because treatment did not result in resolution of clinical signs. Clinical data at the time of presentation, including signalment, weight, the presence of hypercalcemia, and imaging assessment of a mediastinal mass and clinical substage were further evaluated for effect on PFI and survival. When no information was available, a normal finding was not inferred, and the patient was excluded from this analysis. In addition, patient demographics including the dog's age, sex, breed, and clinic where treated were considered as potential confounders.
Descriptive statistics were calculated for all variables collected at baseline. Age at diagnosis was dichotomized at the median for all uni‐ and multivariable analyses. Log rank test and Kaplan‐Meier plots were used to evaluate whether any baseline variable was associated with PFI or survival. Multivariable Cox proportional hazard analyses were performed in a backward stepwise manner. An initial model was constructed with all potential prognostic variables with P values <.20 on univariable analysis as well as the potential confounders breed, clinic, and treatment. Backward selection was conducted until all remaining variables had P values <.05. Finally, reanalysis was conducted adding prognostic indicators one‐by‐one to determine if they were significant in a multivariable setting; only significant variables remained in the final model. Except where otherwise noted, all statistical analyses were performed by SAS 9.26 and P values <.05 were considered significant.
Results
Flow Cytometric Characterization
Sixty‐one of the 67 CD4‐positive cases were characterized by uniform expression of CD3, CD45, low levels of MHC class II (median fluorescence intensity [MFI] 1.2), and absent expression of CD21 (referred to subsequently as CD4+/CD45+). CD5 expression was variable in this group with 26 cases categorized as lacking the expression of CD5, whereas 34 were uniformly CD5 positive. The median percentage of CD5+ cells in those cases categorized as lacking CD5 expression was 2.1% (range 0–20%). The remaining 6 cases had unique phenotypic features characterized by high MHC class II expression (MFI 22), coexpression of CD21 and loss of CD45 expression (referred to subsequently as CD4+/CD45−; Fig 1). The median percentage of cells negative for CD45 was 96% (range 93–98%), whereas the median percentage of cells positive for CD21 was 94% (range 60–97%). All 6 of these cases uniformly expressed both CD3 and CD5.
Figure 1.
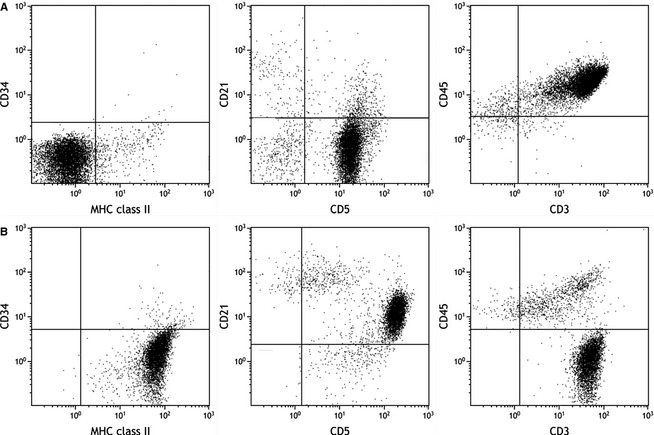
Typical flow cytometric properties of CD4+/CD45+ (A) and CD4+/CD45− (B) subsets of CD4+ lymphoma. CD45 expression is shown in the right panel. CD45− cases expressed high levels of class II MHC compared with CD4+/CD45+ dogs (left panel), and higher levels of CD21 (middle panel).
Patient Demographics
Table 1 shows demographic information for all 67 dogs in the study. For the 61 dogs with CD4+/CD45+ TCL, the median age was 7 years (range 3–13 years). Twenty‐seven cases were female (6 intact, 21 neutered) and 34 cases were male (2 intact, 32 neutered). Mixed breed dogs were the most frequently represented breed (n = 16; 26%) followed by Boxers (n = 13; 21%) and Golden Retrievers (n = 8; 13%). Other breeds represented by >1 case included English Bulldogs, Labrador Retrievers, Shetland Sheepdogs, Rottweilers, and Saint Bernards (all 3%). Median age of the 6 dogs with CD4+/CD45− lymphoma was 8.9 years (range 6–10 years) and 4 were female‐neutered and 2 were male‐neutered. Two CD4+/CD45− dogs were Golden Retrievers, whereas there was 1 each of Australian Shepherd, Bernese Mountain Dog, Chihuahua, and mixed breed.
