Abstract
Background
Measurement of plasma concentration of natriuretic peptides (NPs) is suggested to be of value in diagnosis of cardiac disease in dogs, but many factors other than cardiac status may influence their concentrations. Dog breed potentially is 1 such factor.
Objective
To investigate breed variation in plasma concentrations of pro‐atrial natriuretic peptide 31‐67 (proANP 31‐67) and N‐terminal B‐type natriuretic peptide (NT‐proBNP) in healthy dogs.
Animals
535 healthy, privately owned dogs of 9 breeds were examined at 5 centers as part of the European Union (EU) LUPA project.
Methods
Absence of cardiovascular disease or other clinically relevant organ‐related or systemic disease was ensured by thorough clinical investigation. Plasma concentrations of proANP 31‐67 and NT‐proBNP were measured by commercially available ELISA assays.
Results
Overall significant breed differences were found in proANP 31‐67 (P < .0001) and NT‐proBNP (P < .0001) concentrations. Pair‐wise comparisons between breeds differed in approximately 50% of comparisons for proANP 31‐67 as well as NT‐proBNP concentrations, both when including all centers and within each center. Interquartile range was large for many breeds, especially for NT‐proBNP. Among included breeds, Labrador Retrievers and Newfoundlands had highest median NT‐proBNP concentrations with concentrations 3 times as high as those of Dachshunds. German Shepherds and Cavalier King Charles Spaniels had the highest median proANP 31‐67 concentrations, twice the median concentration in Doberman Pinschers.
Conclusions and Clinical Importance
Considerable interbreed variation in plasma NP concentrations was found in healthy dogs. Intrabreed variation was large in several breeds, especially for NT‐proBNP. Additional studies are needed to establish breed‐specific reference ranges.
Keywords: Canine, Interbreed variation, Intrabreed variation, NT‐proBNP, Plasma, proANP 31‐67
Abbreviations
- ANP
atrial natriuretic peptide
- BNP
B‐type natriuretic peptide
- CHF
congestive heart failure
- EU
European Union
- IQR
interquartile range
- NP
natriuretic peptide
- NT‐proANP
N‐terminal pro‐atrial natriuretic peptide
- NT‐proBNP
N‐terminal pro‐B‐type natriuretic peptide
- proANP 31‐67
pro‐atrial natriuretic peptide 31‐67
Natriuretic peptides (NPs) are used as indicators of cardiac health in humans and have been suggested to be of diagnostic and prognostic value in cardiac disease in dogs. B‐type natriuretic peptide (BNP) and N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) tests currently are widely used as diagnostic and prognostic tools for congestive heart failure (CHF) and left ventricular dysfunction in acute myocardial infarction in humans.1 Increased plasma NP concentrations have been identified in dogs with dilated cardiomyopathy (DCM).2, 3, 4, 5, 6, 7 Furthermore, NP concentrations have been shown to increase with increasing disease severity in dogs with myxomatous mitral valve disease (MMVD),3, 8, 9, 10, 11 and NT‐proBNP has been suggested to be predictive of outcome in dogs with MMVD.9, 12, 13, 14, 15, 16 Natriuretic peptides also have been found useful to distinguish CHF from respiratory disease.12, 17, 18, 19, 20 However, in several studies of dogs, considerable overlap between investigated groups has been identified.2, 3, 8, 9, 11, 17, 18, 19
Both atrial natriuretic peptide (ANP) and B‐type natriuretic peptide (BNP) are synthesized as high‐molecular weight precursors (prohormones), which are cleaved into 2 segments21, 22; 1 biologically active peptide in the C‐terminal region described as ANP or BNP, and 1 biologically inactive N‐terminal segment described as NT‐proANP or NT‐proBNP.1, 21, 22, 23 NT‐proANP is further cleaved into 3 segments; proANP 1‐30, 31‐67, and 68‐98.24 All segments can be analyzed,3, 4, 6, 8, 17, 18, 25 but the N‐terminal segments have been found to have longer half‐lives and may, therefore, be better suited as diagnostic tools.11, 26, 27, 28, 29 Furthermore, midregional proANP, such as proANP 31‐67, has been shown to have high biological stability, potentially because of a lower risk of fragmentation compared to proANP 1‐98.27
A clinically useful diagnostic and prognostic test for canine heart disease requires an upper reference limit for natriuretic peptides in healthy dogs, and cutoff values for dogs with subclinical disease or clinical signs of CHF.29, 30 There are, however, numerous physiologic and pathologic factors in addition to cardiac function that may influence NP concentrations in humans as well as dogs, and concerns exist about how to adjust reference limits and cutoff‐values with regard to these factors.3, 6, 8, 9, 17, 18, 19, 30, 31, 32
Previous studies have indicated potential breed differences in NP concentrations,3, 4, 18 and differences have been shown in concentration of NT‐proBNP between healthy purebred and healthy mixed breed dogs.3 To our knowledge, the influence of breed on NP concentration has not been specifically studied previously. Hence, the aim of this study was to investigate breed variation in the plasma concentrations of proANP 31‐67 and NT‐proBNP in healthy dogs.
