Abstract
Background
Hyperthyroidism has substantial effects on the circulatory system. The cardiac biomarkers NT‐proBNP and troponin I (cTNI) have proven useful in identifying cats with myocardial disease but have not been extensively investigated in hyperthyroidism.
Hypothesis
Plasma NT‐proBNP and cTNI concentrations are higher in cats with primary myocardial disease than in cats with hyperthyroidism and higher in cats with hyperthyroidism than in healthy control cats.
Animals
Twenty‐three hyperthyroid cats, 17 cats with subclinical hypertrophic cardiomyopathy (HCM), and 19 euthyroid, normotensive healthy cats ≥8 years of age. Fourteen of the hyperthyroid cats were re‐evaluated 3 months after administration of radioiodine (131I).
Methods
Complete history, physical examination, complete blood count, serum biochemistries, urinalysis, blood pressure measurement, serum T4 concentration, plasma concentrations of NT‐proBNP and cTNI, and echocardiogram were obtained prospectively from each cat.
Results
Hyperthyroid cats and cats with HCM had plasma NT‐proBNP and cTNI concentrations that were significantly higher than those of healthy cats, but there was no significant difference between hyperthyroid cats and cats with HCM with respect to the concentration of either biomarker. In hyperthyroid cats that were re‐evaluated 3 months after 131I treatment, plasma NT‐proBNP and cTNI concentrations as well as ventricular wall thickness had decreased significantly.
Conclusions and Clinical Importance
Although there may be a role for NT‐proBNP in monitoring the cardiac response to treatment of hyperthyroidism, neither NT‐proBNP nor cTNI distinguish hypertrophy associated with hyperthyroidism from primary HCM. Therefore, the thyroid status of older cats should be ascertained before interpreting NT‐proBNP and cTNI concentrations.
Keywords: Cardiac troponin I, Myocardial disease, NT‐proBNP
Abbreviations
- 131I
radioiodine
- cTNI
cardiac troponin I
- FS
fractional shortening
- HCM
hypertrophic cardiomyopathy
- IVSd
end‐diastolic interventricular septal dimension
- LA/Ao
left atrial to aortic ratio
- LVPWd
end‐diastolic left ventricular caudal wall dimension
- NT‐proBNP
N‐terminal pro brain natriuretic peptide
- TSH
thyroid stimulating hormone
Cardiac abnormalities, including murmurs and gallop sounds, often are detected during physical examination of hyperthyroid cats. Cardiovascular abnormalities in hyperthyroid cats are diverse and comprise subtle, clinically inconsequential myocardial changes as well as severe changes that can be associated with development of heart failure. There is evidence that myocardial abnormalities resolve after treatment in many cats, but persist in others.1, 2 It is relevant that subclinical echocardiographic abnormalities are common in the general feline population; 15.5% of apparently healthy euthyroid cats were found to have cardiomyopathy in a recent study.3 Other investigations have echocardiographically identified left ventricular hypertrophy in 34% of apparently healthy cats and in 40% of apparently healthy cats with murmurs.4, 5 Therefore, it is uncertain whether cardiac abnormalities that persist after resolution of hyperthyroidism are the result of hyperthyroidism or concurrent primary cardiac disease. An inexpensive and readily available method that reliably differentiates thyrotoxic heart disease from primary myocardial disease before treatment of hyperthyroidism would provide prognostic information, guide treatment recommendations, and possibly avoid unnecessary and costly diagnostic evaluation. In addition, it would be useful to have a method to predict the severity of cardiovascular changes caused by hyperthyroidism before definitive treatment.
Primary myocardial disease is a diagnosis of exclusion based on the results of blood pressure determination, metabolic assessment, and echocardiography. The most common form of primary myocardial disease in the cat, hypertrophic cardiomyopathy (HCM), is defined by hypertrophy of a nondilated ventricle that occurs in the absence of metabolic or hemodynamic stimuli such as hyperthyroidism or hypertension.6 Echocardiography is not readily available to all general practitioners. In addition, thyrotoxic heart disease may result in echocardiographic changes similar to those found in cats with primary myocardial disease, making results of echocardiography difficult to interpret. Evaluation of the plasma concentrations of the cardiac biomarkers N‐terminal probrain natriuretic peptide (NT‐proBNP), secreted in response to myocardial stretch, and cardiac troponin I (cTNI), released from the cytosol of damaged cardiomyocytes, may assist with the aforementioned diagnostic challenge.
Many studies of hyperthyroid humans have demonstrated increased plasma concentrations of NT‐proBNP,7, 8, 9 and T4 has been shown to stimulate BNP release from isolated rat cardiocytes.10 Limited studies of hyperthyroid cats have documented increased concentrations of NT‐proBNP and cTNI.11, 12 However, the biomarker profile of cats with hyperthyroidism and its relationship with echocardiographic findings has been incompletely defined. In clinical practice, biomarker concentrations may be determined in diverse clinical circumstances. They may be used in the evaluation of cats with clinical signs suggestive of congestive heart failure as well as in the evaluation of cats that have abnormal heart sounds in the absence of clinical signs. Furthermore, measurement of feline NT‐BNP has been proposed as a screening test for cardiac disease in apparently healthy cats.13, 14 Therefore, it is important to determine if hyperthyroidism affects plasma concentrations of NT‐proBNP and cTNI independent of clinically relevant heart disease.