Summary of Endpoints
After excluding 12 dogs that were treated only with prednisone (1 CD4+/CD45−, 11 CD4+/CD45+), analyses evaluating PFI included 55 dogs. Of these dogs, 2 of 5 (40%) CD4+/CD45− dogs and 43 of 50 (86%) CD4+/CD45+ dogs developed progressive disease. Overall, 10 dogs were censored; 5 were alive without progressive disease at the end of the study period (3 CD4+/CD45−, 2 CD4+/CD45+); 1 CD4+/CD45+ was lost to follow‐up; and 4 CD4+/CD45+ died or were euthanized before progressive disease was noted. All 67 dogs were able to be analyzed for overall survival; 3 of 6 (50%) CD4+/CD45− dogs and 54 of 61 (89%) CD4+/CD45+ died or were euthanized during the study period. Censoring occurred for 7 dogs that were alive at the end of the study period (3 CD4+/CD45−, 4 CD4+/CD45+) and 3 CD4+/CD45+ dogs that were lost to follow‐up. Median follow‐up time did not differ significantly between CD4+/CD45+ dogs and CD4+/CD45− dogs (548 days [95% CI, 361–557] for CD4+/CD45+ dogs, 405 days [95% CI, 185–557] for CD4+/CD45− dogs; log rank P = .8751).
CD45− T‐Cell Lymphomas Exhibit Different Clinical Behavior
Three of the 6 CD4+/CD45− phenotype dogs were alive without progression at the end of the study, suggesting that some of these cases have a more indolent clinical course. Median PFI and OS in the majority CD4+/CD45+ group were 91 days (range 25–557 days) and 159 days (range 4–557 days), respectively. Median PFI and OS of the 6 CD4+/CD45− dogs could not be calculated because 3 of these dogs were alive at 228, 303, and 1043 days of follow‐up (Fig 2). During the study period, 2 CD4+/CD45− dogs developed progressive disease (27 and 107 days, subsequently euthanized for unknown causes at 134 and 188 days) and 1 was treated with prednisone only and euthanized after 166 days, also for unknown reasons. Concurrent work in our laboratory has associated the loss of CD45 expression, high MHC class II expression, and coexpression of CD21 in T‐cell lymphomas with T zone histology and indolent behavior (Seelig et al5). Because of this apparent difference in biologic behavior and our concurrent findings, which identify this phenotype as associated with T zone histology (Seelig et al5), these cases were removed from all subsequent analysis.
Figure 2.
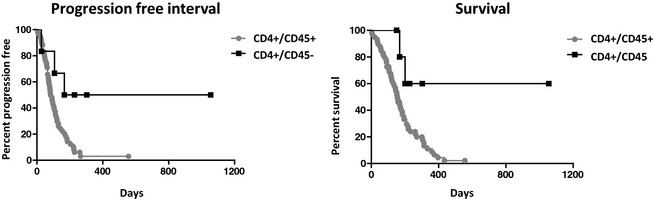
Surface marker expression distinguishes CD4+ lymphomas with distinct clinical courses. Median PFI/survival CD4+/CD45+ = 91/159 days, CD4+/CD45− = undefined/undefined days.
Cell Size and CD5 Expression in CD4+/CD45+ Lymphomas Influences Outcome
When the size of the neoplastic CD4+/CD45+ lymphocytes was divided into small and large based on the median cell size of all cases, there was a significant, modest impact on both PFI and survival in univariable analysis (Table 2). Controlling for treatment, large cell size remained significantly associated with a shortened PFI. Large cell size was also associated with shortened overall survival, controlling for treatment, CD5 status, and substage (Table 2).
Table 2.