Materials and Methods
Animals
Dogs were examined at 5 centers, as part of the EU‐funded LUPA project.33 Centers included University of Liège, Belgium; University of Copenhagen, Denmark; National Veterinary School of Alfort, France; University of Helsinki, Finland, and the Swedish University of Agricultural Sciences, Sweden. The study was approved by an ethical committee in each participating country. Dogs were privately owned, and informed owner consent was obtained. To be included in the study, dogs had to be purebred, healthy, and between 1 and 7 years of age. They also had to have a normal body condition score, and could not be related to each other at parental level. Each center could only include 2–5 breeds, and within center, each breed cohort included dogs of 1 sex only, intact males or females that were spayed or in anestrus, according to the inclusion criteria of the LUPA project. One breed was represented at 4 out of 5 centers. Exclusion criteria consisted of any finding indicating systemic or organ‐related disease observed during the clinical examination described below.
Preparations
Dog owners were instructed to feed their dog only standard commercial dog food 2 weeks before participation in the study to ensure consistent salt intake. On examination day, all dogs were fasted and had no access to water for at least 2 hours before the examination.
Verification of Health Status
Each dog underwent a general physical examination including blood pressure measurement by high‐definition oscillometry, a 5‐minute ECG recording, and an echocardiographic examination. The echocardiographic examination was performed from the right and left sides, by using standardized imaging planes34 and continuous ECG monitoring. The left atrial‐to‐aortic root ratio was quantified from the right 2‐dimensional short‐axis view.35 Pulmonic and aortic flow velocities were measured by spectral Doppler, and the mitral, aortic, pulmonic, and tricuspid valves were screened with color Doppler. The left ventricle was measured by standard M‐mode techniques. All examinations were performed in unsedated dogs.
Sampling Procedures and Storage
Urine samples were collected by voiding and standard urine analysis was performed by dipstick chemistry test, and refractometer for urine specific gravity. Blood sampling was carried out by venipuncture, and blood was collected into 5‐mL EDTA and serum tubes. Routine analysis of hematology and biochemistry including parameters of liver and kidney function, glucose, and serum electrolyte concentrations were performed for verification of health status. EDTA tubes for analysis of natriuretic peptides were centrifuged within 30 minutes of blood sample collection. Plasma was harvested, transferred into plastic cryotubes, and samples were frozen and stored at −80°C. For practical reasons, at 1 center samples were stored at −20°C for a maximum of 2 weeks, after which they were transferred frozen to −80°C and stored for batched analysis. Previous studies have shown that midregional proANP is stable for 3–6 months at −20°C and NT‐proBNP is stable for 4 months at −20°C.27, 36, 37 All samples were later transported frozen to 2 accredited laboratories, 1 laboratory for analysis of proANP 31‐67 and another laboratory for analysis of NT‐proBNP. Protease inhibition was not employed in this study.
Analysis of Natriuretic Peptides
Plasma concentrations of proANP 31‐67 and NT‐proBNP were analyzed by commercially available ELISA assays, according to manufacturers' instructions.,1,2 All samples were analyzed in duplicate by personnel blinded to dog identity, and the mean of the 2 results was used for data analysis. Both assays have been validated for dogs.17, 38
Statistical Analyses
Commercially available software3 was used for all statistical analyses. Data are presented as medians and interquartile ranges (IQR). A value of P < .05 was considered significant for the analyses, unless otherwise indicated.
The nonparametric Kruskal–Wallis test was used to investigate overall differences among breeds, for concentrations of proANP 31‐67 and NT‐proBNP, respectively. If a significant difference was detected, pair‐wise comparisons between breeds were performed by use of the Mann–Whitney U‐test with Bonferroni adjustment, for which a value of P < .0014 was considered significant. The Kruskal–Wallis test also was used to investigate differences among breeds at each center for concentrations of proANP 31‐67 and NT‐proBNP, respectively. At centers including more than 2 breeds, pair‐wise comparisons between breeds were performed by use of the Mann–Whitney U‐test with Bonferroni adjustment, if an overall significant difference was detected.
Interquartile distances were calculated for all dogs as well as per breed for each NP, by subtracting the 25‐percentile value from the 75‐percentile value. Interquartile distances were corrected for differences in median values by dividing the interquartile distance by the median value for all dogs as well as per breed for each NP.
Unilinear regression analyses were performed to evaluate potential associations between breed as well as age, body weight, examination center, and concentrations of proANP 31‐67 and NT‐proBNP, respectively. A subanalysis of the same variables by unilinear regression analysis was performed in the Labrador Retriever breed alone, because this breed included the largest number of dogs, was represented at 4 out of 5 centers and included both female and male dogs. Therefore, sex also was assessed by unilinear regression analysis in the Labrador Retriever breed.
To compensate for the influence of other confounding factors on plasma NP concentrations, a multiple regression analysis was performed, including variables that reached P < .2 in the unilinear regression analysis of all dogs. Analyses were performed in a reverse stepwise manner,39 starting with all variables included in the model and then removing the variable with the highest P value until all remaining variables had a value of P < .05. All variables were assessed only as main effects; no interaction terms were considered in the model.
