Abstract
Background
Peripheral blood hematopoietic cell transplantation (PBHCT) is a feasible treatment option for dogs with B‐cell lymphoma.
Objective
To examine apheresis and PBHCT outcomes in dogs diagnosed with T‐cell lymphoma (TCL).
Animals
Fifteen client‐owned dogs diagnosed with high‐grade TCL.
Methods
After high‐dose cyclophosphamide and rhG‐colony‐stimulating (rhG‐CSF) factor treatment, peripheral blood mononuclear cells were collected using cell separators. The harvested cells then were infused after varying doses of total body irradiation (TBI). Postirradiation adverse effects were managed symptomatically and dogs were discharged upon evidence of hematopoietic engraftment.
Results
More than 2 × 106 CD34+ cells/kg were harvested from 15/15 dogs. Thirteen of 15 (87%) dogs engrafted appropriately, whereas 2 (13%) of the dogs died in the hospital. One dog developed cutaneous B‐cell lymphoma 120 days post‐PBHCT. The median disease‐free interval and overall survival (OS) of the 13 dogs transplanted in first remission from the time of PBHCT were 184 and 240 days, respectively. Stage and substage of disease at diagnosis had no effect on OS. Two of 13 (15%) dogs were alive 741 and 772 days post‐PBHCT.
Conclusions and Clinical Importance
PBHCT may be considered as a treatment option for dogs with TCL.
Keywords: Apheresis, Bone marrow transplantation, CD34+, Engraftment, Total body irradiation
Abbreviations
- BCL
B‐cell lymphoma
- CBC
complete blood count
- CHOP
cyclophosphamide, doxorubicin, vincristine, prednisone
- DFI
disease‐free interval
- G‐CSF
granuloctye colony‐stimulating factor
- HCT
hematocrit
- HDC
high‐dose cyclophosphamide
- LRS
lactated Ringer's solution
- LSA
lymphoma
- PARR
polymerase chain reaction for antigen receptor rearrangements
- PBHCT
peripheral blood hematopoietic cell transplantation
- PBMC
peripheral blood mononuclear cells
- SHC
sterile hemorrhagic cystitis
- TBI
total body irradiation
- TCL
T‐cell lymphoma
- WBC
white blood cells
T‐cell lymphoma (TCL) accounts for approximately 10‐38% of canine non‐Hodgkin's lymphoma (LSA).1, 2 The disease is considered to have a more aggressive clinical course and is associated with a poorer prognosis (6–9 months median survival) when compared to B‐cell lymphoma (BCL; 12–14 months median survival).3
Treatment of all LSA phenotypes historically has involved the administration of multiagent CHOP‐based (cyclophosphamide, doxorubicin, vincristine, prednisone) chemotherapy protocols of varying length.4, 5 In addition, the use of cranial and caudal half‐body irradiation in conjunction with chemotherapy has been described for the treatment of both B‐ and T‐cell LSA.6, 7 In a recent study, 31% of veterinarians treated dogs with TCL using alternative front‐line protocols by including lomustine, procarbazine, and mechlorethamine while discontinuing the use of doxorubicin and cyclophosphamide.8 However, despite these different treatment regimens, complete remission rates and overall survival have remained relatively unchanged over the last decade.9
Autologous peripheral blood hematopoietic cell transplantation (PBHCT) after high‐dose cyclophosphamide and lethal total body irradiation (TBI) for the treatment of canine B‐cell LSA (BCL) has been reported.10 The median overall survival time for dogs transplanted before they relapsed was 531 days, with 33% of the dogs still alive >2 years after PBHCT. In addition, a TerumoBCT (formally CaridianBCT)1 cell separator was able to collect a higher number of both total CD34+ cells and CD34+ cells/kg when compared to a Baxter‐Fenwal CS‐3000 Plus 2 cell separator. Therefore, we reasoned that apheresis using a TerumoBCT cell separator and autologous PBHCT represent a feasible treatment alternative for dogs with TCL.
In this study, we report the apheresis product analysis, toxicities, and treatment outcome of dogs diagnosed with TCL treated with an aggressive myeloablative protocol including high‐dose cyclophosphamide (HDC), mononuclear cell apheresis, and total body irradiation (TBI) followed by autologous PBHCT.
Materials and Methods
Study Design
Between November 2008 and March 2012, 15 dogs definitively diagnosed with high‐grade TCL were enrolled. Dogs in a first and second clinical remission (CR), and 1 dog not in CR, were included. All clients signed a written informed consent form approved by the North Carolina State University (NCSU) Veterinary Health Complex Hospital Board.
Donor Preparation for PBMC Apheresis
Donor preparation, including administration of HDC3 and rhG‐CSF,4 was carried out as previously described.10 All dogs received IV HDC and mesna5 at 500 (5 dogs), 600 (1 dog), 650 (7 dogs), 700 (1 dog), or 750 (1 dog) mg/m2. In some dogs, glutamine6 (30 mg/kg PO q12h) also was administered.
PBMC Apheresis
PBMC aphereses were performed as previously described,10 using either a Baxter‐Fenwal CS‐3000 Plus (1 dog) or TerumoBCT (formally CaridianBCT) (14 dogs) cell separator machine. In 4 dogs weighing <15 kg, the TerumoBCT cell separator was primed to ensure hemodynamic stability during the initial period of apheresis. Three of these dogs have been previously described.11 The priming solution used in the remaining dog (6.7 kg) was 100 mL autologous whole blood and 100 mL 0.9% NaCl. In addition, the rinse back products (purged machine contents after completion of apheresis) in these cases were collected into an empty bag rather than being returned to the dogs. This blood (approximately 400 mL) was subsequently infused into the dogs at approximately 50 mL/h beginning immediately after the aphereses were completed.