Summary of PFI and survival analysis for selected clinical and immunophenotypic characteristics.
| Variable (No. of Patients)a | Univariable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|
| PFI | P Value for PFI Difference | Overall Survival (Range) | P Value for Survival Difference | Hazard Ratio for PFI | Hazard Ratio for Survival | |
| CD5 expression | ||||||
| Positive (34) | 77 (65–111) | .1353 | 143 (92–159) | .0056 | — | 2.5 (1.2–5.1) |
| Negative (26)b | 98 (65–175) | 195 (137–314) | 1 | |||
| Cell size | ||||||
| Small (30) | 121 (73–191) | .0012 | 206 (146–235) | .0146 | 3.3 (1.6–6.6) | 2.2 (1.1–4.3) |
| Large (31) | 77 (44–91) | 130 (91–159) | 1 | 1 | ||
| Multi‐agent tx | ||||||
| MOPP (10) | 131 (35–266) | .0091 | 114 (35–194) | .0003 | 1.6 (0.6–4.1) | 2.0 (0.7–5.3) |
| Single agent (22) | 70 (43–81) | 170 (104–218) | 3.1 (1.5–6.3) | 1.6 (0.7–3.5) | ||
| Prednisone (11) | — | 80 (8–130) | — | 2.7 (1.1–6.7) | ||
| CHOP (18) | 109 (91–191) | 237 (159–312) | 1 | 1 | ||
| Calcium status | ||||||
| High (28) | 83 (65–182) | .5532 | 141 (95–185) | .1323 | — | — |
| Normal (22) | 113 (73–167) | 170 (137–272) | ||||
| Mediastinal mass | ||||||
| Present (15) | 81 (28–113) | .0801 | 130 (40–195) | .1762 | — | — |
| Absent (18) | 140 (65–191) | 155 (91–435) | ||||
| Substage | ||||||
| b (13) | 97 (48–204) | .7495 | 106 (20–195) | .0085 | — | 4.1 (1.7–9.9) |
| a (48) | 91 (69–111) | 164 (137–210) | 1 | |||
Numbers reflect the number of dogs analyzed for overall survival. Fewer animals were analyzed for PFI as the prednisone‐treated patients were not included in this group. Only patients with CD45+ T‐cell lymphoma are included in this table.
Not all values were available for all dogs. For example, in 1 dog, CD5 staining was not performed, so that there are only 60 dogs in the CD5 outcome analysis.
CD5 expression was absent in 43% (26 of 60 with available data) of dogs in the CD4+/CD45+ subset. Absent CD5 was associated with a significant, modest impact on survival in univariable analysis without a significant impact on PFI (Table 2). Controlling for treatment, cell size, and substage, CD5+ status remained significantly associated with a shortened overall survival. All 6 dogs with the CD4+/CD45− phenotype expressed CD5 (not shown in Table 2).
Effect of Clinical Characteristics on Outcome
There were no significant differences comparing PFI and OS in normocalcemic versus hypercalcemic dogs in univariable analysis, nor was there a significant difference in comparing dogs with and without evidence of a mediastinal mass (Table 2). Full staging was not performed in most dogs, and accurate assessment of stage could not be determined in this study. Twenty‐five (37%) of dogs had no staging beyond a CBC and serum biochemical profile before treatment initiation; 38 (57%) dogs had thoracic radiographs; 3 (4%) dogs had abdominal radiographs; 12 (22%) dogs had abdominal ultrasound examinations; and 3 (4%) dogs had bone marrow aspirates performed. Forty‐eight dogs (79%) were clinically well (substage a) and the other 13 (21%) dogs were substage b at the time of diagnosis. Substage did not have a significant impact on PFI, but there was a significant impact on survival (Table 2).
Effect of Treatment on Outcome
The dogs with CD45+ lymphoma were treated with a variety of chemotherapy protocols. Thirty‐one (46%) dogs were treated with a multi agent protocol; 20 (30%) dogs were treated with a CHOP‐based protocol that included vincristine, cyclophosphamide, doxorubicin, prednisone, +/− L‐asparaginase; and 11 (16%) dogs were treated with a MOPP‐based protocol consisting of mechlorethamine, vincristine, procarbazine, prednisone, +/− L‐asparaginase. Twenty‐four (36%) dogs were treated with a single‐agent protocol plus prednisone consisting of CCNU (n = 10; 15%), doxorubicin (n = 12; 18%), or chlorambucil (n = 2; 3%). The remaining 12 (18%) dogs were treated with prednisone only. Three dogs treated at CSU were initially treated with an investigational chemotherapeutic agent, which delayed the initiation of definitive treatment by 8–11 days; the start of treatment for these 3 dogs was recorded as the day they started CHOP (n = 1) or doxorubicin (n = 2) for the purposes of calculating PFI and OS. Response rates were not evaluated in this study because of the variability in assessment between clinics and the retrospective nature of this study.