The distribution of residuals in the multiple regression analysis was tested for normality by Shapiro–Wilk W‐test. The adjusted R 2 is defined as the percentage of the total sum of squares that can be explained by the regression and it also considers the degrees of freedom for variables added. No multiple regression analysis was performed in the Labrador Retriever cohort attributable to the high covariance between center and sex.
Results
In total, 535 dogs of 9 breeds were included in the study. These dogs had passed the general health examination without abnormal findings, no ECG abnormalities were detected, and all echocardiographic variables were within the reference range. Furthermore, no clinically relevant abnormalities were detected in the hematologic or serum biochemistry variables or in the urine analysis. Distribution of breeds and individuals examined at the different centers is shown in Table 1. Each center examined dogs belonging to 2–4 different breeds; some breeds were examined at more than 1 center. Sex distribution was uneven with 416 males and 119 female dogs (Table 1). All males were intact whereas females were spayed or in anestrus. Median age (n = 531) was 3.3 (IQR, 2.6–4.4) years and median body weight (n = 497) was 30.2 (IQR, 23.5–36.0) kg.
Table 1.
Distribution by center of examination, breed, and sex.
| Belgium | Denmark | Finland | France | Sweden | Total | |
|---|---|---|---|---|---|---|
| Box | 15M | 15 | ||||
| BS | 97M | 26M | 123 | |||
| CKCS | 34M | 34 | ||||
| Dach | 26M | 16M | 42 | |||
| Dob | 25M | 25 | ||||
| FinL | 50M | 50 | ||||
| GS | 16M | 60M | 76 | |||
| Lab | 6M | 45F | 29F | 45M | 125 | |
| NF | 45F | 45 | ||||
| Total | 119 | 90 | 136 | 80 | 110 | 535 |
Box, Boxer; BS, Belgian Shepherd; CKCS, Cavalier King Charles Spaniel; Dach, Dachshund; Dob, Doberman Pinscher; FinL, Finnish Lapphund; GS, German Shepherd; Lab, Labrador Retriever; NF, Newfoundland; M, male; F, female.
Natriuretic Peptides
The median concentration of proANP 31‐67 (n = 535) was 889 (IQR, 735–1,065) pmol/L, whereas the median concentration of NT‐proBNP (n = 527) was 638 (IQR, 403–980) pmol/L. Natriuretic peptide concentrations by breed are shown in Figure 1. The overall interquartile distance was 330 pmol/L for proANP 31‐67 and 577 pmol/L for NT‐proBNP. Overall interquartile distance median ratio was 0.37 for proANP 31‐67 and 0.90 for NT‐proBNP.
Figure 1.
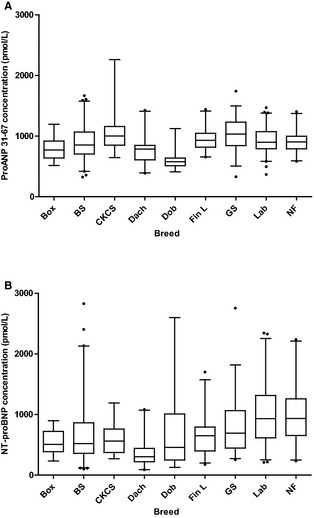
Boxplots showing distribution of proANP 31‐67 (A) and NT‐proBNP (B) by breed. The top, bottom, and line through the middle of each box correspond to the 75th percentile (top quartile), the 25th percentile (bottom quartile) and the 50th percentile (median), respectively. The whiskers extend from the bottom 2.5th percentile to the top 97.5th percentile. Outliers, which are represented by black dots, were included in the statistical analyses. There was an overall significant difference between breeds for both natriuretic peptides (P < .0001). For information on which breeds that differed significantly, see Table 2. Box, Boxer; BS, Belgian Shepherd; CKCS, Cavalier King Charles Spaniel; Dach, Dachshund; Dob, Doberman Pinscher; Fin L, Finnish Lapphund; GS, German Shepherd; Lab, Labrador Retriever; NF, Newfoundland.
Group‐Wise Comparisons, All Dogs
There was an overall significant breed difference for proANP 31‐67 (P < .0001) and NT‐proBNP (P < .0001). Pair‐wise comparisons between breeds showed significant differences in 15 of 36 comparisons of proANP 31‐67 and 18 of 36 comparisons of NT‐proBNP (Table 2). Concentrations of proANP 31‐67 were lowest in Doberman Pinschers, with a median concentration almost half of the median values in German Shepherds and Cavalier King Charles Spaniels, which had the highest concentrations. Concentrations of NT‐proBNP were lowest in Dachshunds, whereas Labrador Retrievers and Newfoundlands had the highest concentrations with median values 3 times the median value in Dachshunds (Fig 1). Interquartile distances and interquartile distance median ratios per breed for both NPs are shown in Figures 2 and 3.
Table 2.
