Abstract
Background
The prevalence of systemic hypertension (SHT) in Shetland Sheepdogs has not been reported.
Hypothesis/Objectives
SHT is common in Shetland Sheepdogs and positively correlated with proteinuria. Measurements of forelimb and hindlimb systolic arterial pressure (SAP) are comparable.
Animals
Seventy‐two clinically healthy, client‐owned Shetland Sheepdogs.
Methods
Forelimb and hindlimb SAP were recorded by Doppler ultrasonography. Proteinuria was quantified by urine dipstick, microalbuminuria, and protein:creatinine ratio (UPC). The relationship of UPC, anxiety, age, weight, and heart rate with forelimb SAP was evaluated.
Results
The mean forelimb and hindlimb SAP were 132 ± 20 and 118 ± 20 mmHg, respectively. The SAP exceeded 160 mmHg in 9 dogs, suggesting 13% prevalence of SHT. Four dogs had a UPC above 0.5; 2 of these had forelimb SAP exceeding 160 mmHg. Correlation of forelimb and hindlimb SAP was poor (r 2 = 0.09; P = .011). Bland–Altman plots revealed substantial bias (−14 mmHg) between limb measurements with clinically unacceptable 95% limits of agreement (−60 to 33 mmHg). There was no correlation between forelimb SAP and UPC (P = .06) or anxiety level (P = .49). Age (P < .0001) and heart rate (P = .038) were significant predictors of forelimb SAP; weight (P = .73) was not.
Conclusions
Prevalence of SHT was 13% and not correlated with proteinuria. Forelimb and hindlimb SAP were poorly correlated; therefore, trends in an individual animal should be monitored using the same measurement site. Additionally, values for Doppler SAP were determined in Shetland Sheepdogs.
Keywords: Chronic kidney disease, Doppler ultrasonography, Proteinuria, Systemic hypertension
Abbreviations
- CKD
chronic kidney disease
- IQR
interquartile range
- SAP
systolic arterial pressure
- SHT
systolic arterial hypertension
- UPC
urine protein:creatinine ratio
Systemic hypertension is defined as an elevation from normal in systolic arterial blood pressure (SAP), diastolic arterial blood pressure, or both. Although isolated diastolic hypertension has been observed in animals, SAP is more commonly evaluated in veterinary medicine, given the devices available for measurement and the belief that systolic arterial hypertension (SHT) is a more important determinant of target organ injury.1 Several methods are available for noninvasive estimation of SAP in dogs, including Doppler ultrasound, standard oscillometry, and high‐definition oscillometry. Each measurement device has potential drawbacks, and few studies have been performed in conscious animals validating these techniques. Two studies suggest that evaluation of SAP by Doppler techniques has less variation and measurement error while also taking less time to acquire in conscious dogs, as compared with standard or high‐definition oscillometry.2, 3 As such, Doppler ultrasound was the technique employed in this study. In addition to method of measurement, clinical experience has questioned the correlation between SAP measured in the forelimb as compared with SAP measurements in the hindlimb of the same dog. Some studies have reported different cut‐off values for diagnosing SHT, depending on whether the measurement was performed on the tail as compared with a limb,4, 5 although the authors are not aware of studies comparing forelimb with hindlimb Doppler SAP measurements in the same dog.