Figure 3 shows PFI and survival curves for all 4 treatment groups. Among CD4+/CD45+ dogs, there was no significant difference in median PFI or survival comparing dogs treated with MOPP with those treated with CHOP in univariable analysis (PFI; MOPP, 131 days; CHOP, 109 days; P = .9480; survival; MOPP, 114 days; CHOP, 237 days; P = .06) or multivariable analysis. Progression‐free interval, but not overall survival, was significantly different between dogs treated with single‐agent chemotherapy and those treated with CHOP (PFI; single, 70 days; CHOP, 109 days; P = .0039). This relationship remained significant in multivariable analysis (PFI hazard ratio [HR], 3.11; 95% CI, 1.53–6.32). Not surprisingly, survival was significantly different between dogs treated with prednisone only compared with those treated with CHOP (survival; prednisone, 80 days; CHOP, 237 days; P < .0001). Controlling for cell size, substage, and CD5 status, prednisone treatment remained significantly different from CHOP in terms of survival (HR, 2.72; 95% CI, 1.11–6.66).
Figure 3.
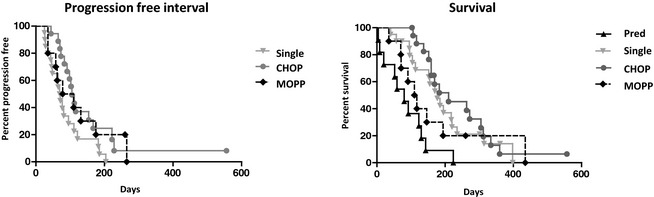
Progression‐free interval (PFI) is not different between treatment groups. Overall survival is significantly different for prednisone only compared with CHOP. Only dogs with CD4+/CD45+ lymphoma were included in this analysis.
Histologic Characterization
Biopsies of 15 CD4+/CD45+ cases were available for review. The consensus histologic classification for 10 cases was peripheral T cell not otherwise specified (PTCL), whereas the remaining 5 cases were classified as lymphoblastic T cell (LBT; Fig 4). All affected lymph nodes were characterized by diffuse effacement by neoplastic cells. Peripheral T cell not otherwise specified and LBT were discriminated primarily based on cell size (intermediate‐sized cells in LBT and intermediate‐to‐large‐sized cells in PTCL), degree of pleomorphism (low in LBT and high in PTCL), and number of mitotic figures (very common in LBT and rare in PTCL). There were no differences in presentation or in clinical outcome between the 2 groups, but small sample size may have precluded detection of a difference. All of the dogs with available tissue had an aggressive clinical course (median PFI = 85 days, median survival = 155 days). Biopsies were not available for any of the 6 dogs with the CD4+/CD45− flow cytometric staining pattern.
Figure 4.
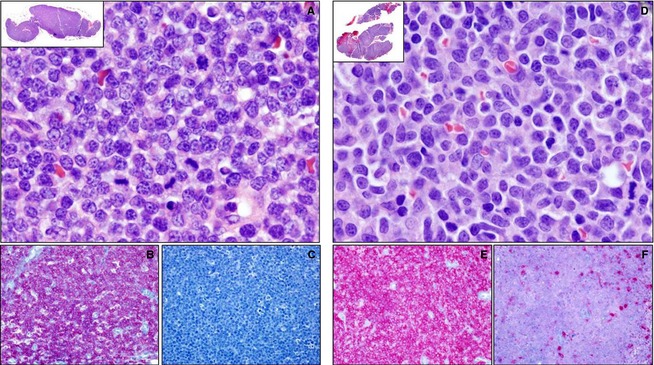
Histologic and immunohistologic features of the 2 morphologic variants of canine CD4+/CD45+ lymphoma. Five of the 15 cases of CD4+/CD45+ lymphoma were histologically classified as lymphoblastic T‐cell lymphoma (LBT, A–C), whereas 10 were classified as peripheral T‐cell lymphoma (PTCL, D–F). On hematoxylin‐and‐eosin–stained sections, cases of LBT (A) were characterized by diffuse effacement of the lymph node architecture by intermediate‐sized cells with uniform round‐to‐oval nuclei and high numbers of mitotic figures, whereas in PTCL (B), the effacing cells were intermediate‐to‐large in size with irregularly shaped nuclei, anisokaryosis, and rare mitotic figures. In both LBT and PTCL, immunohistochemistry confirms the T‐cell phenotype of the neoplastic cells through uniform, heavy anti‐CD3 immunoreactivity (B and E, red) and absent‐to‐scant anti‐Pax5 (C, red) or CD79a (F, red) immunoreactivity.