Pair‐wise comparisons between breeds in plasma concentrations of the natriuretic peptides proANP 31‐67 and NT‐proBNP. The asterisks denote significant differences by using a Bonferroni corrected P value of <.014.
| Box | CKCS | Dach | Dob | FinL | GS | Lab | NF | ||
|---|---|---|---|---|---|---|---|---|---|
| BS | – | – | – | * | – | * | – | – | proANP 31‐67 |
| – | – | * | – | – | – | * | * | NT‐proBNP | |
| – | Box | * | – | – | – | * | – | – | proANP 31‐67 |
| * | – | – | – | * | * | NT‐proBNP | |||
| – | – | CKCS | * | * | – | – | – | – | proANP 31‐67 |
| – | * | – | – | – | * | * | NT‐proBNP | ||
| – | – | – | Dach | * | * | * | * | * | proANP 31‐67 |
| – | – | – | * | * | * | * | NT‐proBNP | ||
| – | – | – | – | Dob | * | * | * | * | proANP 31‐67 |
| – | – | – | – | – | * | * | NT‐proBNP | ||
| – | – | – | – | – | FinL | – | – | – | proANP 31‐67 |
| – | – | – | – | – | * | * | NT‐proBNP | ||
| – | – | – | – | – | – | GS | – | – | proANP 31‐67 |
| – | – | – | – | – | * | – | NT‐proBNP | ||
| – | – | – | – | – | – | – | Lab | – | proANP 31‐67 |
| – | – | – | – | – | – | – | NT‐proBNP |
Box, Boxer; BS, Belgian Shepherd; CKCS, Cavalier King Charles Spaniel; Dach, Dachshund; Dob, Doberman Pinscher; FinL, Finnish Lapphund; GS, German Shepherd; Lab, Labrador Retriever; NF, Newfoundland.
Figure 2.
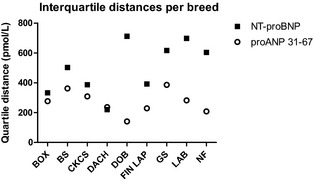
Interquartile distances of NT‐proBNP and proANP 31‐67 displayed by breed. Box, Boxer; BS, Belgian Shepherd; CKCS, Cavalier King Charles Spaniel; Dach, Dachshund; Dob, Doberman Pinscher; Fin Lap, Finnish Lapphund; GS, German Shepherd; Lab, Labrador Retriever; NF, Newfoundland.
Figure 3.
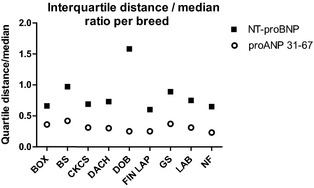
Interquartile distances corrected for median values of NT‐proBNP and proANP 31‐67 displayed by breed. Box, Boxer; BS, Belgian Shepherd; CKCS, Cavalier King Charles Spaniel; Dach, Dachshund; Dob, Doberman Pinscher; Fin Lap, Finnish Lapphund; GS, German Shepherd; Lab, Labrador Retriever; NF, Newfoundland.
Group‐Wise Comparisons, within Center
Overall significant breed differences were found at 3 of the 5 centers for proANP 31‐67 (P ≤ .0006) and at 4 of the 5 centers for NT‐proBNP (P < .003). Pair‐wise comparisons showed significant differences in 8 of 16 comparisons of proANP 31‐67 and 7 of 16 comparisons of NT‐proBNP.
Unilinear Regression Analysis
In the unilinear regression analysis of all dogs, an association was shown between proANP 31‐67 and breed (R 2 = 0.15, P < .0001) as well as center of examination (R 2 = 0.07, P < .0001). NT‐proBNP also was associated with breed (R 2 = 0.16, P < .0001) and center of examination (R 2 = 0.16, P < .0001). Furthermore, NT‐proBNP concentration increased with increasing body weight (R 2 = 0.04, P < .0001). In the separate unilinear regression analysis of Labrador Retrievers, an association was shown between proANP 31‐67 and center of examination (R 2 = 0.11, P = .003) as well as sex (R 2 = 0.05, P = .013), with higher concentrations in male than female dogs. In Labrador Retrievers, NT‐proBNP was associated with center of examination (R 2 = 0.24, P < .0001) and sex (R 2 = 0.24, P < .0001), with higher concentrations in female than male dogs.
Multiple Regression Analysis
The multiple regression analysis of all dogs confirmed an effect of breed (P < .0001) and center (P = .0008) on proANP 31‐67 concentration with an adjusted model R 2 of 0.16. Also for NT‐proBNP concentration, multiple regression analysis confirmed an effect of breed (P < .0001) and center (P < .0001) with an adjusted model R 2 of 0.22.
Discussion
Highly significant overall breed differences were found in plasma concentrations of proANP 31‐67 and NT‐proBNP in healthy dogs examined at 5 centers. Differences were found in approximately 50% of the pair‐wise comparisons between breeds for proANP 31‐67 as well as NT‐proBNP concentrations, both when including all centers and within each center, suggesting that the differences were of genuine clinical importance.
