Abstract
Background
Syncope is a transient loss of consciousness occasionally occurring in dogs with advanced myxomatous mitral valve disease (MMVD).
Objective
(1) To study ECG changes during syncopal episodes in dogs with advanced MMVD and (2) to compare the occurrence of arrhythmias and changes in heart rate variability (HRV) between dogs with advanced MMVD with and without a history of syncope.
Animals
Forty‐three privately owned dogs (<15 kg) with advanced MMVD: 21 with and 22 without a history of syncope.
Methods
Prospective study with dogs recruited for an evaluation including history, physical examination, echocardiography, and arrhythmia and HRV analysis performed on 24‐hour Holter recordings.
Results
A syncopal episode was observed during Holter monitoring in 4 dogs: 3 dogs had sinus rhythm and 1 dog had sinus arrest followed by escape rhythm. An arrhythmia variable representing sinus arrhythmia was significantly lower in dogs with a history of syncope than in those without (P = .008). Eight of 26 HRV variables were significantly different between dogs with and without a history of syncope.
Conclusions and Clinical Importance
Compared with dogs without a history of syncope, dogs with advanced MMVD and a history of syncope did not have a higher occurrence of arrhythmias, but had less sinus arrhythmia, and had changes in HRV variables representing decreased overall HRV, decreased parasympathetic, and increased sympathetic modulation of heart rate.
Keywords: Arrhythmia, Collapse, Heart rate variability
Abbreviations
- ACE‐I
angiotensin‐converting enzyme inhibitor
- APC
atrial premature complex
- AV
atrioventricular
- bpm
beats per minute
- CKCS
Cavalier King Charles Spaniel
- Dig
digoxin
- F
furosemide
- HF
high frequency
- HFn
normalized high frequency
- HR
heart rate
- HRV
heart rate variability
- IVSd
interventricular septal thickness in diastole
- IVSs
interventricular septal thickness in systole
- LA/Ao
left atrium‐to‐aortic root ratio
- LF
low frequency
- LFn
normalized low frequency
- LVIDd
left ventricular internal dimension in diastole
- LVIDs
left ventricular internal dimension in systole
- LVPWd
left ventricular free wall thickness in diastole
- LVPWs
left ventricular free wall thickness in systole
- MEAN
mean of all NN intervals
- MMVD
myxomatous mitral valve disease
- MR
mitral regurgitation
- NN interval
interval between two QRS complex of sinus or supraventricular origin
- PAH
pulmonary hypertension
- PCA
principal component analysis
- Pimo
pimobendan
- PISA
proximal isovelocity surface area
- pNN50
% of successive NN intervals that differ more than 50 ms
- RMSSD
square root of the mean squared differences in successive NN intervals
- SD
standard deviation of the NN intervals
- S
spironolactone
- SVT
supraventricular tachycardia
- TI
triangular index
- TP
total power
- ULF
ultra low frequency
- VLF
very low frequency
- VPC
ventricular premature complexes
- VT
ventricular tachycardia
Syncope is a transient loss of consciousness because of a decreased delivery of essential nutrients to the brain.1, 2 A syncopal episode has a sudden onset, is of short duration (usually a few seconds), and is followed by spontaneous complete recovery.3 In dogs, the cause of syncope is often divided into 3 categories: cardiac, noncardiac, and unknown. A cardiac‐related cause of syncope is suggested to be the most common cause of syncope in dogs.2 Decreased cardiac output leading to cerebral hypoperfusion has been suggested as the cause of cardiac‐related syncope. If this is correct, any cardiac disease leading to decreased cardiac output can potentially cause syncope. Arrhythmias can cause decreased cardiac output and this is suggested to be the most common cause of cardiac‐related syncope.2 Several types of arrhythmias occur in association with syncopal episodes, including sinus arrest, ventricular tachycardia (VT), sick sinus syndrome, and third‐degree atrioventricular (AV) block.2, 4, 5, 6, 7, 8 However, diagnosis of the exact cause of syncope can be challenging and the number of dogs experiencing a syncopal episode during ECG monitoring can vary markedly dependent on the ECG monitoring technique and duration of the recording.4, 5, 8, 9, 10
Syncope has been associated with an increased risk of death in dogs with myxomatous mitral valve disease (MMVD).11, 12, 13 MMVD is the most common heart disease in dogs and mainly affects the mitral valve, which degenerates and becomes insufficient with age, leading to mitral regurgitation (MR).12, 13, 14
Dogs with heart failure secondary to MMVD have decreased heart rate variability (HRV).15, 16, 17 HRV represents rhythmic variations in heart rate (HR) modulated by the autonomic nervous system and is measured from ECG based on the length of NN intervals (interval between 2 QRS complexes of sinus or supraventricular origin).18 The HRV variables total power (TP), standard deviation of NN intervals (SD), and triangular index (TI=total number of NN intervals/maximum number of NN intervals of equal length) represent overall HRV.19, 20
The variables high frequency (HF), square root of mean squared differences in successive NN intervals (RMSSD), and percentage of successive NN intervals differing >50 ms (pNN50) reflect parasympathetic activity, whereas low frequency (LF) reflects both parasympathetic and sympathetic activity.18, 20, 21, 22 Ultra low frequency (ULF) and very low frequency (VLF) are less understood, but are influenced by thermoregulation, physical activity, and the renin‐angiotensin system.18, 23, 24 Decreased HRV has been associated with an increased risk of death in dogs with experimentally induced myocardial infarction and in people with various cardiac diseases.25, 26, 27, 28, 29 Thus, both syncope and decreased HRV are associated with increased risk of death and therefore a history of syncope may be associated with decreased HRV in dogs with MMVD.