Discussion
Our characterization of the majority of the CD4+ lymphomas (CD4+/CD45+) with peripheral lymph node involvement in this case series is consistent with previous studies of canine T‐cell lymphoma. The short median PFI and OS, breed distribution, and incidence of hypercalcemia and mediastinal masses seen in this study are similar to previous reports.12, 13 The over‐representation of Boxers seen here has been reported in previous studies of canine T‐cell lymphoma.5, 8 One study of lymphoma in Boxers utilizing immunocytochemistry reported that 12/22 cases expressed CD4, 6/22 expressed neither CD4 nor CD8, 1/22 expressed both CD4 and CD8, and 1/22 expressed CD8 only.5 Although we cannot conclude that Boxers are particularly prone to CD4+/CD45+ lymphoma without data on the overall breed distribution of canine lymphoma, we note that Boxers were not represented at all in our companion study describing T zone lymphomas (Seelig et al5). The aggressive clinical course described for the majority of CD4+ lymphomas is compatible with earlier work that has given rise to the concept that many dogs with T‐cell lymphoma have poor clinical outcome. Importantly, a small but significant subset of dogs with CD4+ lymphoma in this study had what appeared to be a more indolent clinical course that correlated with a unique surface immunophenotype (CD4+/CD45−).
The presence of hypercalcemia or mediastinal mass did not have an impact on outcome in our cases of CD4+/CD45+ lymphoma; these findings are similar to previous published reports evaluating dogs with T‐cell lymphoma.12, 13 Hypercalcemia and the presence of a mediastinal mass are commonly recognized as negative prognostic factors for dogs with lymphoma14; however, our findings do not support this idea. Rather, these data suggest that hypercalcemia and mediastinal masses occur commonly with CD4+ T‐cell lymphoma and are not likely independent predictors of outcome within this subtype.
Dogs in this study were treated with a variety of chemotherapy protocols resulting in small sample sizes within each treatment group, limiting our ability to draw conclusions regarding the optimal treatment for CD4+ T‐cell lymphoma. However, our study does not support the proposal that doxorubicin‐containing protocols are less efficacious in the treatment of T‐cell lymphoma.12, 15 There was no significant difference in either PFI or OS in dogs treated with MOPP versus a CHOP protocol. This is the first analysis of outcome in T‐cell lymphoma that focuses on a uniform immunophenotype. Possible inclusion of indolent forms of T‐cell lymphoma in previous studies may have influenced prior reported results. A randomized, prospective study evaluating MOPP versus CHOP as treatment for a homogeneous population of dogs with CD4+ T‐cell lymphoma should be conducted to further investigate optimal treatment for this disease.
Although our data show that CD4+/CD45+ T‐cell lymphoma is a highly aggressive form of lymphoma in dogs regardless of the treatment chosen, flow cytometric characteristics did predict small but significant differences in survival. Larger cell size of the neoplastic CD4+/CD45+ lymphomas was associated with a significantly shorter PFI and overall survival. This remained true in multivariable analysis when controlling for other variables such as treatment, CD5 expression, and clinical substage. Large cell size determined by flow cytometry has been shown to be an independent predictor of worse outcome in dogs with circulating neoplastic B cells and in dogs with nodal B cell lymphomas.10, 16 Larger cell size has also been associated with worse outcome in human adult T‐cell lymphoma (ATL) and cutaneous PTCL.17, 18 Loss of the surface expression of the pan‐T cell marker CD5 was a relatively common finding within the CD4+/CD45+ lymphomas. Although both univariable and multivariable analysis demonstrated a modest difference in overall survival between CD5+ and CD5− cases, PFI was not significantly different in either analysis. Loss of CD5 expression has been reported in human cases of PTCL without any apparent effect on clinical outcome.19 The true impact of CD5 expression on the prognosis of canine CD4+/CD45+ lymphomas may require additional studies, but the loss of CD5 expression can serve as a useful marker to classify these cells as neoplastic in ambiguous cases.