Included breeds represented dogs of varying body sizes, temperament, use and genetic background. Strong selection for certain physiologic, morphologic and behavioral traits has created dog breeds with a unique diversity among mammalian species, and specific features are inherited closely within breeds.40 Among the 9 included breeds, Labrador Retrievers and Newfoundlands had the highest median NT‐proBNP concentrations with values 3 times as high as those in Dachshunds. The median proANP 31‐67 concentration instead was highest in German Shepherds and CKCSs, with median concentrations twice the median concentration in Doberman Pinschers. The breed variation could partly be explained by genetic background, as illustrated by the closely related breeds, German and Belgian Shepherds, having very similar distributions of both proANP 31‐67 and NT‐proBNP. Previous studies have shown breed differences in cardiovascular variables such as blood pressure, heart rate, and catecholamine concentrations.41, 42, 43 Tachycardia has been associated with increased plasma concentrations of ANP in people as well as in dogs and has been suggested to depend primarily on increased atrial pressure.44, 45 Both ANP and BNP are released in response to volume expansion and pressure overload. However, their modes of production and release differ, with proANP being stored intact in secretory granules, whereas BNP is constitutively secreted and has little intracellular storage. In healthy dogs, both ANP and BNP are produced in the atria, whereas ventricles as well as atria contribute to the production of ANP and BNP in dogs with CHF.46, 47
All dogs included in this study were confirmed to be healthy by an extensive health examination. Most breeds had higher concentrations of proANP 31‐67 than NT‐proBNP, which is consistent with recent findings in healthy people.27, 48 The interquartile distance was large in several breeds, especially for NT‐proBNP, with 32% of the total number of dogs having NT‐proBNP concentrations higher than a previously proposed value indicative of heart disease in dogs.30 Sample handling differed slightly, with protease inhibitors being used in the previous study, and not in this study, but the same assay was used in both studies. A high individual weekly variability in NT‐proBNP concentration has been shown in healthy dogs,32 making interpretation of a single individual sample uncertain. The lower variability for proANP 31‐67 in combination with high biologic stability, attributable to low risk of fragmentation,27 makes it an interesting molecule for further studies in dogs. Comparing concentrations of proANP 31‐67 in this study to the previously proposed cutoff value of 1,750 pmol/L for CHF in dogs,49 <1% of our included healthy dogs were above the cutoff.
Center of examination was associated with plasma concentration of both proANP 31‐67 and NT‐proBNP in both unilinear and multiple regression analyses. Because of the uneven breed distribution among centers, breed was highly covariate with center. Therefore, group‐wise comparisons between breeds also were performed at each center. The results showed a similar percentage of significant pair‐wise differences as observed when all dogs were included (approximately 50% for both NPs), confirming the presence of breed variation in the examined population.
Because of the uneven sex distribution among centers as well as breeds, sex was not included in the unilinear or multiple regression analyses of all dogs. In the unilinear regression analysis of the Labrador Retriever cohort, an association between sex and NT‐proBNP concentration was found, with higher concentrations in females compared to males, a finding that also has been made in healthy people.48 A weak association also was found between sex and concentration of proANP 31‐67, with higher concentrations in males compared to females. This study was, however, not designed to evaluate sex differences and no multiple regression analysis was performed in the Labrador Retriever cohort, because of the high covariance between center and sex. Additional study into the potential effect of sex on NP concentrations in dogs is warranted.
A strong association between age and NP concentrations has been found in several large studies of humans,31 a finding potentially caused by decreased heart function with greater age. In healthy dogs, NT‐proANP has been shown to be positively associated with age in a small study,50 whereas BNP has been shown to be positively associated with age in dogs with CHF, potentially attributable to the higher incidence of heart disease in older male dogs.18 In this study, no association between age and NP concentration was found. However, the study only included young adult to middle‐aged dogs, and additional studies are needed to investigate a potential association between age and proANP 31‐67 and NT‐proBNP in healthy dogs.
Study Limitations
The study only included 9 breeds and, therefore, cannot be considered representative of the entire dog population. Because of the uneven sex representation and narrow age span of included dogs, results should not be interpreted as reference values. To establish reference values, additional studies evaluating other breeds with an even sex and age representation should be performed. Blood was collected only once from each dog. Therefore, individual variability in NP concentrations could not be assessed. The comparably low R 2‐values of the final multiple regression models for both NPs suggest that factors other than breed and center affect NP concentrations, with 1 potential factor being individual variation.32 All dogs were fed commercial dog food, but differences in salt intake could have occurred, potentially affecting NP concentrations. Sample handling was standardized, although minor differences in short‐term freezing temperature occurred at 1 center. However, NPs have been shown to be stable at the freezing temperatures used,27, 36, 37 and each peptide was analyzed in batches at the same laboratory. Hence, it is unlikely that sample handling had any major effect on the results.
Conclusion
Considerable breed variation exists in concentrations of proANP 31‐67 and NT‐proBNP in healthy dogs. Intrabreed variation was large in several breeds, especially for NT‐proBNP. Additional studies are warranted to establish breed‐specific reference values, in order to improve future use of natriuretic peptides as cardiac biomarkers in dogs.