The first aim of this study was to investigate ECG changes during syncopal episodes in dogs with advanced MMVD. The second aim was to study whether arrhythmia and HRV‐derived variables in dogs with advanced MMVD differed between dogs with and without a history of syncope.
Materials and Methods
Study Population
Privately owned dogs weighing <15 kg were included in the study prospectively. Dogs were included if they (1) showed/had shown clinical signs of heart failure (any or all of dyspnea, cough, exercise intolerance, and restlessness at night) responsive to furosemide therapy; (2) had a left atrium‐to‐aortic root ratio (LA/Ao) >1.528; and (3) had a nonfunctional MR jet occupying more than half of the left atrial area, as assessed by color Doppler mapping. Dogs were included in the syncope group provided (1) they had a history of 1 or more episodes of collapse/fainting; (2) there were no convulsions during the episodes; (3) the episodes were short (usually lasting a few seconds); (4) the episodes were not related to coughing; and (5) the dog appeared normal before and after the episode (ie, no aura or post ictus). Dogs were excluded from the study if they had signs of clinically relevant systemic or organ‐related disease, other than MMVD, based on history, clinical examination, echocardiography, serum biochemistry, and CBC. Furthermore, dogs were excluded from arrhythmia and HRV analysis if they had <20 hours of readable data on the Holter recording. All dogs were examined between April 2007 and November 2010 and were recruited from the Department of Veterinary Clinical and Animal Sciences, Faculty of Life Sciences, University of Copenhagen, Denmark, Din Veterinär Animal Hospital, Sweden, Blå Stjärnans Animal Hospital, Sweden, Clinic for Small Animal Medicine and Surgery, University of Ljubljana, Ljubljana, Slovenia, and Department of Clinical Sciences, Swedish University of Agricultural Sciences, Uppsala, Sweden. All owners gave their consent, and the study was approved by the Danish Animal Welfare Division and the Ethical Committee in Gothenburg and Uppsala, Sweden and the Ethical committee at the Veterinary Administration of the Republic of Slovenia.
Examination Procedure
All dogs were evaluated by case history, physical examination, echocardiography, and Holter monitoring. The Holter monitoring was performed on the same day (n = 40) or within 7 days (n = 3) of the other examinations. In addition, blood pressure was noninvasively measured using a high‐definition oscillometric technique1 in 12 dogs that concurrently participated in another study30 between September 2009 and March 2010.
Echocardiography
Two‐dimensional echocardiography was performed in unsedated dogs placed in right and left lateral recumbency by 4 operators (LHO [n = 20], TF [n = 17], AD [n = 5], and IL [n = 1]). M‐mode echocardiography was performed on the right parasternal short‐axis view and used to assess left ventricular dimensions.31 In this study, left ventricular dimensions are presented as percent increase from expected normal dimensions determined from individual body weight.32 The right parasternal short‐axis view at the level of the aortic valve was used to measure LA/Ao.33 Color Doppler mapping over the mitral and tricuspid valve area was used to measure the proximal isovelocity surface area (PISA), MR, and tricuspid regurgitation (TR).34, 35, 36 MR was evaluated as the maximum regurgitant jet occupying the left atrial area, whereas TR was semiquantitatively classified into four categories: no, mild, moderate, or severe.35, 36 If TR was present, continuous wave Doppler over the tricuspid valve area was used to estimate the peak TR velocity.37
Holter Monitoring
An area intended for electrode placement was prepared by shaving and cleaning the skin with alcohol, and electrodes2 were placed in a 2‐lead precordial system and connected to a Holter recorder (Lifecard CF Digital Holter recorder3).38 The Holter recorder and leads were secured and Holter monitoring was performed in the dog's home environment for at least 24 hours. The owners were instructed to maintain the dogs' daily routines and to note general activities and episodes of syncope in a diary.
Arrhythmia and Heart Rate Variability Analysis
Commercially available software designed for humans was used to perform standardized semiautomatic arrhythmia analyses (Pathfinder digital Holter analysis system V8.7013) and automatic HRV analyses (HRV Tools software package version 1.733). Software settings and protocol have been described previously.39 All Holter recordings were analyzed by the same author (CER), who was blinded to dog identity during the process. All Holter‐derived variables (HRV and arrhythmia variables) were measured over a 24‐hour period. During the 24‐hour period, HRV variables were additionally measured in a 6‐hour nightly period starting 30 minutes after bedtime. Bedtime was identified by use of the diary or, if bed time was not noted in the diary, as the first period of 10 minutes after 9:00 pm with a HR below mean HR. HRV variables were suffixed by 24 or 6 indicating the analysis period (24‐hour or 6‐hour). Either period included >90% valid NN intervals; otherwise, the period was excluded from HRV analysis.