Apheresis Product Analysis
Midprocedure and final harvest products were evaluated as previously described.10 Polymerase chain reaction for antigen receptor rearrangements (PARR)12 analysis was used to detect tumor cell contamination in the 14 dogs with PARR‐positive tumors at diagnosis. When the apheresis product contained >1 × 107 CD34+ cells/kg, one‐half of the product was centrifuged to remove the plasma, and the cells were diluted 1:1 in freezing media containing 20% autologous plasma, 20% Normosol, and 10% dimethyl sulfoxide,7 placed in a freezing press,8 and frozen to −80°C in a controlled rate freezing chamber.13 The remaining apheresis product was refrigerated at 4°C until transplantation.
Mononuclear Cell Product Yield and Collection Efficiency Calculations
Mononuclear cell product yield, CD34+ cells/kg, and mononuclear cells collection efficiencies (MCCE) were calculated as previously described.10
Total Body Irradiation
The day after apheresis, 14 dogs received TBI at a dose rate of 7 cGy/min administered with 6 MV photons from a Varian Clinac 1800,9 as previously described.10 In these dogs, TBI consisting of either two 5 Gy fractions at least 3 hours apart (10 Gy, 3 dogs), two 4 Gy fractions at least 3 hours apart on day 1, and a single 3 Gy fraction on day 2 (11 Gy, 9 dogs), or two 3 Gy fractions at least 3 hours apart on 2 consecutive days (12 Gy, 1 dog). In 1 dog, two 4 Gy fractions at least 3 hours apart were given on day 1 and the machine faulted on day 2 after the dog received approximately 38% of a final 3 Gy fraction (target total dose of 11 Gy). Therefore, because of the 1‐day delay, another 4 Gy dose was administered the next day after the machine was repaired, for a total of 12 Gy. One dog received TBI at a dose rate of 10 cGy/min administered with 6 MV photons from a Varian Clinac 21EX10 with the dose prescribed to the 100% isodose line as previously described,10 although a dose miscalculation resulted in this dog receiving only 8 Gy (of a prescribed 11 Gy dose) over 3 days. This dog received another 6 Gy (two 3 Gy fractions at least 3 hours apart) approximately 93 days after the initial PBHCT. TBI was considered day 0. Immediately after TBI, the apheresis product was warmed to room temperature and administered IV over a period of 1 hour.
Post‐PBHCT Care
Post‐PBHCT care was essentially as previously described.10 Toxicities are described using the Veterinary Co‐operative Oncology Group v1.1 common terminology criteria for adverse events.14 In the event of relapse after PBHCT, the dogs received a variety of chemotherapy protocols at the discretion of the local veterinarian.
Statistical Analysis
All apheresis data were normally distributed as determined by a D'Agostino and Pearson omnibus K2 normality test and Kolmogorov–Smirnov normality test. A Welch's unpaired t‐test was used to calculate significant differences between the dogs in this study (Group 1) and 16 dogs from a previous study diagnosed with BCL that underwent apheresis using a TerumoBCT cell separator and PBHCT (Group 2).10 The relationship between preapheresis parameters, collected CD34+ cells, and MCCE in both groups was estimated by linear regression and correlation analysis. One dog that underwent apheresis using a Baxter‐Fenwal CS‐3000 Plus cell separator was not included in the analyses for Tables 2 and 3. Disease‐free interval (DFI), defined as the time from PBHCT until the time of relapse, and overall survival (OS), defined as the time from PBHCT until death, were calculated using the Kaplan–Meier method. For comparison to historical data, OS also was calculated from the time of diagnosis until death. DFI and OS were compared between the groups using the log rank test. Two dogs that died from treatment‐related causes were censored from the DFI analysis but included in the overall survival analysis.10 Because only 2 dogs had not maintained their first remission before PBHCT, remission status before PBHCT was not considered in this analysis. All statistics were calculated using GraphPad.10 A P‐value ≤.05 was considered significant.
Results
Patient Characteristics
Fifteen dogs, representing 12 breeds, met the inclusion criteria (Table 1). T‐cell phenotype was confirmed using immunohistochemistry (3 dogs), flow cytometry (1 dog), or PARR (11 dogs), and the preponderance of large, immature lymphoblasts with a moderate to high mitotic rate was used to classify the malignancies as high‐grade. Four of these dogs were hypercalcemic at diagnosis, and 3 dogs had a mediastinal mass (2 dogs had both a mediastinal mass and hypercalcemia). Eight dogs had stage 5 disease, 3 dogs had stage 4 disease, and 4 dogs had stage 3 disease. Nine dogs were substage a, whereas 6 dogs were substage b (5/6 substage b dogs had stage 5 disease). One dog was in second remission before PBHCT, whereas 1 dog was not in CR upon arrival at NCSU.
Table 1.