We tested the hypothesis that plasma NT‐proBNP and cTNI concentrations are higher in cats with primary myocardial disease than in cats with hyperthyroidism and higher in cats with hyperthyroidism than in healthy control cats. The primary objective was to describe plasma concentrations of these biomarkers in 3 groups of cats: cats with naturally occurring hyperthyroidism, cats with primary myocardial disease, and healthy older cats. Secondary objectives were to determine if biomarkers could be used to differentiate among these populations and to describe changes in cardiac function and biomarker status after resolution of hyperthyroidism.
Materials and Methods
Animals
Three groups of cats were studied. Group 1 consisted of cats presented to the Veterinary Teaching Hospital of the Virginia‐Maryland Regional College of Veterinary Medicine (VMRCVM) with hyperthyroidism based on compatible clinical findings and serum total T4 concentration above the upper reference limit. Group 2 consisted of euthyroid, normotensive cats presented to the VMRCVM cardiology service for evaluation of suspected heart disease and ultimately confirmed to have HCM diagnosed by echocardiography. Group 3 consisted of VMRCVM staff‐ or student‐owned euthyroid normotensive healthy cats (as determined by history, physical examination, systolic blood pressure, laboratory testing, and echocardiography) ≥8 years of age or older, which acted as controls. The study design called for prospective enrollment of 30 cats in Groups 1 and 2, and 20 cats in Group 3 during a 15‐month enrollment period. All cats were screened for the following exclusion criteria: azotemia (BUN concentration >32 mg/dL and/or plasma creatinine concentration >1.8 mg/dL or both), current or previous congestive heart failure, current or previous treatment of cardiac disease, and presence of a systemic disease other than hyperthyroidism. In addition, cats were excluded from group 1 if they had been treated with antithyroid medication unless they had been treated for <3 weeks and there was clinicopathologic evidence that treatment did not result in euthyroidism. If the latter situation was applicable, oral antithyroid medication was discontinued at least 2 weeks before evaluation. Owners of cats in group 1 were invited to return their cats for re‐evaluation 3 months after administration of 4.5 mCi of radioiodine (131I). The study was approved by the Virginia Tech Animal Care and Use Committee. All owners provided informed consent.
Experimental Protocol
The results of complete history, physical examination, CBC, serum biochemistry panel, urinalysis, blood pressure measurement, serum T4 concentration, plasma NT‐proBNP concentration, plasma cTNI concentration, and echocardiography were evaluated for all cats; exceptions are described below. Orthogonal thoracic radiographs also were obtained from all cats in groups 1 and 2. Results of auscultation by a board‐certified cardiologist were used to determine the presence and intensity of a murmur.
Urine was collected by cystocentesis. Systemic systolic arterial blood pressure was estimated by the Doppler flow meter1 method with a manometric cuff; 5 determinations were recorded and averaged. Systemic hypertension was defined as systolic blood pressure >180 mmHg; cats were excluded from groups 2 and 3 if systemic blood pressure exceeded 180 mmHg. Ten to 12 mL of blood was obtained by jugular venipuncture (7 mL for cats <3 kg body weight) for CBC, biochemistry, T4, NT‐proBNP, and cTNI. Blood for NT‐proBNP was placed in EDTA tubes and immediately centrifuged for 10 min at 1,417 × g. Plasma was immediately harvested, placed in duplicate spray‐dried K2EDTA tubes, and stored at −80°C for up to 4 months before shipping to a reference laboratory for assay. Plasma NT‐proBNP was measured using a commercially available, previously validated horseradish peroxidase, colorimetric end‐point assay for quantitative determination of feline NT‐proBNP2 with a lower limit of detection of 23 pmol/L.15 Samples for cTNI were placed in lithium heparin tubes and immediately centrifuged for 10 min at 1,417 × g. Plasma was immediately harvested, stored in duplicate at −80°C for up to 4 months, and then shipped overnight to the New Bolton Center of the University of Pennsylvania Veterinary School for analysis on a previously validated16 fluorometric analyzer3 with an analytical sensitivity of 0.03 ng/mL. All laboratory measurements other than NT‐proBNP and cTNI were performed by the Virginia‐Maryland Regional College of Veterinary Medicine Veterinary Teaching Hospital laboratory using standard procedures.
Echocardiography was performed as described elsewhere17 and thoracic radiographs were reviewed for evidence of congestive heart failure (eg, pulmonary edema, pleural effusion) by 1 of 2 board‐certified cardiologists (JA or AL). Measurements were made at the time of echocardiography without review of results before 131I treatment. The cardiologist was unaware of results of biomarker testing in all cats. HCM was defined as end‐diastolic wall thickness ≥6 mm affecting >50% of any region of the interventricular septum or the left ventricular caudal wall in the absence of hyperthyroidism or hypertension.3
According to current guidelines from the reference laboratory for interpretation of NT‐proBNP results in asymptomatic cats,4 a concentration of <100 pmol/L indicates that clinically relevant heart disease is unlikely and ≥100 pmol/L indicates that heart disease is likely. Results were stratified into these 2 groups for interpretation.