Arrhythmia variables included:
minimum HR,
mean HR,
maximum HR,
bradycardia (>4 successive sinus complexes at a HR <45 complexes per minute [bpm]),
“dropped beats” (the term represents sinus arrhythmia and is defined as a NN interval >180% longer than the previous NN interval), sinus pauses (NN intervals >2.0 seconds),40
“premature normals” (the term represents sinus arrhythmia and is defined as a premature sinus complex [NN interval 50% shorter than the previous]),41
atrial premature complexes (APC) (a premature normal‐appearing QRS complex conducted by a P wave with abnormal morphology),42
supraventricular tachycardia (SVT) (>3 APCs at a HR >150 bpm, where the first complex is premature while the following should have NN intervals shorter than or equal to the previous NN interval),42, 43
sinus tachycardia (>3 sinus complexes at a HR >150 bpm, where the first complex was premature, while the following have NN intervals shorter than or equal to the previous NN interval),
ventricular premature complex (VPC) (a premature wide and bizarre looking QRS complex, not associated with a P wave, but accompanying a large T wave of opposite polarity),42
ventricular escape complex (wide QRS complexes of different orientation occurring after a sinus pause and not associated with a P wave),42
fusion complex (normal P wave followed by an intermediate shaped QRS complex),44
second‐degree AV block (a P wave not conducting a QRS complex).42
A VPC occurring on the top of the T wave associated with the previous sinus complex was termed RonT.44 More than 5 VPCs at a HR >150 bpm was defined as VT.40, 44 APCs and VPCs were included in the statistical analyses as percentage of the total QRS complexes in 24 hours. HRV analyses consisted of both frequency‐ and time domain analysis. Complexes of ventricular origin were excluded from the HRV analyses, thus NN intervals included in the HRV analyses comprised sinus complexes, APCs, and SVTs. The frequency domain variables included TP (0–0.4 Hz), ULF (0–0.00333 Hz), VLF (0.00333–0.04 Hz), LF (0.04–0.15 Hz), HF (0.15–0.4 Hz), normalized LF (LFn), normalized HF (HFn), and the ratio between HF and LF (HF/LF). LFn and HFn were calculated as LFn = LF/(TP‐ULF‐VLF) × 100 and HFn = HF/(TP‐ULF‐VLF) × 100).4 The time domain variables included MEAN (mean of all NN intervals), pNN50, RMSSD, SD, and TI.4
Statistical Analyses
Statistical analyses were performed by commercially available software.5 Syncope and nonsyncope dogs were compared for each of the 38 Holter‐derived variables (9 arrhythmia, 3 HR, and 26 HRV variables). We needed to allow for multiple significance tests, but as these variables were highly correlated, a Bonferroni correction would be very inefficient and conservative. We therefore used principal component analysis (PCA) to form a single combination of the 38 variables to give a single primary outcome variable, the first principal component. This was tested for influence of a history of syncope (yes/no) using a 2 sample t‐test and for an influence of CKCS (yes/no), age, sex (male/female), and interaction between a history of syncope and CKCS using analyses of variance. If the composite primary outcome variable was influenced by a history of syncope (yes/no), Student's t‐test was used to identify Holter‐derived variables differing between dogs with and dogs without a history of syncope. Student's t‐test was also used to test for differences in characteristics between dogs with and without a history of syncope. Variables were tested for normal distribution using Shapiro‐Wilks test and Normal quantile plot, and a logarithmic transformation was applied if necessary. The significance level was P < .05.
Results
The study included 21 dogs with and 22 dogs without a history of syncope. The frequency of the syncopal episodes varied between the dogs: Six of the 21 dogs had 1 or multiple syncopal episodes on the same day and within a month, they were presented for examination. Syncope had been observed on several occasions in the remaining 15 dogs. The occurrence in these 15 dogs varied from daily syncopal episodes to having syncopal episodes only every second month, but the majority of dogs had 1 weekly episode of syncope. Of 21 dogs with a history of syncope, 4 dogs experienced an episode during the Holter recording. One dog had sinus arrest followed by an escape rhythm during the syncopal episode (Fig 1), while the remaining 3 dogs showed sinus rhythm with HR ranging from 131 to 171 bpm (Fig 2). The dog with sinus arrest and escape rhythm during the syncopal episode was excluded from the statistical analyses because of <20 hours readable data on the Holter recording. Thus, the statistical analyses included 20 dogs with and 22 dogs without a history of syncope (Tables 1, 2). Five dogs did not have serum biochemistry and CBC measured. Peak TR velocity was only measured in dogs included in the study after September 2009. During the 24‐hour Holter monitoring, both dogs with and without a history of syncope had RonTs (without a history of syncope n = 10, with a history of syncope n = 5), ventricular couplets (0, 4), ventricular triplets (1, 1), ventricular bigeminy (2, 1), VT (0, 1), ventricular escape complexes (1, 1), and fusion complexes (2, 2). Logarithmic transformations were applied to all 9 arrhythmia variables, minimum HR, 14 frequency (TP24 + 6, ULF24 + 6, VLF24 + 6, LF24 + 6, HF24 + 6, LFn24 + 6, and HF/LF24 + 6), and 2 time domain (TI24 + 6) HRV variables. The first principal component contained 46% of the total variance and the second principal component 15%. Further statistical analysis of the first principal component showed a significant difference between dogs with and without a history of syncope (P = .025), but no evidence of an association with CKCS (yes/no), age, sex (male/female), or an interaction between a history of syncope and CKCS.
Figure 1.
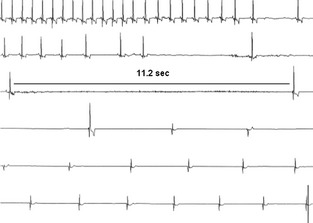
Electrocardiogram (ECG) from a 7.5‐year‐old Cavalier King Charles Spaniel with advanced myxomatous mitral valve disease. The ECG strip shows 75 seconds of a single precordial lead from the 24‐hour Holter recording displayed chronologically and centered around a syncopal episode. The ECG is showing sinus rhythm with HR 146 bpm, followed by a decrease in HR and sinus pauses. Hereafter, the dog has sinus arrest followed by an escape rhythm. bpm, beats per minute; HR, heart rate.
Figure 2.