T‐cell lymphoma case details before PBHCT.
| Signalment | |
| Number of dogs | 15 |
| Breeds | 12 (2 Box, 2 GR, 2 LR) |
| Age (months) | Mean, 63.6 (range, 36–108) |
| Spay/neuter | 9NM, 1IM, 4SF, 1IF |
| Weight (kg) | Mean, 28.4 (range, 6.7–54.4) |
| T‐cell Lymphoma (15) | 14 LN FNA/biopsy, 1 liver/spleen FNA |
| Diagnosis | |
| Stage III | 4 |
| Stage IV | 3 |
| Stage V | 8 |
| Substage a | 9 |
| Substage b | 6 |
| Relapse before PBHCT | 1 |
| Remission before PBHCT | 14 |
| Days from dx to PBHCT | Mean 121 (range, 61–217) |
| PARR + tumors | 14 |
Box, Boxer; GR, Golden Retriever; LR, Labrador Retriever; NM, neutered male; IM, intact male; SF, spayed female; IF, intact female; LN FNA, lymph node fine needle aspirate; PBHCT, peripheral blood hematopoietic cell transplantation; PARR, PCR for antigen receptor rearrangements.
Toxicity
Acute HDC Toxicity
Seven of 15 (47%) dogs (1 at 500 mg/m2, 1 at 600 mg/m2, 3 at 650 mg/m2, 1 at 700 mg/m2, and 1 at 750 mg/m2) were reported to have experienced an adverse event. One dog experienced grade‐2 lethargy and inappetence only, whereas 6 dogs developed grade‐4 neutropenia. Of the 6 neutropenic dogs, 3 also developed grade‐3 thrombocytopenia, 3 became febrile, and 3 exhibited grade‐2 gastrointestinal (GI) signs. Four of these dogs were hospitalized and given supportive care, including fluid administration, antibiotics, and antiemetics. Within 24 hours of hospitalization, all 4 dogs were discharged and cytopenias resolved within 7 days. Only 1 dog (650 mg/m2 HDC) exhibited clinical signs consistent with sterile hemorrhagic cystitis (SHC) and this dog eventually required surgery to repair a ruptured urinary bladder, presumably secondary to severe SHC (moderate to marked transmural necrotizing cystisis). Regardless of the severity of HDC toxicities, all dogs recovered and were able to proceed with rhG‐CSF treatment and PBMC apheresis.
Acute TBI Hematologic Toxicity
All dogs developed grade‐4 neutropenia and thrombocytopenia as previously described.10 Grade‐4 neutropenia (<500/μL) lasted from 3 to 11 days (median, 6 days) whereas grade‐4 thrombocytopenia (<25 × 103/μL) persisted for 0–21 days (median, 8 days), with 8 (53%) dogs having grade‐4 thrombocytopenia at discharge. No statistically significant difference in either duration or grade of neutropenia and thrombocytopenia was observed between dogs that received 10 Gy or 11 Gy of irradiation (P = .583). Nine of 15 (60%) dogs experienced grade‐2 petechiation, ecchymosis, or both, 1 (7%) dog developed hematuria, and 1 (7%) dog developed epistaxis. Fifteen (100%) dogs received irradiated15 (20 Gy) blood products which included DEA 1.1‐negative fresh whole blood, donor‐matched fresh whole blood, frozen platelet concentrate, donor‐matched packed red blood cells, and fresh platelet concentrate. There was no statistically significant difference in the median duration of hospitalization for dogs receiving 10 Gy of TBI (median, 18 days; range, 14–23 days) when compared to dogs receiving 11 Gy of TBI (median, 22.5 days; range, 17–34 days; P = .1874).
Acute TBI Gastrointestinal Toxicity
Fourteen of 15 (93%) dogs experienced GI adverse events, including vomiting, diarrhea, inappetence, or some combination of these. Six (40%) dogs vomited during their hospitalization (3 grade‐2, 3 grade‐3). Eleven (73%) dogs also developed diarrhea (3 grade‐1, 4 grade‐2, 4 grade‐3). Inappetence developed in 10 (67%) dogs for a variable amount of time (3 grade‐1, 2 grade‐2, 3 grade‐3, 2 grade‐4). Three (20%) dogs also received nutritional support including partial parental nutrition (1), total parental nutrition (1), or a combination of partial parental nutrition and amino acid supplementation (1).
Chronic TBI Toxicity
One (7%) dog developed multiple SC nodules 120 days post‐PBHCT that were diagnosed as cutaneous B‐cell LSA by flow cytometry. This dog continued to develop additional SC nodules despite receiving a variety of chemotherapeutic agents and local irradiation, and was euthanized because of progressive B‐cell LSA 605 days post‐PBHCT.
TBI‐Related Mortality
Two (13%) dogs died shortly after PBHCT. One dog that received 11 Gy TBI (two 4 Gy fractions at least 3 hours apart on day 1, and a single 3 Gy fraction on day 2) died of clinical signs consistent with sepsis 13 days post‐PBHCT. One dog, that was not in remission upon arrival at NSCU, received doxorubicin11 (40 mg/m2 IV) and melphalan12 (30 mg/m2 PO) followed by rhG‐CSF (5 μg/kg SC) and plerixafor13 (240 μg/kg SC) 1 week later. After apheresis and 12 Gy TBI (two 3 Gy fractions at least 3 hours apart on 2 consecutive days), the dog died of clinical signs consistent with severe GI toxicity 4 days post‐PBHCT.
Apheresis
Effects of rhG‐CSF Treatment
As reported previously,10 no signs of adverse effects were seen in any dogs receiving rhG‐CSF before apheresis. In all dogs, preapheresis WBC (mean, 45,340 ± 15,963 cells/μL) and mononuclear cell counts (mean, 3,181 ± 1,872 cells/μL) were much higher than our hospital's normal reference range (WBC reference interval, 4,390–11,610 cells/μL; mean, 8,000 cells/μL; monocyte reference interval, 75–850 cells/μL; mean, 462 cells/μL; Table 2). There was no statistically significant difference when comparing these dogs to a previous report of 16 dogs with BCL harvested using a TerumoBCT cell separator10 with respect to preapheresis WBC count (P = .1761) and monocyte count (P = .0623). All rhG‐CSF‐treated dogs underwent apheresis.