Statistical Analysis
Statistical analysis was performed with commercial software.5 Normal probability plots demonstrated that age, weight, blood pressure, thyroid hormone concentration, heart rate, and echocardiographic variables followed a normal distribution whereas biomarker concentrations were skewed. Subsequently, one‐way ANOVA followed by Tukey‐Kramer's procedure for multiple comparisons was used to compare normally distributed variables between groups. Residual plots from each of the ANOVA models were inspected to verify that the errors were normally distributed with a constant variance. Fisher's exact test was used to compare groups with respect to frequency of male sex, murmurs and supraphysiologic biomarker concentrations. Differences in biomarker concentrations between groups were evaluated by a Kruskal–Wallis one‐way ANOVA followed by Dunn's test for multiple comparisons. Change in biomarker concentrations after treatment with radioiodine was evaluated with a Wilcoxon signed rank test. Correlations were assessed using Spearman rank correlation coefficients. Statistical significance was set to P < .05.
Samples with a T4 concentration <6.4 nmol/L were arbitrarily assigned a value of 6.3 nmol/L for data analysis; samples with a T4 of greater than 193 nmol/L were assigned a value of 194 nmol/L. Likewise, samples with an NT‐proBNP concentration less than 24 pmol/L were arbitrarily assigned a value of 23 pmol/L; the single sample with an NT‐proBNP concentration of greater than 1,500 pmol/L was assigned a value of 1,501 pmol/L.
Results
After a 15‐month recruitment period, 23 cats were recruited for group 1, 17 for group 2, and 19 for group 3. Caseload was insufficient to reach full study enrollment during the allotted time period. Group 1 included 11 castrated male and 12 spayed female cats. Twenty of these cats were domestic shorthairs, and there was 1 cat each of domestic longhair, Norwegian Forest Cat, and Himalayan. Group 2 included 12 castrated males, 4 spayed females, and 1 intact female cat. Ten of these cats were domestic shorthairs, 6 were domestic longhairs, and 1 was a Persian. Group 3 included 11 castrated male and 8 spayed female cats. Fifteen of these cats were domestic shorthairs, 2 were domestic longhairs, 1 was a Scottish Fold, and 1 was a Siamese. There were significantly more males in group 2 than in group 1 (P = .02), but the sex distribution of neither group differed significantly from the control group (P = .20 between groups 1 and 3; P = .07 between groups 2 and 3).
The mean ± SD age was 12.4 ± 2.2 years for group 1 (range, 9–17 years), 7.7 ± 4.6 years for group 2 (range, 1–15 years), and 10.3 ± 2.2 years for group 3 (range, 8–16 years). Group 2 was significantly younger than group 1 (P < .001) and group 3 (P = .028). There was no significant difference in age between groups 1 and 3 (P = .08). The mean ± SD body weight was 4.3 ± 1.1 kg for group 1 (range, 2.3–6.1 kg), 5.1 ± 1.5 kg for group 2 (range, 2.7–7.0 kg), and 4.7 ± 1.0 kg for group 3 (range, 2.5–6.9). Body weight was not significantly different between groups (P = .06 between groups 1 and 2; P = .52 between groups 1 and 3; P = .46 between groups 2 and 3).
The average systolic blood pressure of group 2 was lower than that of groups 1 or 3 (Table 1). Four of 22 of the group 1 cats for which blood pressure was recorded had average systolic pressures >180 mmHg. Three of these cats returned for 3‐month re‐evaluation; 2 of them were still hypertensive and the third had become normotensive. No cat developed hypertension after treatment with radioiodine.
Table 1.
Descriptive statistics of groups 1 (hyperthyroid), 2 (HCM), and 3 (control).
| T4 (nmol/L) | Blood Pressure (mmHg) | Heart Rate (bpm) | % of group with murmur | M‐mode IVSd (mm) | M‐mode LVPWd (mm) | %FS | M‐mode LA:Ao | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Group 1 (n = 23) | 107.6a | 53.2 | 157a | 22 | 224a | 30 | 61a | 5.3a | 0.8 | 6.0a | 1.6 | 60a | 10 | 1.4a | 0.3 |
| Group 2 (n = 17) | 25.7b | 6.9 | 125b | 26 | 175b | 21 | 94b | 6.1b | 0.8 | 6.4a | 1.4 | 60a | 7 | 1.3a | 0.2 |
| Group 3 (n = 19) | 26.1b | 5.5 | 144a | 21 | 195c | 29 | 21c | 4.4c | 0.5 | 4.6b | 0.6 | 60a | 7 | 1.3a | 0.2 |
SD, standard deviation; IVSd, interventricular septal thickness during diastole; LVPWd, left ventricular caudal wall thickness during diastole; LA:Ao, ratio of left atrial to aortic diameter.
Significant (P < .05) differences are denoted by different superscript letters within columns.
A murmur was present in 14/23 (61%) of group 1 cats, 16/17 (94%) of group 2 cats, and 4/19 (21%) of group 3 cats. Thyroid hormone concentrations and heart rate were significantly higher in group 1 than in groups 2 or 3 (Table 1).