A consistent finding in the CD4+/CD45+ lymphomas in this study was a very low expression level of MHC class II. Decreased surface expression of MHC class II has been commonly associated with a worse clinical outcome in human lymphoma20, 21 and there is evidence that this is true in canine B‐cell lymphoma as well.10 The diminished MHC class II expression may prevent adequate tumor immunosurveillance, which may have played a role in the poor outcome of the majority CD4+/CD45+, MHC class II low cases in this study.22
Cells from the CD4+/CD45+ cases expressed surface CD3 and expressed either no MHC class II or only low levels. By analogy with human T‐cell lymphoproliferative disorders, these features suggest that the normal counterpart of this neoplasm is a T cell that has completed its maturation, and is a single positive thymocyte or a mature peripheral T cell.23 Comparisons with human disease should be made cautiously, however, because expression of MHC class II is constitutive in dogs24, whereas it is a feature only of activated human T cells.25 This phenotype contrasts substantially with that described for T zone lymphoma, which expresses high levels of class II MHC, CD25, and CD21, all of which point to an activated phenotype (Seelig et al5). More studies are needed to definitively establish the normal counterpart of these cells, but such knowledge should help us better understand the genesis of this disease.
The subset of 15 CD4+/CD45+ cases that had tissue available for histologic assessment was categorized as either PTCL or LBT. Recognizing the limitations of small sample numbers, there were no obvious differences in clinical presentation or outcome between these 2 histologic subtypes. A recent paper examining gene expression profiles in a small number of canine lymphoma cases suggests that both PTCL and LBT have similar gene expression profiles and may represent a continuum of the same disease.26 Work comparing surface phenotype with the Kiel cytologic classification in a small subset of canine T‐cell lymphomas found that 5/8 small clear cell (T zone correlate) and 5/5 pleomorphic mixed small and large (PTCL correlate) tumors expressed surface CD4.4 Interestingly, 5/8 of the small clear cell/T zone cases had dim or absent CD45 expression and one of the small clear cell/T zone cases expressed low levels of CD21. Although we did not have tissue available from any of the CD4+/CD45− cases, work from our laboratory presented in a concurrent manuscript and a recent report has shown that the surface phenotype of this group (CD45−/MHC class II high/CD21+) correlates with T zone histology (Seelig et al5).6
In this study, we have demonstrated that the majority of canine CD4+ T‐cell lymphomas with peripheral lymph node involvement have an aggressive clinical course and share many of the clinical features commonly reported in previous studies of T‐cell lymphoma. These lymphomas expressed low levels of MHC class II and variable levels of the pan‐T cell marker CD5 and were histologically classified as both PTCL and LBT. Importantly, we have identified a small subset of CD4+ T‐cell lymphomas that express a unique surface phenotype characterized by an absence of CD45, coexpression of CD21, and high MHC class II expression that appear to have a much more indolent overall clinical course. Our concurrent study (Seelig et al5) and a recent publication6 indicated that this phenotype is consistent with T zone lymphoma. Three dogs with the CD4+/CD45− phenotype had overall survival times similar to the CD4+/CD45+ cases and, although we do not know the exact reason these animals were euthanized, this could be compatible with the recent suggestion that indolent lymphomas may behave more aggressively if diagnosed at more advanced stages.27
The 2 diseases described in this study and in the companion paper (Seelig et al5) represent 2 ends of the spectrum of T‐cell disease. T zone lymphoma is generally characterized by a prolonged clinical course, and there are data to support the idea that CHOP chemotherapy is no more effective than treatment with prednisone and chlorambucil.28 CD4+/CD45+ T‐cell lymphoma is aggressive, and although multi drug treatment extends survival, the majority of patients die of their disease by 300 days. This study demonstrates the practical utility of flow cytometry as a noninvasive diagnostic tool and reiterates the importance of subclassification of canine lymphomas beyond B versus T‐cell phenotype.