Table 2.
Apheresis characteristics of dogs with T‐ and B‐cell lymphoma collected with a CaridianBCT cell separator.
| Weight (kg) | Coll Length (m) | TBV | ACD‐A (mL) | Pre Aph WBC/μL | PreAph Mono/μL | Pre Aph Hct | Post Aph WBC/μL | Post Aph Mono/μL | Post Aph Hct | |
|---|---|---|---|---|---|---|---|---|---|---|
| TCLa (n = 14) | ||||||||||
| Range | 6.7–54.4 | 181–395 | 2.6–4.8 | 322–1,115 | 20,210–75,550 | 606–6,800 | 29.7–51.5 | 21,200–77,020 | 432–3,569 | 24.5–50 |
| Mean ± SD | 28.5 ± 13.0 | 308 ± 56 | 3.3 ± 0.7 | 752 ± 306 | 45,340 ± 15,963 | 3,181 ± 1,872 | 40.2 ± 5.7 | 46,365 ± 16,698 | 1,655 ± 853 | 37.3 ± 8.3 |
| BCL10 (n = 16) | ||||||||||
| Range | 11.3–55.6 | 240–420 | 2.3–4.2 | 299–1,625 | 36,600–85,060 | 1,935–10,618 | 31.5–48.2 | 31,820–89,180 | 1,273–8,000 | 17.9–55.3 |
| Mean ± SD | 33.0 ± 8.6 | 323 ± 59 | 3.2 ± 0.6 | 1,071 ± 351 | 54,951 ± 16,723 | 4,708 ± 2,453 | 41.2 ± 5.0 | 59,140 ± 16,531 | 3,245 ± 1,775 | 40.7 ± 10.3 |
| TCL versus BCL aphereses | ||||||||||
| P‐value | .2721 | .7365 | .7563 | .0262 | .1761 | .0623 | .7491 | .0442 | .1994 | .1954 |
TCL, T‐cell lymphoma; BCL, B‐cell lymphoma; TBV, total blood volume; ACD‐A, acid‐dextrose formula; Pre Aph, preapheresis; Post Aph, postapheresis; Mono, mononuclear cells; WBC, white blood cells; Hct, hematocrit.
Does not include 1 dog who underwent apheresis using a Baxter‐Fenwal CS‐3000 Plus cell separator.
Apheresis Procedure
All 15 dogs completed the apheresis. There was no statistically significant difference when comparing these dogs to a previous report of 16 dogs with BCL harvested using a TerumoBCT cell separator10 with respect to collection duration (P = .7365), total blood volume processed (P = .7563), postapheresis monocyte count (P = .0636), and Hct (P = .1954; Table 2). There was a statistically significant difference when comparing postapheresis WBC counts (P = .0442) and the amount of ACD‐A infused during the procedure (P = .0262).
Effects of Apheresis
Fourteen of 15 dogs tolerated the procedure. One dog developed clinically relevant bleeding from the jugular catheter site during the last 15 minutes of the procedure. The dog's platelet count (preapheresis, 177 × 103 cells/μL; postapheresis, 30 × 103 cells/μL) and hematocrit (preapheresis, 45%; postapheresis 20%) decreased substantially during the procedure. A coagulation panel immediately postapheresis was abnormal (PT, no clot after 40 s; PTT, no clot formation after 120 s; d‐dimer, >2,000 ng/mL). A coagulation panel the next day was normal (PT, 8.1 s; PTT 13 s, d‐dimer <250 ng/mL) except for grade‐4 thrombocytopenia (26 × 103 cells/μL), although all signs of bleeding slowly resolved during the day without medical management. One dog experienced mild generalized urticaria immediately after the procedure that responded appropriately to diphenhydramine administration. For the 4 dogs weighing <15 kg, machine priming with a variety of fluids was sufficient to maintain cardiovascular stability and the aphereses were completed uneventfully.
Apheresis Cell Yield
Table 3 compares the apheresis products of these dogs with a previous report of 16 dogs with BCL harvested using a TerumoBCT cell separator.10 There were no statistically significant differences when comparing the mean WBC (P = .6836), red blood cells (P = .4089), or mononuclear cells (P = .8369) harvested. In addition, we found no statistically significant difference in the number of CD34+ cells/kg harvested (P = .3690) and the cell separator MCCE (P = .7643) between the 2 groups. There was a statistically significant difference when comparing the mean number of platelets (P = .0422) harvested. PARR analysis was negative in 13/14 harvests for which the original tumor was PARR‐positive. The one dog with a PARR‐positive harvest also had PARR‐positive blood at presentation to NCSU. Two other dogs with PARR‐positive blood at presentation to NCSU had PARR‐negative harvests.
Table 3.