A recent American College of Veterinary Internal Medicine expert consensus statement1 on SHT in dogs advocated for the development of breed‐specific normal values, because epidemiologic studies suggested that breed accounted for important variation (15.2%) in SAP.6 However, reported breed‐specific studies have been limited to Irish Wolfhounds,7 Kangal dogs,8 Greyhounds,9 and a large‐scale epidemiologic study that sorted dogs into breed categories (eg, sight hounds, retrievers).6 In addition to breed, age has inconsistently been documented as a positive correlate to SAP in dogs.6, 7
Chronic kidney disease (CKD) is a known cause of SHT in dogs; additionally, SHT as a comorbidity in CKD has been reported to be a risk factor for development of uremic crises and to result in reduced survival as compared with nonhypertensive dogs with CKD.1, 10, 11 Prior reports of CKD have also documented a correlation between SAP and degree of proteinuria.10 We have the clinical impression that the Shetland Sheepdog is overrepresented among dogs with SHT and CKD and a recent retrospective study reported this breed as the fourth most common breed affected with CKD.12 Familial kidney disease has not yet been described in Shetland Sheepdogs, although 2 cases of bilateral renal agenesis have been reported.13
These reports and our clinical interest in the breed stimulated the hypothesis that SHT may be identified in clinically healthy Shetland Sheepdogs. To address the hypothesis that SHT is prevalent in these dogs, we measured SAP in a convenience sample of 72 clinically healthy Shetland Sheepdogs. A second hypothesis was that SAP would correlate with proteinuria and age in this breed as has been previously reported in other populations.6, 7, 10 In addition, we addressed the hypothesis that the values for SAP obtained from 2 limbs were not significantly different. Thus, the goals of this study were the determination of SHT prevalence in a general population of healthy Shetland Sheepdogs and a comparison of limb sites for determination of SAP. Additional study goals included exploring the relationships between SAP and other factors that might influence SAP, namely age, weight, gender, anxiety level, and protein content in the urine. Although SHT may arise for many reasons (CKD, primary or essential hypertension, adrenal disease, etc), a full screening of potential causes for SHT in these dogs was beyond the scope of this preliminary study.
Materials and Methods
Study Population
A convenience sample of 72 apparently healthy Shetland Sheepdogs were enrolled in this study. Recruitment was directed toward local breeders, Shetland Sheepdog interest groups, and rescue organizations in an effort to enroll dogs of varying age and sex. Dogs exhibiting signs of illness or extreme anxiousness, or those known to have a systemic disease, were excluded from the study. Dogs receiving prescription medications were excluded; standard preventative medications against heartworm infection and fleas were allowed. Client surveys were used to identify clinical signs suggestive of underlying kidney disease; animals with atypical urinary habits or reported to have abnormal water or food consumption were excluded.
Blood Pressure Assessment
All SAP measurements were performed by the same trained observer (J.V.). Each dog was allowed to acclimate to the environment for at least 10 minutes prior to evaluation. The dog was placed in right lateral recumbency and the limb circumference measured to determine appropriate cuff sizing. The cuff size was chosen using 40% of the limb circumference as the optimal cuff diameter. Limbs were measured at the mid‐point of the antebrachium for the left forelimb, or just below the tarsus for the left hindlimb. The client was encouraged to stay with his/her dog and considerable effort was made to minimize anxiety before data collection. The Doppler ultrasound crystal was placed over the median palmar or medial plantar superficial artery for the forelimb and hindlimb measurements, respectively. The acoustic signal was amplified with a standard Doppler ultrasonic flow detector.1 The limb was held perpendicular to the body and at a vertical elevation roughly corresponding to the xiphoid to approximate the position of the heart in the thorax. The cuff was gradually inflated until the acoustic signal was inaudible and then slowly deflated until the acoustic signal returned. The pressure at which the acoustic signal was first audible during cuff deflation was recorded as the SAP. The first reading was discarded and the next 5 or more consecutive measurements that yielded <15% variability were recorded and averaged as the dog's SAP. The limb acquisition sequence for SAP measurement was varied with the intent to randomly perform either the hindlimb or the forelimb first. However, upon study completion, it became clear that the two groups were not equal as 23 dogs had the forelimb measurement performed first and 49 dogs had the hindlimb measurement performed first. The second measurement (forelimb or hindlimb, dependent upon which was first performed) was obtained in the same manner and within 5 minutes of the previous limb's measurement. A qualitative assessment of the dog's anxiety/excitement level was also recorded at the time of SAP measurement by the same examiner (J.V.). Anxiety was scored on a subjective scale of 4 increments, from 0 to 3, where 0 signified calm (the dog remained in lateral recumbency without restraint); 1 signified mild anxiety (the dog required light manual restraint); 2 signified moderate anxiety (the dog required light manual restraint as well as active verbal intervention, petting, or both from the owner to remain in lateral recumbency); and 3 signified high anxiety (the dog was vocalizing, requiring owner intervention or the dog required active restraint to maintain the lateral recumbent position).