Acknowledgments
Conflict of Interest: Authors disclose no conflict of interest.
Presented in part at the American College of Veterinary Pathology/Veterinary Clinical Pathology Annual Meeting, December 2, 2012, Seattle, WA
Footnotes
AbD Serotec, Raleigh, NC
Beckman Coulter Inc, Fullerton, CA
Leica Biosystems, Buffalo Grove, IL
Dako Inc, Carpinteria, CA
Seelig D, Avery, P, et al. Canine T zone lymphoma: Unique immunophenotypic features, outcome and population characteristics. Journal of Veterinary Internal Medicine
SAS Institute Inc, Cary, NC
References
- 1. Ponce F, Magnol JP, Ledieu D, et al. Prognostic significance of morphological subtypes in canine malignant lymphomas during chemotherapy. Vet J 2004;167:158–166. [DOI] [PubMed] [Google Scholar]
- 2. Valli VE, Vernau W, de Lorimier L‐P, et al. Canine indolent nodular lymphoma. Vet Pathol 2006;43:241–256. [DOI] [PubMed] [Google Scholar]
- 3. Sozmen M, Tasca S, Carli E, et al. Use of fine needle aspirates and flow cytometry for the diagnosis, classification, and immunophenotyping of canine lymphomas. J Vet Diagn Invest 2005;17:323–330. [DOI] [PubMed] [Google Scholar]
- 4. Gelain ME, Mazzilli M, Riondato F, et al. Aberrant phenotypes and quantitative antigen expression in different subtypes of canine lymphoma by flow cytometry. Vet Immunol Immunopathol 2008;121:179–188. [DOI] [PubMed] [Google Scholar]
- 5. Lurie DM, Milner RJ, Suter SE, et al. Immunophenotypic and cytomorphologic subclassification of T‐cell lymphoma in the Boxer breed. Vet Immunol Immunopathol 2008;125:102–110. [DOI] [PubMed] [Google Scholar]
- 6. Martini V, Poggi A, Riondato F, et al. Flow‐cytometric detection of phenotypic aberrancies in canine small clear cell lymphoma. Vet Comp Oncol 2013; Published on line 5‐31‐13. [DOI] [PubMed] [Google Scholar]
- 7. Culmsee K, Simon D, Mischke R, et al. Possibilities of flow cytometric analysis for immunophenotypic characterization of canine lymphoma. J Vet Med A Physiol Pathol Clin Med 2001;48:199–206. [DOI] [PubMed] [Google Scholar]
- 8. Lurie DM, Lucroy MD, Griffey SM, et al. T‐cell‐derived malignant lymphoma in the Boxer breed. Vet Comp Oncol 2004;2:171–175. [DOI] [PubMed] [Google Scholar]
- 9. Ruslander DA, Gebhard DH, Tompkins MB, et al. Immunophenotypic characterization of canine lymphoproliferative disorders. In Vivo 1997;11:169–172. [PubMed] [Google Scholar]
- 10. Rao S, Lana S, Eickhoff J, et al. Class II major histocompatibility complex expression and cell size independently predict survival in canine B‐cell lymphoma. J Vet Intern Med 2011;25:1097–1105. [DOI] [PubMed] [Google Scholar]
- 11. Valli VE, San Myint M, Barthel A, et al. Classification of canine malignant lymphomas according to the World Health Organization criteria. Vet Pathol 2011;48:198–211. [DOI] [PubMed] [Google Scholar]
- 12. Brodsky EM, Maudlin GN, Lachowicz JL, et al. Asparaginase and MOPP treatment of dogs with lymphoma. J Vet Intern Med 2009;23:578–584. [DOI] [PubMed] [Google Scholar]
- 13. Rebhun RB, Kent MS, Borrofka SA, et al. CHOP chemotherapy for the treatment of canine multicentric T‐cell lymphoma. Vet Comp Oncol 2011;9:38–44. [DOI] [PubMed] [Google Scholar]
- 14. Vail DM, Pinkerton ME, Young KM. Hematopoietic tumors In: Withrow SJ, Vail DM, Page RL, eds. Small Animal Clinical Oncology, 5th ed St. Louis, MO: Elseiver; 2013:608–688. [Google Scholar]