The median NT‐proBNP and cTNI concentrations were significantly higher in groups 1 and 2 than in group 3 (Table 2; Figs 1, 2). When a receiver‐operating characteristic curve was evaluated, sensitivity and specificity of NT‐proBNP concentration was optimal at a cut‐point of 62 pmol/L for which sensitivity was 78% and specificity was 66%. Because the manufacturer of the NT‐proBNP assay states that plasma NT‐proBNP concentrations >100 pmol/L indicate “heart disease likely,” results also were evaluated using this cut‐point, which had a sensitivity of 56% and specificity of 81%. Ten of 23 (43%) group 1 cats, 10/18 (55%) group 2 cats, and 1/19 (5%) group 3 cats would have been classified as “heart disease likely” based on an NT‐proBNP concentration >100 pmol/L. Of the 13 group 1 cats classified as echocardiographically normal, 6 (46%) had cTNI concentrations above the reference range, and 5 (38%) had NT‐proBNP concentrations in the “heart disease likely” range (Table 2). Seven cats (4 and 3 in HCM and hyperthyroid groups, respectively) had IVSd, LVPWd, or both measurements >7.0 mm; of these 7 cats, 1 had an NT‐proBNP concentration <100 pmol/L (23 pmol/L).
Table 2.
Cardiac biomarker concentrations within group 1 (hyperthyroid), 2 (HCM), and 3 (control).
| Group | Median (range) NT‐proBNP | Median (range) cTNI | # of Cats with NT‐proBNP | # of Cats with cTNI | ||||
|---|---|---|---|---|---|---|---|---|
| <100 pmol/L | >100 pmol/L | ≤62 pmol/L | >62 pmol/L | ≤0.16 ng/mL | >0.16 ng/mL | |||
| 1 | 92 pmol/La (<24–718) | 0.17 ng/mLa (0.0–0.153) | 13 | 10 | 9 | 14 | 11 | 12 |
| 2 | 112 pmol/La (<24–>1,500) | 0.14 ng/mLa (0.0–0.59) | 7 | 10 | 3 | 14 | 11 | 6 |
| 3 | 60 pmol/Lb (<24–176) | 0.02 ng/mLb (0.0–0.23) | 18 | 1 | 15 | 4 | 18 | 1 |
Significant (P < .05) differences are denoted by different superscript letters within columns.
Figure 1.
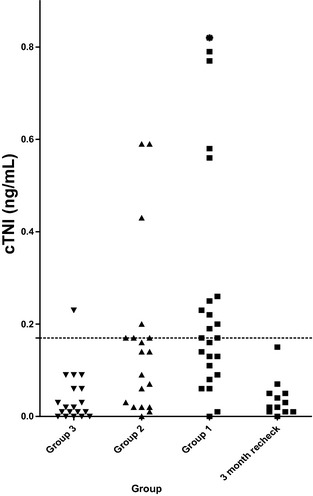
Cardiac troponin I (cTNI) concentrations in group 3 (control), group 2 (cardiac), group 1 (hyperthyroid), and a group of cats from group 1 sampled 3 months after treatment of hyperthyroidism with 131I. The asterisk represents a cat in group 1 with a cTNI of 1.53 ng/mL. The dotted line represents 0.16 ng/mL, the upper reference limit.
Figure 2.
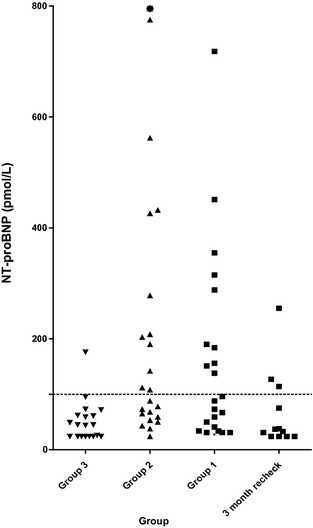
N‐terminal pro brain natriuretic peptide (NT‐proBNP) concentrations in group 3 (control), group 2 (HCM), group 1 (hyperthyroid), and a group of cats from group 1 sampled 3 months after treatment of hyperthyroidism with 131I. The asterisk represents a cat in group 2 with an NT‐proBNP of >1,500 pmol/L. The dotted line represents 100 pmol/L.
Plasma cTNI was above the upper reference limit (>0.16 ng/mL)14 in 12/23 (52%) group 1 cats, 6/17 (35%) group 2 cats, and 1/19 (5%) group 3 cats. There was no significant difference between proportions of cats with increased plasma cTNI in groups 1 and 2 (P = .35), but the percentage of group with increased plasma cTNI (P = .001 and P = .03, respectively) for each of group 1 and group 2 was significantly higher than the control group. Of the 7 cats with IVSd, LVPWd, or both >7.0 mm, 2 (29%) had cTNI concentrations <0.16 ng/mL. The highest cTNI and NT‐proBNP concentrations in group 3 were present in 2 different cats.