Acknowledgments
We thank IDEXX for providing the assays used for analysis of proANP 31‐67 and NT‐proBNP. We also thank the Universities involved for their efforts that allowed successful completion of the study. We are grateful to all dog owners for their willingness to enroll their dogs, and we thank Yukihide Momozawa for his contribution to the study. The study was funded by the European Commission (FP7‐LUPA, GA‐201370).
Conflict of Interest: Authors disclose no conflict of interest.
The planning of the study started in 2007. The study protocol was finalized in 2008. There was a follow‐up meeting in 2010. This article was finalized and approved by the investigators in May 2013. The publication committee consisted of Katja Höglund, Karin Sjöstrand, Gerhard Wess, Ingrid Ljungvall and Jens Häggström.
Presented in part at the 22nd European College of Veterinary Internal Medicine – Companion Animal Congress, Maastricht, The Netherlands, September 2012.
Footnotes
ProANP 31‐67: ELISA VETSIGN Canine CardioSCREEN, Biomedica, Vienna, Austria
NT‐proBNP: ELISA Cardiopet proBNP test, IDEXX Laboratories, Westbrook, ME
JMP, version 9.0.0, SAS Institute Inc, Cary, NC
References
- 1. Maisel A, Mueller C, Adams K, Jr. , et al. State of the art: Using natriuretic peptide levels in clinical practice. Eur J Heart Fail 2008;10:824–839. [DOI] [PubMed] [Google Scholar]
- 2. Wess G, Butz V, Mahling M, et al. Evaluation of N‐terminal pro‐B‐type natriuretic peptide as a diagnostic marker of various stages of cardiomyopathy in Doberman Pinschers. Am J Vet Res 2011;72:642–649. [DOI] [PubMed] [Google Scholar]
- 3. Oyama MA, Fox PR, Rush JE, et al. Clinical utility of serum N‐terminal pro‐B‐type natriuretic peptide concentration for identifying cardiac disease in dogs and assessing disease severity. J Am Vet Med Assoc 2008;232:1496–1503. [DOI] [PubMed] [Google Scholar]
- 4. Oyama MA, Sisson DD, Solter PF. Prospective screening for occult cardiomyopathy in dogs by measurement of plasma atrial natriuretic peptide, B‐type natriuretic peptide, and cardiac troponin‐I concentrations. Am J Vet Res 2007;68:42–47. [DOI] [PubMed] [Google Scholar]
- 5. Singletary GE, Morris NA, Lynne O'Sullivan M, et al. Prospective evaluation of NT‐proBNP assay to detect occult dilated cardiomyopathy and predict survival in Doberman Pinschers. J Vet Intern Med 2012;26:1330–1336. [DOI] [PubMed] [Google Scholar]
- 6. O'Sullivan ML, O'Grady MR, Minors SL. Plasma big endothelin‐1, atrial natriuretic peptide, aldosterone, and norepinephrine concentrations in normal Doberman Pinschers and Doberman Pinschers with dilated cardiomyopathy. J Vet Intern Med 2007;21:92–99. [DOI] [PubMed] [Google Scholar]
- 7. Tidholm A, Häggström J, Hansson K. Effects of dilated cardiomyopathy on the renin‐angiotensin‐aldosterone system, atrial natriuretic peptide activity, and thyroid hormone concentrations in dogs. Am J Vet Res 2001;62:961–967. [DOI] [PubMed] [Google Scholar]
- 8. Takemura N, Toda N, Miyagawa Y, et al. Evaluation of plasma N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) concentrations in dogs with mitral valve insufficiency. J Vet Med Sci 2009;71:925–929. [DOI] [PubMed] [Google Scholar]
- 9. Tarnow I, Olsen LH, Kvart C, et al. Predictive value of natriuretic peptides in dogs with mitral valve disease. Vet J 2009;180:195–201. [DOI] [PubMed] [Google Scholar]
- 10. Wolf J, Gerlach N, Weber K, et al. Lowered N‐terminal pro‐B‐type natriuretic peptide levels in response to treatment predict survival in dogs with symptomatic mitral valve disease. J Vet Cardiol 2012;14:399–408. [DOI] [PubMed] [Google Scholar]
- 11. Häggström J, Hansson K, Kvart C, et al. Relationship between different natriuretic peptides and severity of naturally acquired mitral regurgitation in dogs with chronic myxomatous valve disease. J Vet Cardiol 2000;2:7–16. [DOI] [PubMed] [Google Scholar]
- 12. Reynolds CA, Brown DC, Rush JE, et al. Prediction of first onset of congestive heart failure in dogs with degenerative mitral valve disease: The PREDICT cohort study. J Vet Cardiol 2012;14:193–202. [DOI] [PubMed] [Google Scholar]
- 13. Chetboul V, Serres F, Tissier R, et al. Association of plasma N‐terminal pro‐B‐type natriuretic peptide concentration with mitral regurgitation severity and outcome in dogs with asymptomatic degenerative mitral valve disease. J Vet Intern Med 2009;23:984–994. [DOI] [PubMed] [Google Scholar]