- 15. Beaver LM, Strottner G, Klein MK. Response rate after administration of a single dose of doxorubicin in dogs with B‐cell or T‐cell lymphoma: 41 cases (2006‐2008). J Am Vet Med Assoc 2010;237:1052–1055. [DOI] [PubMed] [Google Scholar]
- 16. Williams MJ, Avery AC, Lana SE, et al. Canine lymphoproliferative disease characterized by lymphocytosis: Immunophenotypic markers of prognosis. J Vet Intern Med 2008;22:596–601. [DOI] [PubMed] [Google Scholar]
- 17. Bekkenk MW, Vermeer MH, Jansen PM, et al. Peripheral T‐cell lymphomas unspecified presenting in the skin: Analysis of prognostic factors in a group of 82 patients. Blood 2003;102:2213–2219. [DOI] [PubMed] [Google Scholar]
- 18. Bittencourt AL, da Gracas Vieira M, Brites CR, et al. Adult T‐cell leukemia/lymphoma in Bahia, Brazil: Analysis of prognostic factors in a group of 70 patients. Am J Clin Pathol 2007;128:875–882. [DOI] [PubMed] [Google Scholar]
- 19. Gallamini A, Stelitano C, Calvi R, et al. Peripheral T‐cell lymphoma unspecified (PTCL‐U): A new prognostic model from a retrospective multicentric clinical study. Blood 2004;103:2474–2479. [DOI] [PubMed] [Google Scholar]
- 20. Veelken H, Vik Dannheim S, Schulte Moenting J, et al. Immunophenotype as prognostic factor for diffuse large B‐cell lymphoma in patients undergoing clinical risk‐adapted therapy. Ann Oncol 2007;18:931–939. [DOI] [PubMed] [Google Scholar]
- 21. Rimsza LM, Farinha P, Fuchs DA, et al. HLA‐DR protein status predicts survival in patients with diffuse large B‐cell lymphoma treated on the MACOP‐B chemotherapy regimen. Leuk Lymphoma 2007;48:542–546. [DOI] [PubMed] [Google Scholar]
- 22. Rimsza LM, Roberts RA, Miller TP, et al. Loss of MHC class II gene and protein expression in diffuse large B‐cell lymphoma is related to decreased tumor immunosurveillance and poor patient survival regardless of other prognostic factors: A follow‐up study from the Leukemia and Lymphoma Molecular Profiling Project. Blood 2004;103:4251–4258. [DOI] [PubMed] [Google Scholar]
- 23. Han X, Bueso‐Ramos CE. Precursor T‐cell acute lymphoblastic leukemia/lymphoblastic lymphoma and acute biphenotypic leukemias. Am J Clin Pathol 2007;127:528–544. [DOI] [PubMed] [Google Scholar]
- 24. Doveren RF, Buurman WA, Schutte B, et al. Class II antigens on canine T lymphocytes. Tissue Antigens 1985;25:255–265. [DOI] [PubMed] [Google Scholar]
- 25. Holling TM, van der Stoep N, Quinten E, et al. Activated human T cells accomplish MHC class II expression through T cell‐specific occupation of class II transactivator promoter III. J Immunol 2002;168:763–770. [DOI] [PubMed] [Google Scholar]
- 26. Frantz AM, Sarver AL, Ito D, et al. Molecular profiling reveals prognostically significant subtypes of canine lymphoma. Vet Pathol 2013;50:693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aresu L, Martini V, Rossi F, et al. Canine indolent and aggressive lymphoma: Clinical spectrum with histologic correlation. Vet Comp Oncol 2013; epub June 20, 2013. [DOI] [PubMed] [Google Scholar]
- 28. Flood‐Knapik KE, Durham AC, Gregor TP, et al. Clinical, histopathological and immunohistochemical characterization of canine indolent lymphoma. Vet Comp Oncol 2012;11:272–286. [DOI] [PubMed] [Google Scholar]