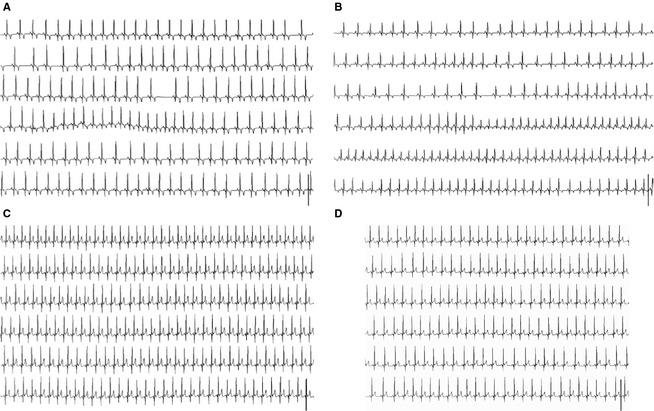
Electrocardiogram (ECG) from 3 small breed dogs with advanced myxomatous mitral valve disease during a syncopal episode. The ECG strips show 75 seconds of a single precordial lead from the 24‐hour Holter recording displayed chronologically and centered around a syncopal episode. (A) Jack Russell Terrier having a syncopal episode showing sinus rhythm at HR = 131 bpm. (B) Dachshund having sinus rhythm with a shift in HR from 126 to 181 bpm. (C) and (D) ECG showing 2 syncopal episodes from a Miniature Poodle with sinus rhythm at HR 171 and 136 bpm, respectively. bpm, beats per minute; HR, heart rate.
Table 1.
Characteristics of 42 dogs with advanced myxomatous mitral valve disease.
| Characteristic/Group | With a History of Syncope | Without a History of Syncope |
|---|---|---|
| N | 20 | 22 |
| CKCS, n | 6 | 10 |
| Sex, female/male | 7/13 | 7/15 |
| Age, years (n = 20 + 22) | 11.2 ± 2.0 | 11.3 ± 2.3 |
| Body weight, kg (n = 18 + 22) | 8.3 ± 1.9 | 9.5 ± 2.7 |
| Systolic blood pressure, mmHg (n = 5 + 7) | 145.0 ± 34.8 | 150.4 ± 16.4 |
| Diastolic blood pressure, mmHg (n = 5 + 7) | 78.0 ± 7.0 | 78.4 ± 5.7 |
| IVSd, % (n = 15 + 19)a | 6.2 ± 25.0 | 11.7 ± 17.0 |
| IVSs, % (n = 15 + 19)a | 14.2 ± 17.7 | 11.5 ± 20.3 |
| LVIDd, % (n = 17 + 22) | 45.4 ± 15.2 | 39.0 ± 22.4 |
| LVIDs, % (n = 15 + 19) | 42.0 ± 27.0 | 29.2 ± 23.8 |
| LVPWd, % (n = 15 + 19) | 12.3 ± 16.4 | 16.2 ± 16.1 |
| LVPWs, % (n = 15 + 19)a | 3.0 ± 16.3 | ‐0.1 ± 13.2 |
| LA/Ao (n = 17 + 22) | 2.2 ± 0.3 | 2.0 ± 0.5 |
| PISA, mm (n = 13 + 22) | 13.3 ± 6.0 | 14.1 ± 5.1 |
| FS, % (n = 15 + 19) | 36.8 ± 8.7 | 38.8 ± 5,2 |
| TR, no/mild/moderate/severe (n = 10 + 19) | 0/7/1/2 | 4/8/4/3 |
| peak TR velocity, m/s (n = 12 + 8) | 3.3 ± 1.0 | 3.3 ± 0.7 |
| PAH, normal/equivocal/hypertensive (n = 12 + 8)b | 1/4/7 | 0/1/7 |
Values are shown as mean ± SD. Characteristic is suffixed by (n, number of dogs with a history of syncope + number of dogs without a history of syncope). In addition to CKCS, dogs with a history of syncope comprised 3 Dachshunds, 2 Jack Russell Terriers, 2 Pekingeses, 1 King Charles Spaniel, 1 Maltese, 1 Miniature Poodle, 1 Chinese Chrested, 1 Bichon Frisé, 1 Bichon Havanais, and a mixed breed dog. Dogs without a history of syncope in addition to CKCS comprised 3 Dachshunds, 2 Jack Russell Terriers, 1 King Charles Spaniel, 1 Maltese, 2 Norfolk Terriers, 1 Bichon Frisé, 1 Boston Terrier, and 1 Bedlington Terrier. Using a Student's t‐test, none of the characteristics was significantly different between dogs with and without a history of syncope.
CKCS, Cavalier King Charles Spaniel; d, diastole; FS, fraction shortening; IVS, percentage increase in interventricular septal thickness; LA/Ao, left atrium‐to‐aortic root ratio; LVID, percentage increase in left ventricular internal dimension; LVPW, percentage increase in left ventricular free wall thickness; PAH, pulmonary hypertension; PISA, proximal isovelocity surface area; TR, tricuspid regurgitation; s, systole.
Variable was logarithmic transformed to obtain normal distribution.
PAH is categorized according to peak TR velocity (normal <2.5 m/s, equivocal 2.5–3.0 m/s, and hypertensive >3.0 m/s).50
Table 2.
Medical treatment of 42 dogs with advanced myxomatous mitral valve disease.
| Medical Treatment | A History of Syncope, n (%) | No History of Syncope, n (%) |
|---|---|---|
| F | 3 (15.0) | 0 (0) |
| Pimo | 0 (0) | 1 (4.5)a |
| F + ACE‐I | 4 (20.0) | 3 (13.6) |
| F + Pimo | 1 (5.0) | 2 (9.0) |
| F + Dig | 1 (5.0) | 0 (0) |
| F + ACE‐I + ß‐blocker | 2 (10.0) | 0 (0) |
| F + ACE‐I + Pimo | 7 (35.0) | 7 (31.8) |
| F + ACE‐I + S | 0 (0) | 1 (4.5) |
| F + Pimo + Dig | 0 (0) | 1 (4.5) |
| F + Pimo + S | 0 (0) | 1 (4.5) |
| F + ACE‐I + Pimo + S | 1 (5.0) | 2 (13.6) |
| F + ACE‐I + Pimo + S + Dig | 0 (0) | 4 (18.2) |
| F + ACE‐I + Pimo + S + Dig + β‐blocker | 1 (5.0) | 0 (0) |
F, furosemide; ACE‐I, angiotensin‐converting enzyme inhibitor; Pimo, pimobendan; S, spironolactone; Dig, digoxin
This dog was started on and responded to furosemide treatment shortly after the examinations performed for this study.