- 14. Serres F, Pouchelon JL, Poujol L, et al. Plasma N‐terminal pro‐B‐type natriuretic peptide concentration helps to predict survival in dogs with symptomatic degenerative mitral valve disease regardless of and in combination with the initial clinical status at admission. J Vet Cardiol 2009;11:103–121. [DOI] [PubMed] [Google Scholar]
- 15. Hezzell MJ, Boswood A, Chang YM, et al. The combined prognostic potential of serum high‐sensitivity cardiac troponin I and N‐terminal pro‐B‐type natriuretic peptide concentrations in dogs with degenerative mitral valve disease. J Vet Intern Med 2012;26:302–311. [DOI] [PubMed] [Google Scholar]
- 16. Moonarmart W, Boswood A, Luis Fuentes V, et al. N‐terminal pro B‐type natriuretic peptide and left ventricular diameter independently predict mortality in dogs with mitral valve disease. J Small Anim Pract 2010;51:84–96. [DOI] [PubMed] [Google Scholar]
- 17. Boswood A, Dukes‐McEwan J, Loureiro J, et al. The diagnostic accuracy of different natriuretic peptides in the investigation of canine cardiac disease. J Small Anim Pract 2008;49:26–32. [DOI] [PubMed] [Google Scholar]
- 18. DeFrancesco TC, Rush JE, Rozanski EA, et al. Prospective clinical evaluation of an ELISA B‐type natriuretic peptide assay in the diagnosis of congestive heart failure in dogs presenting with cough or dyspnea. J Vet Intern Med 2007;21:243–250. [DOI] [PubMed] [Google Scholar]
- 19. Prosek R, Sisson DD, Oyama MA, et al. Distinguishing cardiac and noncardiac dyspnea in 48 dogs using plasma atrial natriuretic factor, B‐type natriuretic factor, endothelin, and cardiac troponin‐I. J Vet Intern Med 2007;21:238–242. [DOI] [PubMed] [Google Scholar]
- 20. Oyama MA, Rush JE, Rozanski EA, et al. Assessment of serum N‐terminal pro‐B‐type natriuretic peptide concentration for differentiation of congestive heart failure from primary respiratory tract disease as the cause of respiratory signs in dogs. J Am Vet Med Assoc 2009;235:1319–1325. [DOI] [PubMed] [Google Scholar]
- 21. Saito Y, Nakao K, Itoh H, et al. Brain natriuretic peptide is a novel cardiac hormone. Biochem Biophys Res Comm 1989;158:360–368. [DOI] [PubMed] [Google Scholar]
- 22. Yasue H, Yoshimura M, Sumida H, et al. Localization and mechanism of secretion of B‐type natriuretic peptide in comparison with those of A‐type natriuretic peptide in normal subjects and patients with heart failure. Circulation 1994;90:195–203. [DOI] [PubMed] [Google Scholar]
- 23. Thibault G, Garcia R, Gutkowska J, et al. The propeptide Asn1‐Tyr126 is the storage form of rat atrial natriuretic factor. Biochem J 1987;241:265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Winters CJ, Sallman AL, Baker BJ, et al. The N‐terminus and a 4,000‐MW peptide from the midportion of the N‐terminus of the atrial natriuretic factor prohormone each circulate in humans and increase in congestive heart failure. Circulation 1989;80:438–449. [DOI] [PubMed] [Google Scholar]
- 25. Sagnella GA. Measurement and importance of plasma brain natriuretic peptide and related peptides. Ann Clin Biochem 2001;38:83–93. [DOI] [PubMed] [Google Scholar]
- 26. Buckley MG, Marcus NJ, Yacoub MH. Cardiac peptide stability, aprotinin and room temperature: Importance for assessing cardiac function in clinical practice. Clin Sci 1999;97:689–695. [PubMed] [Google Scholar]
- 27. Morgenthaler NG, Struck J, Thomas B, et al. Immunoluminometric assay for the midregion of pro‐atrial natriuretic peptide in human plasma. Clin Chem 2004;50:234–236. [DOI] [PubMed] [Google Scholar]
- 28. Pemberton CJ, Johnson ML, Yandle TG, et al. Deconvolution analysis of cardiac natriuretic peptides during acute volume overload. Hypertension 2000;36:355–359. [DOI] [PubMed] [Google Scholar]
- 29. Sagnella GA. Measurement and significance of circulating natriuretic peptides in cardiovascular disease. Clin Sci 1998;95:519–529. [DOI] [PubMed] [Google Scholar]
- 30. Ettinger SJ, Farace G, Forney SD, et al. Evaluation of plasma N‐terminal pro‐B‐type natriuretic peptide concentrations in dogs with and without cardiac disease. J Am Vet Med Assoc 2012;240:171–180. [DOI] [PubMed] [Google Scholar]