Apheresis product counts of dogs with T‐ and B‐cell lymphoma using a CaridianBCT cell separator.
| WBCs (Cells/μL) | Plts (Cells/μL) | Grans (%) | Hct (%) | Mono (Cells/μL) | CD34+ (%) | CD34+ (Cells/kg) | MCCE (%) | |
|---|---|---|---|---|---|---|---|---|
| TCLa (n = 14) | ||||||||
| Range | 13,770–357,300 | 92 x 103–1,373 x 103 | 0–45 | 2.1–4.5 | 10,643–110,763 | 1.3–6.7 | 2 x 106–42 x 106 | 3.6–68.8 |
| Mean ± SD | 92,217 ± 87,051 | 725 x 103 ± 403 x 103 | 18.0 ± 16.2 | 5.7 ± 0.7 | 43,846 ± 34,105 | 2.4 ± 1.4 | 1.4 x 107 ± 1.2 x 107 | 36.2 ± 18 |
| BCL10 (n = 16) | ||||||||
| Range | 18,310–313,560 | 85 x 103–2,833 x 103 | 3.0–4.7 | 1.4–6.2 | 4,944–131,695 | 0.2–4.9 | 3.5 x 106–54.5 x 106 | 8.6–76 |
| Mean ± SD | 105,646 ± 91,341 | 1,195 x 103 ± 750 x 103 | 14.8 ± 13.2 | 3.3 ± 1.2 | 59,639 ± 42,036 | 2.4 ± 1.4 | 1.8 x 107 ± 1.4 x 107 | 37.6 ± 19.3 |
| TCL versus BCL aphereses | ||||||||
| P‐value | .6836 | .0422 | .6243 | .4089 | .8369 | .9878 | .3690 | .7643 |
TCL, T‐cell lymphoma; BCL, B‐cell lymphoma; WBC, white blood cells; Plts, platelets; Grans, granulocytes; Hct, hematocrit; Mono, mononuclear cells; MCCE, mononuclear cell collection efficiency.
Does not include 1 dog who underwent apheresis using a Baxter‐Fenwal CS‐3000 Plus cell separator.
Factors Affecting Apheresis Yield
We examined the effect of preapheresis Hct on the machine MCCE because we reported previously that preapheresis Hct was moderately correlated (R 2 = 0.3371, P = .0184) with increased MCCE in dogs with BCL.10 In the TCL dogs, this correlation was not evident (R 2 = 0.1280, P = .2091; Fig 1A). When combining these data from both groups of dogs (n = 30), however, preapheresis Hct was correlated (R 2 = 0.2021, P = .0127) with machine MCCE (Fig 1B). Concordant with previous results,10 preapheresis leukocyte counts did not affect the machine MCCE (R 2 = 0.01627, P = .6639) or the number of CD34+ cells/kg (R 2 = 0.05644, P = .4135; data not shown). Finally, concordant with previous results,10 preapheresis monocyte count was not significantly correlated with the number of CD34+ cells/kg harvested (R 2 = 0.00385, P = .9516; Fig 1C), although when combining the 2 groups of dogs there was a significant correlation (R 2 = 0.2119, P = .0105; Fig 1D).
Figure 1.
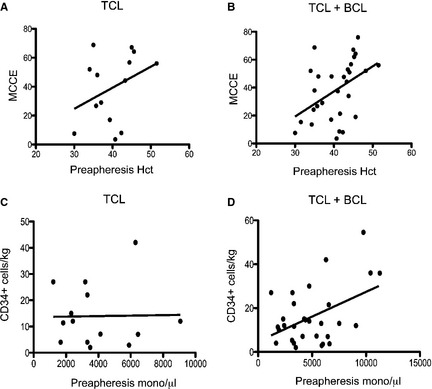
Correlation between preapheresis blood cell parameters and harvest outcomes. (A) Linear regression analysis did not show a correlation in TCL dogs between preapheresis Hct and MCCE (R 2 = 0.1280, P = .2091) or (C) preapheresis mononuclear cell counts and the number of CD34+ cells/kg harvested (R 2 = 0.00385, P = .9516). (B) Linear regression analysis did show a correlation between preapheresis Hct and MCCE (R 2 = 0.2021, P = .0127) and (D) preapheresis mononuclear cell counts and the number of CD34+ cells/kg harvested (R 2 = 0.2119, P = .0105) when TCL and BCL 10 cases were combined.
PBHCT Outcome
Platelet Recovery
We examined the effects of the number of transplanted CD34+ cells/kg on platelet recovery at day +14 after PBHCT. Contrary to previous findings,10 there was no statistically significant difference (P = .1983) in platelet counts at discharge in dogs receiving <6 × 106 CD34+ cells/kg (mean, 43,000 ± 57,000/μL) compared with dogs receiving ≥6 × 106 CD34+ cells/kg (mean, 119,000 ± 123,000/μL), although only 3/4 dogs that received <6 × 106 CD34+ cells/kg were included in this analysis (1 dog died in the hospital). When we combined these data with the previously reported BCL dogs, the difference in day +14 platelet counts became significant (P = .0234). There was no statistically significant difference in day +14 platelet counts when examining TCL dogs that received <1 × 107 CD34+ cells/kg versus >1 × 107 cells/kg alone or when combining these data with the previously reported BCL dogs.
Remission Duration
The median DFI for the 13 dogs that did not relapse before PBHCT and were in CR at the time of PBHCT from the time of PBHCT to relapse was 184 days (range, 28–738 days; Fig 2A). Three (23%) of these 13 dogs relapsed within 4 months of PBHCT, 3 (23%) dogs relapsed between 4 and 8 months after PBHCT, 5 (38%) dogs relapsed after 8 months of PBHCT (at 8.3, 10, 12.9, 18.3, and 24.6 months), and 1 (8%) dog had not relapsed after 24.7 months. There was no statistically significant difference (P = .7665) in DFI between dogs that received 10 or 11 Gy TBI. When we compared the median DFI between the dogs with TCL in this study and the previously reported BCL dogs,10 there was no statistically significant difference (P = .0704) between the 2 groups (184 days versus 511 days, respectively; Fig 2B). One dog that had relapsed and was in clinical remission before PBHCT remained disease‐free for 78 days, whereas 1 dog not in clinical remission on presentation at NCSU died in the hospital.