The re‐evaluation group comprised 6 castrated males and 8 spayed females. Twelve were domestic shorthairs, 1 was a domestic longhair, and 1 was a Norwegian Forest Cat. Myocardial thickness as well as percent fractional shortening (%FS) significantly decreased after 131I treatment (Table 3). Four of the 7 cats in the re‐evaluation group that had a murmur on initial examination had a murmur auscultated after treatment. One cat initially without a murmur developed one after treatment. Median plasma NT‐proBNP and cTNI concentrations significantly decreased 3 months after treatment with 131I. The cat with the highest NT‐proBNP at re‐evaluation was followed up for an additional 11 months. At that time, wall thickness remained abnormal (LVPWd, 8.5 mm) despite euthyroidism. Plasma cTNI concentration was within the normal range for all cats at the 3‐month re‐evaluation.
Table 3.
Changes to selected variables before and 3 months after treatment of hyperthyroidism with 131I.
| Before 131I | 3‐Month Re‐evaluation | P‐Value | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| T4 (nmol/L) | 112.7 | 49.3 | 23.2 | 11.7 | <.001 |
| M‐mode IVSd (mm) | 5.3 | 0.9 | 4.6 | 0.8 | .019 |
| M‐mode LVPWd (mm) | 6.0 | 1.6 | 5.1 | 1.2 | .001 |
| %FS | 56 | 9 | 50 | 9 | .006 |
| M‐mode LA:Ao | 1.4 | 0.2 | 1.3 | 0.2 | .51 |
| Median | Range | Median | Range | P‐Value | |
|---|---|---|---|---|---|
| NT‐proBNP (pmol/L) | 70 | 31–718 | 37.5 | <24–255 | .002 |
| cTNI (ng/mL) | 0.14 | 0.0–1.53 | 0.04 | 0.0–0.15 | .001 |
SD, standard deviation; IVSd, interventricular septal thickness during diastole; LVPWd, left ventricular caudal wall thickness during diastole; FS, fractional shortening; LA:Ao, ratio of left atrial to aortic diameter.
Neither biomarker was significantly correlated with end‐diastolic internal dimension (LVIDd) of the left ventricle, %FS, or systolic blood pressure (Table 4). Concentrations of both biomarkers were significantly but weakly correlated with IVSd and LVPWd (measured in both 2‐dimensional and M‐mode), left atrial diameter, LA/Ao, and intensity of murmur. When group 2 was excluded from the analysis, T4 was significantly positively correlated with both biomarkers, IVSd and LVPWd.
Table 4.
Correlation of cTNI and NT‐proBNP to selected variables.
| cTNI | NT‐proBNP | # of Observations cTNI/NT‐proBNP | T4 | |||||
|---|---|---|---|---|---|---|---|---|
| Spearman's correlation coefficient | P‐value | Spearman's correlation coefficient | P‐value | Spearman's correlation coefficient | P‐value | # of observations | ||
| M‐mode IVSd | 0.301 | .02 | 0.447 | <.001 | 59 | 0.507 | <.001 | 42 |
| M‐mode LVPWd | 0.471 | <.001 | 0.419 | <.001 | 59 | 0.500 | <.001 | 42 |
| M‐mode LA:Ao | 0.274 | .04 | 0.417 | .001 | 59 | 0.281 | .07 | 42 |
| %FS | −0.044 | .74 | −0.032 | .81 | 59 | −0.051 | .74 | 42 |
| HR | 0.253 | .05 | −0.293 | .02 | 59 | |||
| Intensity of murmur | 0.370 | .004 | 0.477 | <.001 | 58 | |||
| BP | 0.078 | .56 | −0.079 | .55 | 58 | |||
| T4 | 0.634 | <.001 | 0.461 | .002 | 42 | |||
HR refers to heart rate; BP refers to systolic blood pressure via the Doppler method. Group 2 (HCM) was excluded from the analysis for the correlation of T4 to each biomarker. IVSd, interventricular septal thickness during diastole; LVPWd, left ventricular caudal wall thickness during diastole; LA:Ao, ratio of left atrial to aortic diameter; FS, fractional shortening; HR, heart rate; BP, systolic blood pressure.
Two of the cats (14%) had low serum total T4 concentrations (<6.4 nmol/L) at their 3‐month re‐evaluation after 131I administration. Neither cat was azotemic or exhibited clinical signs of hypothyroidism, and cTNI and NT‐proBNP concentrations in both cats were normal. One cat still had high serum total T4 concentration at the 3‐month re‐evaluation, but its T4 had decreased from 149 to 43.6 nmol/L (reference range, 16.0–37.7 nmol/L), clinical signs of hyperthyroidism had resolved, cTNI had decreased from 1.53 to 0.08 ng/mL, and body weight had increased by 0.8 kg.
Blood pressure was not recorded in 3 cats. Blood pressure was inadvertently not recorded in 1 cat in group 1 and in 2 cats at the 3‐month recheck. One cat in group 2 did not have sufficient urine in its bladder to collect a urine sample. A CBC was not performed on 1 cat in group 2 because the sample clotted before analysis.