- 31. Balion CM, Santaguida P, McKelvie R, et al. Physiological, pathological, pharmacological, biochemical and hematological factors affecting BNP and NT‐proBNP. Clin Biochem 2008;41:231–239. [DOI] [PubMed] [Google Scholar]
- 32. Kellihan HB, Oyama MA, Reynolds CA, et al. Weekly variability of plasma and serum NT‐proBNP measurements in normal dogs. J Vet Cardiol 2009;11(Suppl 1):S93–S97. [DOI] [PubMed] [Google Scholar]
- 33. Lequarre AS, Andersson L, Andre C, et al. LUPA: A European initiative taking advantage of the canine genome architecture for unravelling complex disorders in both human and dogs. Vet J 2011;189:155–159. [DOI] [PubMed] [Google Scholar]
- 34. Thomas WP, Gaber CE, Jacobs GJ, et al. Recommendations for standards in transthoracic two‐dimensional echocardiography in the dog and cat. Echocardiography Committee of the Specialty of Cardiology, American College of Veterinary Internal Medicine. J Vet Intern Med 1993;7:247–252. [DOI] [PubMed] [Google Scholar]
- 35. Hansson K, Häggström J, Kvart C, et al. Left atrial to aortic root indices using two‐dimensional and M‐mode echocardiography in Cavalier King Charles Spaniels with and without left atrial enlargement. Vet Radiol Ultrasound 2002;43:568–575. [DOI] [PubMed] [Google Scholar]
- 36. Hunt PJ, Richards AM, Nicholls MG, et al. Immunoreactive amino‐terminal pro‐brain natriuretic peptide (NT‐PROBNP): A new marker of cardiac impairment. Clin Endocrinol 1997;47:287–296. [DOI] [PubMed] [Google Scholar]
- 37. Mueller T, Gegenhuber A, Dieplinger B, et al. Long‐term stability of endogenous B‐type natriuretic peptide (BNP) and amino terminal proBNP (NT‐proBNP) in frozen plasma samples. Clin Chem Lab Med 2004;42:942–944. [DOI] [PubMed] [Google Scholar]
- 38. Schellenberg S, Grenacher B, Kaufmann K, et al. Analytical validation of commercial immunoassays for the measurement of cardiovascular peptides in the dog. Vet J 2008;178:85–90. [DOI] [PubMed] [Google Scholar]
- 39. Bland M. Multifactorial methods In: Bland M, ed. An Introduction to Medical Statistics, 2nd ed Oxford: Oxford University Press; 1995:322–323. [Google Scholar]
- 40. Parker HG, Ostrander EA. Canine genomics and genetics: Running with the pack. PLoS Genet 2005;1:e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bodey AR, Michell AR. Epidemiological study of blood pressure in domestic dogs. J Small Anim Pract 1996;37:116–125. [DOI] [PubMed] [Google Scholar]
- 42. Rasmussen CE, Vesterholm S, Ludvigsen TP, et al. Holter monitoring in clinically healthy Cavalier King Charles Spaniels, Wire‐haired Dachshunds, and Cairn Terriers. J Vet Intern Med 2011;25:460–468. [DOI] [PubMed] [Google Scholar]
- 43. Höglund K, Hanås S, Carnabuci C, et al. Blood pressure, heart rate, and urinary catecholamines in healthy dogs subjected to different clinical settings. J Vet Intern Med 2012;26:1300–1308. [DOI] [PubMed] [Google Scholar]
- 44. Walsh KP, Williams TD, Spiteri C, et al. Role of atrial pressure and rate in release of atrial natriuretic peptide. Am J Physiol 1988;254:R607–R610. [DOI] [PubMed] [Google Scholar]
- 45. Crozier IG, Ikram H, Nicholls MG. The pattern of atrial natriuretic peptide release during ventricular tachycardia in man. Clin Exp Pharmacol Physiol 1987;14:597–604. [DOI] [PubMed] [Google Scholar]
- 46. Luchner A, Borgeson DD, Grantham JA, et al. Relationship between left ventricular wall stress and ANP gene expression during the evolution of rapid ventricular pacing‐induced heart failure in the dog. Eur J Heart Fail 2000;2:379–386. [DOI] [PubMed] [Google Scholar]
- 47. Luchner A, Stevens TL, Borgeson DD, et al. Differential atrial and ventricular expression of myocardial BNP during evolution of heart failure. Am J Physiol 1998;274:H1684–H1689. [DOI] [PubMed] [Google Scholar]
- 48. Hess G, Runkel S, Zdunek D, et al. Reference interval determination for N‐terminal‐B‐type natriuretic peptide (NT‐proBNP): A study in blood donors. Clin Chim Acta 2005;360:187–193. [DOI] [PubMed] [Google Scholar]
- 49. Boswood A, Attree S, Page K. Clinical validation of a proANP 31‐67 fragment ELISA in the diagnosis of heart failure in the dog. J Small Anim Pract 2003;44:104–108. [DOI] [PubMed] [Google Scholar]
- 50. Eriksson AS, Jarvinen AK, Eklund KK, et al. Effect of age and body weight on neurohumoral variables in healthy Cavalier King Charles Spaniels. Am J Vet Res 2001;62:1818–1824. [DOI] [PubMed] [Google Scholar]