Figure 2.
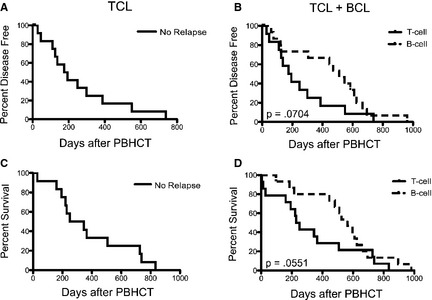
(A) Disease‐free interval of TCL and (B) TCL and BCL 10 (P = .0704) cases combined. (C) Overall survival of TCL and (D) TCL and BCL 10 (P = .0551) cases combined.
Overall Survival
The median OS for the 13 dogs that were in CR at the time of PBHCT from the time of PBHCT was 239.5 days (range, 4–738 days; Fig 2C). When we compared the median OS between the dogs with TCL in this study and the previously reported BCL dogs,10 there was no statistically significant difference (P = .0551) between the 2 groups (240 days versus 531 days, respectively; Fig 2D). The median OS from the time of diagnosis to death of the 13 TCL dogs in CR at the time of PBHCT was 602 days (range, 246–928 days), with 2 (14%) dogs still alive at 741 and 772 days. Stage and substage of disease at diagnosis had no effect on OS (P = .5862 and P = .1303, respectively).
Discussion
Similar to our previous study using PBHCT to treat dogs with BCL,10 an adequate number of peripheral blood CD34+ cells could be harvested using a TerumoBCT cell separator for HCT after myeloablative treatment. In addition, the majority of dogs tolerated the toxicities associated with escalating doses of HDC and TBI and achieved complete hematologic reconstitution after transplantation.
In an effort to decrease the potential for minimal residual disease in the blood of the affected dogs, HDC dose intensification was incrementally increased throughout the study. As such, 9 dogs received ≥650 mg/m2 of cyclophosphamide, with 1 dog receiving 750 mg/m2. Although some dogs developed grade‐4 neutropenia, only 4 dogs required brief hospitalization (1 received 500 mg/m2, 1 received 600 mg/m2, 2 received 650 mg/m2), only 1 dog developed cyclophosphamide‐induced cystitis, and all dogs were able to proceed with apheresis. These results suggest that cyclophosphamide can be administered to dogs in amounts up to 3.75 times the standard dosage if appropriate protocols are used and supportive care is given if needed.
In a previous report of PBHCT of dogs with BCL,10 the relapse rate was approximately 65% when 10 Gy of TBI was used to achieve molecular remission. In this study, we continued to escalate the dose of TBI because a higher dose of irradiation should, theoretically, lead to a better clinical outcome16, 17, 18 and because TCL is a more aggressive disease than BCL. Therefore, we administered >10 Gy TBI to 12 dogs while incorporating more fractionated protocols in an effort to decrease the associated GI toxicities. In addition, many dogs received enteral glutamine, an antioxidant that may protect the GI tract from the adverse effects associated with radiation treatment.19, 20 Although 2 dogs in this study died in the hospital, only 1 dog, that was not in CR at the time of presentation to NCSU and subsequently received high‐dose chemotherapy 1 week before TBI, had evidence of severe GI toxicity at necropsy. Therefore, we conclude that dogs can routinely tolerate >10 Gy TBI when administered in multiday fractionated protocols. In future PBHCT studies, we also will utilize lower dose rates (regardless of overall dose) because it appears that both radiation dose rate and overall dose are the most important factors in acute toxicity of the canine GI tract associated with TBI.16
Overall, there were only minor differences between the TCL dogs in this study and the BCL dogs in a previous study10 with respect to apheresis characteristics and apheresis product counts. When we examined a variety of factors affecting apheresis cell yield, preapheresis Hct was not significantly correlated with increased MCCE and preapheresis monocyte count was not significantly correlated with the number of CD34+ cells/kg harvested as we found in the BCL dogs. The lack of correlation between the preapheresis monocyte count and number of CD34+ cells/kg harvested is most likely a reflection of the lower overall number of monocytes before the procedure in the TCL dogs, whereas the reason for the lack of correlation between preapheresis Hct and increased MCCE in these dogs is not clear. However, when combining the data from both studies, these correlations again became significant. Importantly, rhG‐CSF administration was well tolerated and the resultant apheresis products contained >2 × 106 CD34+ cells/kg (target cell dose) in all TCL dogs.