Discussion
The results of this study are consistent with others that show that cardiac biomarkers NT‐proBNP and cTNI are increased in cats with HCM and in cats with hyperthyroidism.11, 12 However, demonstration that neither of these biomarkers can be used to distinguish hyperthyroid cats from cats with those with primary cardiomyopathy is arguably the most important clinically relevant finding. Therefore, the thyroid status of cats, particularly older cats, should be ascertained before interpreting results of biomarker testing. In order to differentiate abnormalities resulting from hyperthyroidism from those caused by HCM, hyperthyroid cats with increased NT‐proBNP or cTNI or those with echocardiographic abnormalities should be re‐evaluated ≥3 months after resolution of hyperthyroidism.
To the authors' knowledge, previous descriptions of cTNI in cats with HCM have included cats currently or previously in congestive heart failure. We deliberately chose a study population without heart failure in order to more closely approximate the dilemma faced by veterinary practitioners when biomarker concentrations are determined during evaluation of hyperthyroid cats. Although the HCM population in this study had significantly higher cTNI concentrations than normal cats, our median plasma cTNI concentration of 0.14 ng/mL is lower than the median concentrations ranging from 0.66 to 1.59 ng/mL that others have reported.18, 19, 20, 21 In addition to including cats in congestive heart failure, two of the previous studies19, 21 used a different assay than that used in the current study, possibly accounting for some of the discrepancy.22 Cardiac troponin I concentrations in normal cats in the current study were consistent with those previously published.16 Because only 35% of our HCM cats had increased cTNI concentrations, this biomarker in isolation is not a sensitive indicator of cardiomyopathy. Results of this study support the findings of Connolly et al12 that cTNI concentrations are increased in hyperthyroid cats and decrease after treatment of hyperthyroidism.
Forty‐one percent of cats with HCM in this study had plasma NT‐proBNP concentrations in the “cardiac disease unlikely” range based on the manufacturer's guidelines for interpretation, and 18% of cats with HCM fell below the ROC‐optimized cut‐point of 62 pmol/L. Although concentrations in HCM cats were higher than those of normal cats, the low sensitivity of NT‐proBNP for detection of HCM in the current study contrasts with the findings of others13, 21 who concluded that NT‐proBNP is a sensitive and specific screening test for cats with HCM. Although one of these studies13 included cats in congestive heart failure (in which myocardial stretch would be expected to markedly increase NT‐proBNP concentrations), the other23 included only asymptomatic cats. The latter study included cats with restrictive, dilated, and unclassified forms of cardiomyopathy in contrast to the present study, which only included cats with HCM. The authors are not aware of comparisons of NT‐proBNP concentrations among cats with different forms of cardiomyopathy. Results of the current study are more consistent with those that have demonstrated increased NT‐proBNP primarily in cats with severe HCM.24, 25 However, because both of these studies only included Maine Coon cats with hereditary HCM, it is difficult to make direct comparisons. The population of HCM cats in the current study was relatively mildly affected, likely because of the fact that cats with current or previous congestive heart failure were excluded. This may have contributed to the low prevalence of increased cTNI and NT‐proBNP in the present study. In addition, the relatively small numbers of cats in each group, particularly those with more severe HCM, is a limitation of the study.
Inclusion of hypertensive cats is a limitation of the study because hypertension is associated with increased concentrations of both biomarkers in humans.26, 27 In hypertensive cats with concurrent chronic kidney disease, NT‐proBNP is markedly increased.28 However, because hypertension is often present in hyperthyroid cats, we judged it inappropriate to exclude clinical cases based on hypertension alone. When the data were analyzed with the hypertensive cats excluded, statistical conclusions remained the same (data not shown). In addition, in the present study, there was no correlation between blood pressure and either biomarker. Three of the 4 hypertensive hyperthyroid cats had LVPWd >6 mm, thus it is likely that their biomarkers were increased at least in part secondary to cardiac changes. None of the hypertensive cats had retinal lesions suggestive of end‐organ damage, indicating that persistent severe hypertension was unlikely in these cats. “White‐coat” hypertension is another possible explanation for the hypertension.
Two different board‐certified cardiologists performed the echocardiograms, a factor that may have introduced variability. However, serial examinations were performed by the same cardiologist in all but 2 cats, for which the images from the first study were re‐measured by the cardiologist who performed the 3 month re‐evaluation. Images were obtained in a standardized fashion. Because many of the cats had asymmetrical hypertrophy, our data may not reflect the thickest part of the myocardium for each cat; some cats may therefore have been underclassified with regard to the severity of their cardiomyopathy. Changes in echocardiographic parameters after treatment of hyperthyroidism previously have been reported.1, 2, 12 The results of this study support previous findings that thyrotoxic myocardial hypertrophy is largely reversible. Significant decreases were found in myocardial thickness and fractional shortening in cats examined 3 months after 131I treatment. Thyroxine stimulates BNP release from cultured rat atrial and ventricular myocytes,10 indicating a direct effect independent of myocardial stretch and other cardiovascular factors. Interestingly, in the current study, 62% of the hyperthyroid cats echocardiographically classified as normal had increased NT‐proBNP, cTNI, or both. Although no firm conclusion can be drawn, this finding may serve as further support of a direct effect of hyperthyroidism on biomarker concentrations. However, because T4, echocardiographic measurements, and plasma biomarker concentrations all improved after treatment with radioiodine in the present study, it is difficult to separate direct effects of T4 on plasma biomarker concentrations from changes in these biomarkers induced by improvements in cardiac structure and function.