Urinalysis
Morning urine samples were collected from 61 dogs via free catch; 11 owners allowed urine collection by cystocentesis. Urine samples were analyzed by digital refractometry2 to determine urine specific gravity. The degree of proteinuria was assessed by qualitative chemistry dipstick,3 a semiquantitative microalbuminuria assay,4 and by urine protein:creatinine ratio (UPC).5 All samples were analyzed the day of recovery for specific gravity, dipstick, and microalbuminuria. UPC was analyzed within 48 hours of sampling, refrigerated before analysis, and performed in batches.
Statistical Analyses
Statistical analyses were performed with commercially available software.6,7 Descriptive statistics were calculated for all data and normality was assessed with the D'Agostino & Pearson normality test. Normally distributed data are presented as mean ± SD and range; data that failed normality are reported as median with interquartile range (IQR) and total range. Forelimb versus hindlimb SAP was compared by linear regression and the Pearson correlation coefficient (r). Forelimb versus hindlimb SAP measurements were also evaluated for bias and for limits of agreement by the method of Bland and Altman.14 A t‐test was employed to analyze the order of measurement to identify any systematic difference created by the sampling order. Specifically, the forelimb SAP measurements were compared between those dogs that had the forelimb measured first and those that had the forelimb measured second; the same was done for the hindlimb SAP measurements. In addition, comparisons were made of all first SAP measurements compared with all second SAP measurements. Finally, the difference between the first and second measurement was compared for both groups (whether hindlimb first or forelimb first). A multiple linear regression model evaluated the relationship between the dependent variable SAP and the predictors age, weight, and heart rate. Data were transformed to a normal distribution before entering into the model. UPC could not be transformed to normality, given the presence of outliers and therefore was not included in the model. The relationships between anxiety level and UPC to SAP were evaluated by the Spearman rank correlation (ρ). The comparison between male and female SAP and the comparison between the first or second SAP measurement were made using Student's t‐test. Significance was defined as P < .05.
Results
Seventy‐two Shetland Sheepdogs were enrolled with the sample comprised of 34 males (4 intact) and 38 females (5 intact). The median age was 66 months (IQR: 34–119 months) with a range of 4–164 months. The mean weight was 11.3 ± 3.6 kg and the median body condition score (on a 1–5 point scale) was 3 with a range of 2–4.5. Color variation of the Shetland Sheepdogs included sable (n = 47), tricolor (12), blue merle (7), bi‐black (3), tan & white (1), mahogany sable (1), and cryptic blue (1).
Blood pressure was obtained in all dogs from both the forelimb (mean: 132 ± 20 mmHg; range: 101–183 mmHg) and the hindlimb (mean: 118 ±20 mmHg; range: 76–164 mmHg). Median perceived anxiety score during SAP measurement was 1 (IQR: 1–2; range: 0–3). Nine dogs (13%) had a forelimb or hindlimb SAP that exceeded 160 mmHg, a value that has been reported to be compatible with a diagnosis of SHT and a moderate risk for target organ injury.1 These 9 dogs were considered as suspect hypertensive dogs and determination of the 95% confidence intervals for SAP was repeated with these 9 cases removed from analysis. The forelimb SAP, hindlimb SAP, and averaged forelimb and hindlimb SAP for all dogs, and for the 63 dogs with SAP < 160 mmHg, are presented in Table 1. Paired forelimb/hindlimb measurements correlated poorly (r 2 = 0.09, P = .011; Fig 1). Bland‐Altman analysis (Fig 2) of forelimb versus hindlimb SAP showed substantial bias (−14 ± 24 mmHg) with wide limits of agreement (95% limits of agreement, −60 mmHg to 33 mmHg). In evaluating the order of measurement (eg, whether forelimb first or hindlimb first), the values for the first SAP measurement site were not significantly different than the second SAP measurement site (P = .47; Fig 3). The values for the forelimb SAP also did not differ whether they were performed first or second (P = .15); similarly, the values for the hindlimb SAP were not significantly different whether they were performed first or second (P = .73; Fig 3). Considering the poor correlation between the 2 sites, and a prior study that suggested a stronger correlation between noninvasive forelimb SAP and directly recorded pressure,15 all further statistical comparisons were made using forelimb SAP. The multiple linear regression model found age (t statistic = 4.76, P < .0001) and heart rate (t statistic = 2.11, P = .038) as significant independent predictors of forelimb SAP. Weight was not a significant predictor of forelimb SAP (t statistic = −0.34, P = .73). There was no significant collinearity between the variables used in the model. Anxiety level was not correlated with forelimb SAP (P = .49). There was no difference in forelimb SAP between male and female sex (P = .13).