When apheresis products contain >1 × 107 CD34+ cells/kg, we routinely cryopreserve one‐half of the product in the event a dog relapses and the owners want to perform a second PBHCT. The cryopreserved product was given to 1 dog that relapsed approximately 93 days after PBHCT after additional chemotherapy (doxorubicin [30 mg/m2] and melphalan [30 mg/m2]) and TBI (6 Gy). Complete hematologic reconstitution was again achieved, and the dog remained in clinical and molecular remission for an additional 225 days. This case underscores the utility of apheresis product cryopreservation and corroborates data from humans showing the feasibility of second hematopoietic cell transplants in patients relapsing after prior PBHCT.21, 22
One dog developed a second cancer after PBHCT. Interestingly, this dog developed a B‐cell malignancy whereas a dog in a previous report10 with B‐cell lymphoma developed epitheliotropic T‐cell lymphoma. Late second malignancies after total body irradiation have been reported in both dogs23 and humans.24
Two factors that are consistently identified as having negative prognostic importance in dogs with lymphoma are immunophenotype and WHO substage.25, 26 Therefore, dogs with a T‐cell immunophenotype and substage b disease tend to have substantially shorter remission and survival durations. Interestingly, in this study where 40% of the TCL dogs were WHO substage b, the median OS, when defined as time from diagnosis to death, was 602 days, with 2 dogs (1 Stage 3A, 1 Stage 5b) still alive at manuscript preparation. Factors that may have positively influenced the survival of the dogs in this study include the higher doses of HDC and TBI used in the pre‐PBHCT conditioning regimen and the unknown number of dogs with low‐grade tumors27 because a LN biopsy was obtained in only 3 dogs (all 3 dogs were diagnosed with an aggressive T‐cell lymphoma called peripheral T‐cell lymphoma). In addition, because 87% of the dogs maintained a first remission (median, 3.7 months; range, 2–5.8 months) before transplantation, a positive selection bias in this population may have influenced our results. Regardless, it appears that PBHCT achieved a longer OS (602 days) in dogs with TCL than previously reported studies (159 days3 and 235 days28).
In summary, we show that an adequate number of peripheral blood CD34+ cells can be routinely harvested from dogs with TCL and used for autologous PBHCT. Based on this small number of dogs, it appears that, although PBHCT does not lead to a statistically significant increase in DFI in dogs with TCL, it can lead to a longer median OS when compared to historical studies using multiagent chemotherapy regimens. Because 1 dog in this study achieved postmyeloablative hematopoietic reconstitution after a second PBHCT using a cryopreserved autologous apheresis product, we suggest, that, similar to human protocols,29 cryopreserved canine apheresis products can be routinely used for both autologous and allogeneic PBHCT. Based on an apparent increase in the median OS of the dogs in this study (with 2 dogs alive >2 years after diagnosis), PBHCT may be considered as a viable alternative treatment for dogs with high‐grade TCL.
Acknowledgments
The authors thank the faculty, house officers, students, and staff at the NCSU Veterinary Health Complex for their dedication to the transplant patients. We also thank Linda English and Dr Mary Tompkins (Clinical Immunology) for flow cytometry analyses and the Clinical Pathology staff for CBC analyses.
This work was performed at the North Carolina State College Veterinary Health Complex. This work was not supported by a grant.
Conflict of Interest Declaration: The authors disclose no conflict of interest.
Footnotes
TerumoBCT, Lakewood, CO
Baxter Healthcare Corporation, Deerfield, IL
Cytoxan, Cyclophosphamide for Injection, USP; Baxter Healthcare Corporation, Deerfield, IL
Neupogen; Amgen, Thousand Oaks, CA
Mesnex; Sagent Pharmaceuticals, Schaumburg, IL
L‐glutamine; Vitamin Shoppe, North Bergen, NJ
DMSO; Amresco, Solon, OH
Gambro DF‐700 Bag Press; ThermoFisher Scientific, Hudson, NH
Varian, Palo Alto, CA
GraphPad Software, San Diego, CA
Adriamycin; Bedford Laboratories, Bedford, OH
Alkeran; GlaxoSmithKline, Brentford, London, UK
Mozobil; Genzyme Corporation, Cambridge, MA
References
- 1. Fournel‐Fleury C, Ponce F, Felman P, et al. Canine T‐cell lymphomas: A morphological, immunological, and clinical study of 46 new cases. Vet Pathol 2002;39:92–109. [DOI] [PubMed] [Google Scholar]
- 2. Bienzle D, Vernau W. The diagnostic assessment of canine lymphoma: Implications for treatment. Clin Lab Med 2011;31:21–39. [DOI] [PubMed] [Google Scholar]
- 3. Vail DM. Hematopoietic tumors In: Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Internal Medicine, 7th ed Philadelphia, PA: WB Saunders; 2010:2148–2163. [Google Scholar]
- 4. Keller ET, MacEwen EG, Rosenthal RC. Evaluation of prognostic factors and sequential combination chemotherapy with doxorubicin for canine lymphoma. J Vet Intern Med 1993;7:289–295. [DOI] [PubMed] [Google Scholar]
- 5. Garrett LD, Thamm DH, Chun R, et al. Evaluation of a 6‐month chemotherapy protocol with no maintenance therapy for dogs with lymphoma. J Vet Intern Med 2002;16:704–709. [DOI] [PubMed] [Google Scholar]