The changes in M‐mode echocardiographic variables that were associated with administration of 131I were small in magnitude. Because the design of this study did not allow comparison to a control group, these changes may reflect day‐to‐day measurement variation, not changes in the variables of interest. Given that the reported SD for repeated M‐mode measurements of ventricular wall thickness from healthy cats is ~0.5 mm, these variables might vary as much as 1.3 mm independent of a treatment effect.29, 30 However, despite their small magnitude, changes in end‐diastolic measurement of ventricular wall thickness were, in general, directionally consistent and are biologically plausible.
The duration required for resolution of cardiac changes induced by hyperthyroidism after treatment with radioiodine is unknown. The myocardial thickness of the cat in the re‐evaluation group with the NT‐proBNP concentration that remained increased potentially could return to normal after a more prolonged period of euthyroidism. However, based on the responses of the other 13 cats and persistence of ventricular hypertrophy for almost 1 year after resolution of hyperthyroidism, it is likely that this particular cat had coexisting HCM in addition to resolving thyrotoxic cardiomyopathy. These results indicate a potential use for NT‐proBNP to monitor the cardiac response to treatment of hyperthyroidism. A NT‐proBNP that remains increased 3 months after resolution of hyperthyroidism may indicate underlying cardiomyopathy and an echocardiogram should be recommended in this subset of cats. Additional prospective evaluation of hyperthyroid cats with severe hypertrophy is needed to explore this recommendation.
In summary, the cardiac biomarkers NT‐pro BNP and cTNI are increased in hyperthyroid cats, but in the majority of cases returned to within the normal range 3 months after treatment with 131I. These biomarkers did not facilitate differentiation between cats with primary HCM and cats with thyrotoxic cardiomyopathy.
Acknowledgments
The authors thank the US Army Veterinary Corps for funding Dr Sangster's residency program, Dr Stephen Werre for statistical assistance, and Ms Dana Calicott, LVT, for assistance in data collection. This study was funded by the Virginia Veterinary Memorial Fund; NT‐proBNP assays were performed by the IDEXX Corporation free of charge.
Conflict of Interest: The authors disclose no conflict of interest.
These data were presented in part as an abstract at the 2013 ACVIM Forum, Seattle, WA.
Footnotes
Park's Doppler Flow Detector, Aloha, OR
Cardiopet proBNP, IDEXX Laboratories, Westbrook, ME
Stratus® CS stat fluorometric analyzer, Dade Behring Inc, Newark, DE
Service Update, January 2012, IDEXX Reference Laboratories
SAS Version 9.2, Cary, NC
References
- 1. Bond BR, Fox PR, Peterson ME, et al. Echocardiographic findings in 103 cats with hyperthyroidism. J Am Vet Med Assoc 1988;192:1546–1549. [PubMed] [Google Scholar]
- 2. Weichselbaum RC, Feeney DA, Jessen CR. Relationship between selected echocardiographic variables before and after radioiodine treatment in 91 hyperthyroid cats. Vet Radiol Ultrasound 2005;46:506–513. [DOI] [PubMed] [Google Scholar]
- 3. Paige CF, Abbott JA, Elvinger F, et al. Prevalence of cardiomyopathy in apparently healthy cats. J Am Vet Med Assoc 2009;234:1398–1403. [DOI] [PubMed] [Google Scholar]
- 4. Nakamura RK, Rishniw M, King MK, et al. Prevalence of echocardiographic evidence of cardiac disease in apparently healthy cats with murmurs. J Feline Med Surg 2011;13:266–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wagner T, Fuentes VL, Payne JR, et al. Comparison of auscultatory and echocardiographic findings in healthy adult cats. J Vet Cardiol 2010;12:171–182. [DOI] [PubMed] [Google Scholar]
- 6. Trehiou‐Sechi E, Tissier R, Gouni V, et al. Comparative echocardiographic and clinical features of hypertrophic cardiomyopathy in 5 breeds of cats: A retrospective analysis of 344 cases (2001‐2011). J Vet Intern Med 2012;26:532–541. [DOI] [PubMed] [Google Scholar]
- 7. Schultz M, Faber J, Kistorp C, et al. N‐terminal‐pro‐B‐type natriuretic peptide (NT‐pro‐BNP) in different thyroid function states. Clin Endocrinol (Oxf) 2004;60:54–59. [DOI] [PubMed] [Google Scholar]
- 8. Ozmen B, Ozmen D, Parildar Z, et al. Serum N‐terminal‐pro‐B‐type natriuretic peptide (NT‐pro‐BNP) levels in hyperthyroidism and hypothyroidism. Endocr Res 2007;32:1–8. [DOI] [PubMed] [Google Scholar]
- 9. Ertugrul DT, Gursoy A, Sahin M, et al. Evaluation of brain natriuretic peptide levels in hyperthyroidism and hypothyroidism. J Natl Med Assoc 2008;100:401–405. [DOI] [PubMed] [Google Scholar]