Nine variables, representing parasympathetic influence on HR, were significantly lower in dogs with a history of syncope compared with dogs without a history of syncope: “Dropped beats” (dogs with a history of syncope episodes 1,213 ± 1,245 (mean ± SD), dogs without a history of syncope 3,715 ± 4,140 episodes, P = .0082), HF24 (9,111 ± 8,548 ms2, 17,714 ± 16,258 ms2, P = .026), HF6 (13,511 ±12,365 ms2, 25,479 ± 20,934 ms2, P = .032), HFn24 (54.71 ± 16.21 nu, 64.58 ± 13.54 nu, P = .038) (Fig 3), RMSSD24 (114.6 ± 59.0 ms, 159.0 ± 70.6 ms, P = .034), RMSSD6 (150.3 ± 75.5 ms, 212.4 ± 82.9 ms, P = .015), MEAN6 (572.5 ± 97.2 ms, 635.1 ± 99.5 ms, P = .046), pNN506 (42.86 ± 22.27%, 57.74 ± 17.61%, P = .021), and SD6 (146.7 ±60.5 ms, 190.7 ± 66.1 ms, P = .031). LFn24, representing sympathetic influence on HR, was significantly higher in dogs with a history of syncope (25.40 ± 12.86 nu) compared with dogs without a history of syncope (17.4 ± 12.86 nu, P = .039) (Fig 3).
Figure 3.
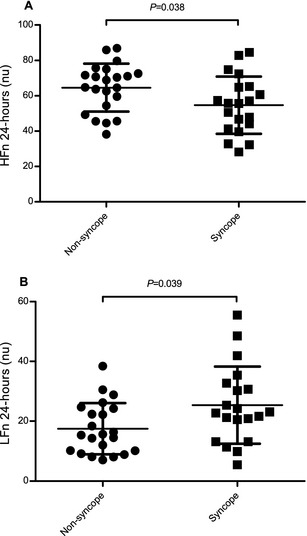
In dogs with advanced myxomatous mitral valve disease, 9 variables representing the parasympathetic modulation of heart rate (HR) (including (A) HFn24) were significantly lower in dogs with a history of syncope compared with dogs without a history of syncope. However, 1 variable, (B) LFn24, suggested to represent both the parasympathetic and sympathetic modulation of HR, was significantly higher in dogs with a history of syncope. HFn24, high frequency measured over a 24‐hour period; LFn24, normalized low frequency measured over a 24‐hour period.
Discussion
A syncopal episode was only observed in 4 dogs during Holter monitoring (1 arrhythmic and 3 nonarrhythmic events). “Dropped beats” and Holter‐derived variables representing parasympathetic modulation of HR were significantly lower and LFn24 was significantly higher in dogs with a history of syncope than in dogs without. These findings suggest a lower parasympathetic and higher sympathetic modulation of HR in dogs with a history of syncope and MMVD.
One of 4 dogs in this study had an arrhythmic event during a syncopal episode. The arrhythmic event might be caused by MMVD. However, given the nature of the arrhythmia, the differential diagnoses of intrinsic sinus nodal disease and cardioinhibitory neuromediated syncope should also be considered.
Both arrhythmic and nonarrhythmic events occur during syncopal episodes in dogs with MMVD during 24‐hour Holter monitoring.5, 10 Of 44 dogs with a history of syncope (12 with MMVD), a syncopal episode during Holter monitoring occurred in 11 dogs (25%), 2 of which had VT (18.2%), 2 had a bradyarrhythmia (18.2%), and 7 had a nonarrhythmic event (63.6%).5 Of 112 dogs (23 with MMVD), 22 (19.6%) dogs had a syncopal episode during Holter monitoring, 3 of which had bradyarrhythmia (13.6%), 2 had VT (9.1%), 8 had neurally mediated bradyarrhythmia (36.4%), and 9 had a nonarrhythmic event (40.9%).10 When comparing the present and the 2 above‐mentioned retrospective studies,5, 10 it appears that the majority of syncopal episodes in dogs with MMVD could be related to nonarrhythmic events. However, because of the limited number of dogs with a syncopal episode during Holter monitoring in this study, the described ECG changes might not be fully representative of the entire population of dogs with advanced MMVD and a history of syncope.
In humans, the procedure most commonly used to differentiate between an arrhythmic and a nonarrhythmic cause of syncope is to document an association between an arrhythmia and a syncopal episode.3 However, in humans, some authors have established cutoff values for arrhythmia variables, which are considered diagnostic for an arrhythmic cause of syncope, even though no syncopal episode occur during ECG monitoring.45, 46 In dogs with a history of syncope, occurrence of a significant arrhythmia during Holter monitoring not associated with a syncopal episode has been suggested to point toward an arrhythmic cause of the syncopal episode.2 Dogs with a history of syncope in this study, however, had the same occurrence of arrhythmias as dogs without a history of syncope. This finding suggests that syncope in dogs with advanced MMVD is not related to arrhythmic events. However, this suggestion cannot be confirmed by this study.