- 6. Williams LE, Johnson JL, Hauck ML, et al. Chemotherapy followed by half‐body radiation therapy for canine lymphoma. J Vet Intern Med 2004;18:703–709. [DOI] [PubMed] [Google Scholar]
- 7. Lurie DM, Gordon IK, Theon AP, et al. Sequential low‐dose rate half‐body irradiation and chemotherapy for the treatment of canine multicentric lymphoma. J Vet Intern Med 2009;23:1064–1070. [DOI] [PubMed] [Google Scholar]
- 8. Regan RC, Kaplan MSW, Bailey DB. Diagnostic evaluation and treatment recommendations for dogs with substage‐a high‐grade multicentric lymphoma: Results of a survey of veterinarians. Vet Comp Oncol 2013. Dec;11(4):287–295. Epub 2012 Mar 2. [DOI] [PubMed] [Google Scholar]
- 9. Legendre AM. Treatment of dogs with lymphoma: A work in progress. J Vet Intern Med 2007;21:1166–1167. [DOI] [PubMed] [Google Scholar]
- 10. Willcox JL, Puritt A, Suter SE. Autologous peripheral blood hematopoietic cell transplantation in dogs with B‐cell lymphoma. J Vet Intern Med 2012;26:1155–1163. [DOI] [PubMed] [Google Scholar]
- 11. Posner LP, Willcox JL, Suter SE. Apheresis in three dogs weighing <14 kg. Vet Anaesth Analg 2013. Jul;40(4):403–409. Epub 2013 Mar 1. [DOI] [PubMed] [Google Scholar]
- 12. Burnett RC, Vernau W, Modiano JF, et al. Diagnosis of canine lymphoid neoplasia using clonal rearrangements of antigen receptor genes. Vet Pathol 2003;40:32–41. [DOI] [PubMed] [Google Scholar]
- 13. Appelbaum FR, Herzig GP, Graw RG, et al. Study of cell dose and storage time on engraftment of cryopreserved autologous bone marrow in a canine model. Transplantation 1976;26:245–248. [DOI] [PubMed] [Google Scholar]
- 14. VCOG . Veterinary cooperative oncology group‐common terminology criteria for adverse events (VCOG‐CTCAE) following chemotherapy of biological antineoplastic therapy in dogs and cats v1.1. Vet Comp Oncol 2011. Jul 20; doi: 10.1111/j.1476‐5829.2011.00283.x. [DOI] [PubMed] [Google Scholar]
- 15. Bean MA, Storb R, Graham T, et al. Prevention of transfusion‐induced sensitization to minor histocompatibility antigens on DLA‐identical canine marrow grafts by gamma irradiation of marrow donor blood. Transplantation 1991;52:956–960. [DOI] [PubMed] [Google Scholar]
- 16. Deeg HJ, Storb R, Longton G, et al. Single dose or fractionated total body irradiation and autologous marrow transplantation in dogs: Effects of exposure rate, fraction size, and fractionation interval on acute and delayed toxicity. Int J Radiat Oncol Biol Phys 1988;15:647–653. [DOI] [PubMed] [Google Scholar]
- 17. McAfee SL, Powell SN, Colby C, et al. Dose‐escalated total body irradiation and autologous stem cell transplantation for refractory hematologic malignancy. Int J Radiat Oncol Biol Phys 2002;53:151–156. [DOI] [PubMed] [Google Scholar]
- 18. Yahalom J, Gulati SC, Toia M, et al. Accelerated hyperfractionated total‐lymphoid irradiation, high‐dose chemotherapy, and autologous bone marrow transplantation for refractory and relapsing patients with Hodgkin's disease. J Clin Oncol 1993;11:1062–1070. [DOI] [PubMed] [Google Scholar]
- 19. Campos FG, Waltzberg DL, Mucerino DR, et al. Protective effects of glutamine enriched diets on acute actinic enteritis. Nutr Hosp 1996;11:167–177. [PubMed] [Google Scholar]
- 20. Anderson PM, Ramsay NKC, Shu Xo, et al. Effect of low‐dose oral glutamine on painful stomatitis during bone marrow transplantation. Bone Marrow Transplant 1998;22:339–344. [DOI] [PubMed] [Google Scholar]
- 21. Smith SM, van Besien K, Carreras J, et al. Second autologous stem cell transplantation for relapsed lymphoma after prior autologous transplant. Biol Blood Marrow Transplant 2008;14:904–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim J‐W, Kim B‐S, Bang S‐M, et al. Allogeneic stem cell transplantation in patients with non‐Hodgkin lymphoma who experienced relapse or progression after autologous stem cell transplantation. Ann Hematol 2011;90:1409–1418. [DOI] [PubMed] [Google Scholar]
- 23. Deeg HJ, Prentice R, Fritz TE, et al. Increased incidence of malignant tumors in dogs after total body irradiation and marrow tranpslantation. Int J Radiat Oncol Biol Phys 1983;9:1505–1511. [DOI] [PubMed] [Google Scholar]
- 24. Brown JR, Yeckes H, Friedberg JE, et al. Increasing incidence of late second malignancies after conditioning with cyclophosphamide and total‐body irradiation and autologous bone marrow transplantation for non‐Hodgkin's lymphoma. J Clin Oncol 2005;23:2208–2214. [DOI] [PubMed] [Google Scholar]
- 25. Greenlee PG, Filippa DA, Quimby FW, et al. Lymphoma in dogs: A morphologic, immunologic, and clinical study. Cancer 1990;66:480–490. [DOI] [PubMed] [Google Scholar]
- 26. Teske E, van Heerde P, Rutteman FR, et al. Prognostic factors for treatment of malignant lymphoma in dogs. J Am Vet Med Assoc 1994;205:1722–1728. [PubMed] [Google Scholar]
- 27. Flood‐Knapik KE, Durham AC, Gregor TP, et al. Clinical, histopathological, and immunohistochemical characterization of canine indolent lymphoma. Vet Comp Oncol 2013. Dec;11(4):272–286. Epub 2012 Feb 2. [DOI] [PubMed] [Google Scholar]
- 28. Rebhun RB, Kent MS, Borrofka SAEB, et al. CHOP chemotherapy for the treatment of canine multicentric T‐cell lymphoma. Vet Comp Oncol 2010;9:38–44. [DOI] [PubMed] [Google Scholar]
- 29. Berz D, McCormack EM, Winer ES, et al. Cryopreservation of hematopoietic stem cells. Am J Hematol 2007;82:463–472. [DOI] [PMC free article] [PubMed] [Google Scholar]