- 10. Kohno M, Horio T, Yasunari K, et al. Stimulation of brain natriuretic peptide release from the heart by thyroid hormone. Metabolism 1993;42:1059–1064. [DOI] [PubMed] [Google Scholar]
- 11. Menaut P, Connolly DJ, Volk A, et al. Circulating natriuretic peptide concentrations in hyperthyroid cats. J Small Anim Pract 2012;53:673–678. [DOI] [PubMed] [Google Scholar]
- 12. Connolly DJ, Guitian J, Boswood A, et al. Serum troponin I levels in hyperthyroid cats before and after treatment with radioactive iodine. J Feline Med Surg 2005;7:289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wess G, Daisenberger P, Mahling M, et al. Utility of measuring plasma N‐terminal pro‐brain natriuretic peptide in detecting hypertrophic cardiomyopathy and differentiating grades of severity in cats. Vet Clin Pathol 2011;40:237–244. [DOI] [PubMed] [Google Scholar]
- 14. Tominaga Y, Miyagawa Y, Toda N, et al. The diagnostic significance of plasma N‐terminal pro‐B‐type natriuretic peptide concentration in asymptomatic cats with cardiac enlargement. J Vet Med Sci 2011;73:971–975. [DOI] [PubMed] [Google Scholar]
- 15. Fox PR, Oyama MA, Reynolds C, et al. Utility of plasma N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) to distinguish between congestive heart failure and non‐cardiac causes of acute dyspnea in cats. J Vet Cardiol 2009;11(Suppl. 1):S51–S61. [DOI] [PubMed] [Google Scholar]
- 16. Sleeper MM, Clifford CA, Laster LL. Cardiac troponin I in the normal dog and cat. J Vet Intern Med 2001;15:501–503. [DOI] [PubMed] [Google Scholar]
- 17. Thomas WP, Gaber CE, Jacobs GJ, et al. Recommendations for standards in transthoracic two‐dimensional echocardiography in the dog and cat. J Vet Intern Med 1993;7:247–252. [DOI] [PubMed] [Google Scholar]
- 18. Herndon WE, Kittleson MD, Sanderson K, et al. Cardiac troponin I in feline hypertrophic cardiomyopathy. J Vet Intern Med 2002;16:558–564. [DOI] [PubMed] [Google Scholar]
- 19. Connolly DJ, Cannata J, Boswood A, et al. Cardiac troponin I in cats with hypertrophic cardiomyopathy. J Feline Med Surg 2003;5:209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Herndon WE, Rishniw M, Schrope D, et al. Assessment of plasma cardiac troponin I concentration as a means to differentiate cardiac and noncardiac causes of dyspnea in cats. J Am Vet Med Assoc 2008;233:1261–1264. [DOI] [PubMed] [Google Scholar]
- 21. Connolly DJ, Brodbelt DC, Copeland H, et al. Assessment of the diagnostic accuracy of circulating cardiac troponin I concentration to distinguish between cats with cardiac and non‐cardiac causes of respiratory distress. J Vet Cardiol 2009;11:71–78. [DOI] [PubMed] [Google Scholar]
- 22. Adin DB, Oyama MA, Sleeper MM, et al. Comparison of canine cardiac troponin I concentrations as determined by 3 analyzers. J Vet Intern Med 2006;20:1136–1142. [DOI] [PubMed] [Google Scholar]
- 23. Fox PR, Rush JE, Reynolds CA, et al. Multicenter evaluation of plasma N‐terminal probrain natriuretic peptide (NT‐pro BNP) as a biochemical screening test for asymptomatic (occult) cardiomyopathy in cats. J Vet Intern Med 2011;25:1010–1016. [DOI] [PubMed] [Google Scholar]
- 24. Hsu A, Kittleson MD, Paling A. Investigation into the use of plasma NT‐proBNP concentration to screen for feline hypertrophic cardiomyopathy. J Vet Cardiol 2009;11(Suppl. 1):S63–S70. [DOI] [PubMed] [Google Scholar]
- 25. Singh MK, Cocchiaro MF, Kittleson MD. NT‐proBNP measurement fails to reliably identify subclinical hypertrophic cardiomyopathy in Maine Coon cats. J Feline Med Surg 2010;12:942–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nunes JP. Cardiac troponin I in systemic diseases. A possible role for myocardial strain. Rev Port Cardiol 2001;20:785–788. [PubMed] [Google Scholar]
- 27. Pahle AS, Sorli D, Omland T, et al. Impact of systemic hypertension on the diagnostic performance of B‐type natriuretic peptide in patients with acute dyspnea. Am J Cardiol 2009;104:966–971. [DOI] [PubMed] [Google Scholar]
- 28. Lalor SM, Connolly DJ, Elliott J, et al. Plasma concentrations of natriuretic peptides in normal cats and normotensive and hypertensive cats with chronic kidney disease. J Vet Cardiol 2009;11(Suppl. 1):S71–S79. [DOI] [PubMed] [Google Scholar]
- 29. Bland JM, Altman DG. Measurement error. BMJ 1996;313:744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chetboul V, Concordet D, Pouchelon JL, et al. Effects of inter‐ and intra‐observer variability on echocardiographic measurements in awake cats. J Vet Med A 2003;50:326–331. [DOI] [PubMed] [Google Scholar]