In this study, the significant changes in arrhythmia and HRV variables indicate that dogs with a history of syncope have a lower occurrence of sinus arrhythmia, decreased overall HRV, and decreased parasympathetic modulation of HR.18, 19, 20, 21, 22 In addition, a higher LFn24 was found in dogs with a history of syncope. LF can be influenced by both parasympathetic and sympathetic modulation of HR,18 but, as several other HRV variables indicate decreased parasympathetic modulation of HR in dogs with a history of syncope in this study, it is most likely that the high LFn24 reflects a higher sympathetic modulation of HR. Thus, the results indicate that dogs with a history of syncope and advanced MMVD can be characterized by a decreased parasympathetic and, to a lesser extent, an increased sympathetic modulation of HR.
In humans and dogs with various stages of different heart diseases, both syncope and decreased HRV are associated with increased risk of death.11, 19, 20, 25, 27, 28, 47, 48 Therefore, an association between a history of syncope and decreased HRV could be suspected. Even so, there are multiple explanations for the association between a history of syncope and decreased HRV. Syncope might be caused by decreased HRV, but both syncope and decreased HRV could also be a result of the complex pathophysiologic processes in MMVD. Given that a previous study has shown that the parasympathetic tone decreases with echocardiographic indices of MMVD,15 the HRV findings in this study might suggest that dogs with syncope have a more advanced stage of MMVD than do dogs without. This is also suggested in a study of survival characteristic in dogs with MMVD, showing that syncope, LA/Ao>1.7, and E‐wave transmitral peak velocity (Emax) >1.2 m/s were significantly associated with increased risk of death.11 However, this is in conflict with the echocardiographic findings in our study where little difference in echocardiographic variables was observed between dogs with and without a history of syncope (Table 1).
Two other important matters to consider in the syncopal etiology are blood pressure and pulmonary hypertension (PAH). Dogs with PAH often present with a history of syncope and PAH has also been associated with MMVD.49, 50, 51, 52 In this study, blood pressure and PAH assessed via peak TR velocity did not differ much between dogs with and without a history of syncope (Table 1). However, this study cannot fully evaluate the role of PAH or blood pressure in the etiology of syncope in dogs with advanced MMVD because of the low numbers available for analysis.
In summary, among dogs with advanced MMVD, a history of syncope was not associated with a higher occurrence of arrhythmias, but with decreased occurrence of sinus arrhythmia, decreased overall HRV, decreased parasympathetic, and increased sympathetic modulation of HR.
Limitations
Dogs were evaluated in 5 locations by 4 different echocardiographic operators, although a standard protocol was used. Radiographic examination was not included in the standard protocol and therefore not performed in all dogs. In the dogs where it was not performed, this examination would have strengthened the diagnosis (for instance by helping to exclude respiratory diseases). Another limitation is that blood pressure and peak TR velocity were only measured in some of the dogs. Furthermore, dogs received different types of medication, including β‐blockers and digoxin, potentially influencing Holter‐derived variables. The dogs did not follow a standardized exercise scheme, but their daily routines. This could potentially create large variation in the HRV results, although all dogs had compromised health resulting in very limited exercise. The Holter analysis software was designed for humans and therefore adds some limitations to the Holter analysis, because APCs and SVT were included in the HRV analyses and the HRV analysis software excludes sinus pauses >2.5 seconds, which are common in dogs.38
Acknowledgments
The study was supported by the Danish Council of Independent Research, Medical Sciences (project no. 271‐08‐0998 and 271‐05‐03335) and Synergy in Human and Animal Research (SHARE), Faculty of Life Sciences, University of Copenhagen. The authors thank Christina Kjempff, Birgitte Holle, Vibeke Christensen, Bettina Dahl, Dennis Jensen, and Hanne Carlsson at the Department of Basic Animal and Veterinary Sciences, University of Copenhagen, Frederiksberg, Denmark for their technical assistance.
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Preliminary results of this study were presented as a research abstract at the European College of Veterinary Internal Medicine (ECVIM) Congress in Porto, Portugal, 2009
Footnotes
VET Memodiagnostic HDO monitor, S+B medVET, Babenhausen, Germany
3M Red Dot electrodes, 3M, St. Paul, MN
SPACELABS Healthcare Company (previously Delmar Reynolds), Issaquah, WA
HRV Tools. Installation and instruction manual. ©2004 Delmar Reynolds Medical Limited, Hertford, England. Drawing No. 038/0369/0 Issue 2 CN 4657. Part No 18‐0369
Stata version 10, Stata Corp, College Station, TX and GraphPad Prism 5.00, GraphPad Software, La Jolla, CA
References
- 1. Grubb BP, Samoil D, Kosinski D, et al. Cerebral syncope: Loss of consciousness associated with cerebral vasoconstriction in the absence of systemic hypotension. Pace 1998;21:652–658. [DOI] [PubMed] [Google Scholar]
- 2. Kittleson MD. Syncope In: Kittleson MD, Kienle RD, eds. Small Animal Cardiovascular Medicine. St. Louis, MO: Mosby; 1998:495–501. [Google Scholar]
- 3. Moya A, Sutton R, Ammirati F, et al. Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J 2009;30:2631–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bright JM, Cali JV. Clinical usefulness of cardiac event recording in dogs and cats examined because of syncope, episodic collapse, or intermittent weakness: 60 cases (1997–1999). J Am Vet Med Assoc 2000;216:1110–1114. [DOI] [PubMed] [Google Scholar]
- 5. Miller RH, Lehmkuhl LB, Bonagura JD, Beall MJ. Retrospective analysis of the clinical utility of ambulatory electrocardiographic (Holter) recordings in syncopal dogs: 44 Cases (1991–1995). J Vet Intern Med 1999;13:111–122. [DOI] [PubMed] [Google Scholar]
- 6. Beckett SD, Branch CE, Robertson BT. Syncopal attacks and sudden death in dogs: Mechanisms and etiologies. J Am Anim Hosp Assoc 1978;14:378–386. [Google Scholar]
- 7. Santilli RA, Perego M, Crosara S, et al. Utility of 12‐lead electrocardiogram for differentiating paraoxysmal supraventricular tachycardia in dogs. J Vet Intern Med 2008;22:915–923. [DOI] [PubMed] [Google Scholar]
- 8. Santilli RA, Ferasin L, Voghera SG, Perego M. Evaluation of the diagnostic value of an implantable loop recorder in dogs with unexplained syncope. J Am Vet Med Assoc 2010;236:78–82. [DOI] [PubMed] [Google Scholar]
- 9. James R, Summerfield N, Loureiro J, et al. Implantable loop recorders: A viable diagnostic tool in veterinary medicine. J Small Anim Pract 2008;49:546–570. [DOI] [PubMed] [Google Scholar]
- 10. Santilli R, Perego M. Utility of Holter monitoring in patients with arrhythmogenic syncope: A retrospective study on 112 cases. Veterinaria 2009;23:13–18. Italian. [Google Scholar]
- 11. Borgarelli M, Savarino P, Crosara S, et al. Survival characteristics and prognostic variables of dogs with mitral regurgitation attributable to myxomatous valve disease. J Vet Intern Med 2008;22:120–128. [DOI] [PubMed] [Google Scholar]
- 12. Buchanan JW. Chronic valvular disease (endocardiosis) in dogs. Adv Vet Sci Comp Med 1977;21:75–106. [PubMed] [Google Scholar]
- 13. Detweiler DK, Patterson DF, Hubben K, Botts RD. The prevalence of spontaneously occurring cardiovascular disease in dogs. Am J Public Health C 1961;51:228–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Patterson DF, Detweiler DK, Hubben K, Botts RP. Spontaneous abnormal cardiac arrhythmias and conduction disturbances in the dog. A clinical and pathologic study of 3,000 dogs. Am J Vet Res 1961;22:355–369. [PubMed] [Google Scholar]
- 15. Rasmussen CE, Falk T, Zois NE, et al. Heart rate, heart rate variability, and arrhythmias in dogs with myxomatous mitral valve disease. J Vet Intern Med 2012;26:76–84. [DOI] [PubMed] [Google Scholar]
- 16. Doxey S, Boswood A. Differences between breeds of dogs in a measure of heart rate variability. Vet Rec 2004;154:713–717. [DOI] [PubMed] [Google Scholar]
- 17. Häggström J, Hamlin RL, Hansson K, Kvart C. Heart rate variability in relation to severity of mitral regurgitation in Cavalier King Charles Spaniels. J Small Anim Pract 1996;37:69–75. [DOI] [PubMed] [Google Scholar]
- 18. Akselrod S, Gordon D, Ubel FA, et al. Power spectrum analysis of heart rate fluctuation: A Quantative probe of beat‐to‐beat cardiovascular control. Science 1981;213:220–222. [DOI] [PubMed] [Google Scholar]
- 19. Stein PK, Kleiger RE. Insights from the study of heart rate variability. Annu Rev Med 1999;50:249–261. [DOI] [PubMed] [Google Scholar]
- 20. Malik M. Task force of the European Society of Cardiology and the North American Society of Pacing Electrophysiology: Heart rate variability—Standards of measurements, physiological interpretation, and clinical use. Circulation 1996;93:1043–1065. [PubMed] [Google Scholar]
- 21. Pagani M, Lombardi F, Guzzetti S, et al. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho‐vagal interaction in man and conscious dog. Circ Res 1986;59:178–193. [DOI] [PubMed] [Google Scholar]
- 22. Strauss‐Blasche G, Moser M, Voica M, et al. Relative timing of inspiration and expiration affects respiratory sinus arrhythmia. Clin Exp Pharmacol Physiol 2000;27:601–606. [DOI] [PubMed] [Google Scholar]
- 23. Saul JP. Beat‐to‐beat variations of heart rate reflect modulation of cardiac autonomic outflow. News Physiol Sci 1990;5:32–37. [Google Scholar]
- 24. Serrador JM, Finlayson HC, Hughson RL. Physical activity is a major contributor to the ultra low frequency components of heart rate variability. Heart 1999;82:9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Binder T, Frey B, Porenta G, et al. Prognostic value of heart rate variability in patients awaiting cardiac transplantation. Pace 1992;15:2215–2220. [DOI] [PubMed] [Google Scholar]
- 26. Dougherty CM, Burr RL. Comparison of heart rate variability in survivors and nonsurvivors of sudden cardiac arrest. Am J Cardiol 1992;70:441–448. [DOI] [PubMed] [Google Scholar]
- 27. Hull SS, Evans AR, Vanoli E, et al. Heart rate variability before and after myocardial infarction in conscious dogs at high and low risk of sudden death. J Am Coll Cardiol 1990;16:978–985. [DOI] [PubMed] [Google Scholar]
- 28. Stein KM, Borer JS, Hochreiter C, et al. Prognostic value and physiological correlates of heart rate variability in chronic severe mitral regurgitation. Circulation 1993;88:127–155. [DOI] [PubMed] [Google Scholar]
- 29. Stein PK. Assessing heart rate variability from real‐world holter reports. Card Electrophysiol Rev 2002;6:239–244. [DOI] [PubMed] [Google Scholar]
- 30. Moesgaard SG, Klostergard C, Zois NE, et al. Flow‐mediated vasodilation measurements in Cavalier King Charles Spaniels with increasing myxomatous mitral valve disease. J Vet Intern Med 2012;26:61–68. [DOI] [PubMed] [Google Scholar]
- 31. Thomas WP. Two‐dimensional, real‐time echocardiography in the dog: Technique and anatomic validation. Vet Radiol 1984;25:50–64. [Google Scholar]
- 32. Cornell CC, Kittleson MD, Della Torre P, et al. Allometric scaling of M‐mode cardiac measurements in normal adult dogs. J Vet Intern Med 2004;18:311–321. [DOI] [PubMed] [Google Scholar]
- 33. Häggström J, Hansson K, Karlberg BE, et al. Plasma concentration of atrial natriuretic peptide in relation to severity of mitral regurgitation in Cavalier King Charles Spaniels. Am J Vet Res 1994;55:698–703. [PubMed] [Google Scholar]
- 34. Oyama M. Advances in echocardiography. Vet Clin North Am Small Anim Pract 2004;34:1083–1104. [DOI] [PubMed] [Google Scholar]
- 35. Suzuki Y, Kambara H, Kadota K, et al. Detection and evaluation of tricuspid regurgitation using a real‐time, two‐dimensional, color‐coded, doppler flow imaging system: Comparison with contrast two‐dimensional echocardiography and right ventriculography. Am J Cardiol 1986;57:811–815. [DOI] [PubMed] [Google Scholar]
- 36. Pedersen HD, Häggström J, Falk T, et al. Auscultation in mild mitral regurgitation in dogs: Observer variation, effects of physical maneuvers, and agreement with color Doppler echocardiography and phonocardiography. J Vet Intern Med 1999;13:56–64. [PubMed] [Google Scholar]
- 37. Schober KE, Baade H. Doppler echocardiographic prediction of pulmonary hypertension in West Highland White Terriers with chronic pulmonary disease. J Vet Intern Med 2006;20:912–920. [DOI] [PubMed] [Google Scholar]
- 38. Petrie JP. Practical application of Holter monitoring in dogs and cats. Clin Tech Small Anim Pract 2005;20:173–181. [DOI] [PubMed] [Google Scholar]
- 39. Rasmussen CE, Vesterholm S, Ludvigsen TP, et al. Holter monitoring in clinically healthy Cavalier King Charles Spaniels, Wire‐haired Dachshunds, and Cairn Terriers. J Vet Intern Med 2011;25:460–468. [DOI] [PubMed] [Google Scholar]
- 40. Hall LW, Dunn JK, Delaney M, Shapiro LM. Ambulatory electrocardiography in dogs. Vet Rec 1991;129:213–216. [DOI] [PubMed] [Google Scholar]
- 41. Pedersen HD. Mitral Valve Prolapse in the Dog. Pathogenesis, Pathophysiology, Diagnosis and Comparative Aspects of Early Myxomatous Mitral Valve Disease. Copenhagen, Denmark: The Royal Veterinary and Agricultural University; 2000. Thesis. [Google Scholar]
- 42. Kittleson MD. Diagnosis and treatment of arrhythmias (dysrhythmias) In: Kittleson MD, Kienle RD, eds. Small Animal Cardiovascular Medicine. St. Louis, MO: Mosby; 1998:449–494. [Google Scholar]
- 43. Kligfield P, Devereux RB. Arrhythmia in mitral valve prolapse In: Podrid PJ, Kowey PR, eds. Cardiac Arrhythmias: Mechanisms, Diagnosis and Management. Philiadelphia, PA: Williams and Wilkins; 1995:1253–1266. [Google Scholar]
- 44. Tilley LP. Analysis of common canine cardiac arrhythmias In: Cann CC, Hunsberger SL, eds. Essentials of Canine and Feline Electrocardiography. Malvern, PA: Lea & Febiger; 1992:127–207. [Google Scholar]
- 45. Krahn AD, Klein GJ, Yee R, Skanes AC. Detection of asymptomatic arrhythmias in unexplained syncope. Am Heart J 2004;148:326–332. [DOI] [PubMed] [Google Scholar]
- 46. Moya A, Brignole M, Sutton R, et al. Reproducibility of electrocardiographic findings in patients with suspected reflex neurally‐mediated syncope. Am J Cardiol 2008;102:1518–1523. [DOI] [PubMed] [Google Scholar]
- 47. Kapoor WN. Evaluation and outcome of patients with syncope. Medicine (Baltimore) 1990;69:160–175. [DOI] [PubMed] [Google Scholar]
- 48. Soteriades ES, Evans JC, Larson MG, et al. Incidence and prognosis of syncope. N Engl J Med 2002;347:878–885. [DOI] [PubMed] [Google Scholar]
- 49. Johnson L, Boon J, Orton EC. Clinical characteristics of 53 dogs with Doppler‐derived evidence of pulmonary hypertension: 1992–1996. J Vet Intern Med 1999;13:440–447. [DOI] [PubMed] [Google Scholar]
- 50. Kellum HB, Stepien RL. Sildenafil citrate therapy in 22 dogs with pulmonary hypertension. J Vet Intern Med 2007;21:1258–1264. [DOI] [PubMed] [Google Scholar]
- 51. Pyle RL, Abbott J, MacLean H. Pulmonary hypertension and cardiovascular sequelae in 54 dogs. Intern J Appl Res Vet Med 2004;2:99–109. [Google Scholar]
- 52. Serres F, Chetboul V, Gouni V, et al. Diagnostic value of echo‐Doppler and tissue Doppler imaging in dogs with pulmonary hypertension. J Vet Intern Med 2007;21:1280–1289. [DOI] [PubMed] [Google Scholar]