Table 1.
Systolic arterial pressure (SAP) measurements from 72 apparently healthy Shetland Sheepdogs. The mean (±SD) as well as the 95% confidence intervals of forelimb SAP, hindlimb SAP, and averaged forelimb/hindlimb SAP for all dogs as well as for the 63 dogs with SAP < 160 mmHg are shown.
| SAP in All Dogs (N = 72) | All Dogs with SAP < 160 mmHg (N = 63) | |||||
|---|---|---|---|---|---|---|
| Forelimb (mmHg) | Hindlimb (mmHg) | Averaged Forelimb and Hindlimb (mmHg) | Forelimb (mmHg) | Hindlimb (mmHg) | Averaged Forelimb and Hindlimb (mmHg) | |
| Mean ± SD | 132 ± 20 | 118 ± 20 | 125 ± 16 | 127 ± 15 | 117 ± 19 | 122 ± 13 |
| Lower 95% CI | 102 | 81 | 100 | 102 | 79 | 99 |
| Upper 95% CI | 177 | 159 | 166 | 157 | 153 | 152 |
SAP, systolic arterial pressure; SD, standard deviation; CI, confidence interval.
Figure 1.
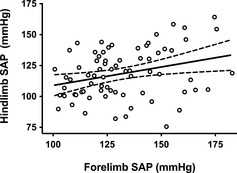
Scatterplot and linear regression of forelimb and hindlimb systolic arterial pressure (SAP) obtained from 72 healthy Shetland Sheepdogs. The best‐fit regression line (solid) and 95% confidence intervals for the regression line (dotted lines) are shown. Forelimb and hindlimb systolic arterial pressure were weakly correlated (Pearsons r 2 = 0.09; P = .011).
Figure 2.
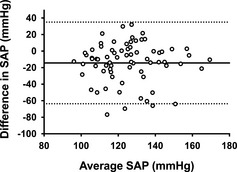
Bland‐Altman plot of the difference compared to the average for each forelimb and hindlimb systolic arterial pressure (SAP) measurement from 72 healthy Shetland Sheepdogs. The horizontal solid line represents the mean difference or bias of −14 mmHg in systolic arterial pressure between the hindlimb minus forelimb methods. The dashed lines represent ± 1.96 SD, or the 95% limits of agreement (−60 to 33 mmHg).
Figure 3.
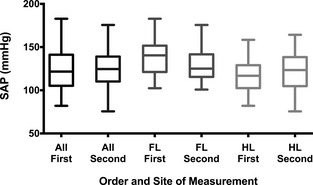
Box and whiskers plot of systemic arterial pressure (SAP) measurements by order of measurement. The boxes extend from the 25th to the 75th percentile with the median shown by the midline; the whiskers are drawn from the lowest to the highest value (eg, entire range). The left 2 boxes in black show all the measurements of SAP obtained first (All First) as compared with all SAP measurements obtained second (All Second). The middle 2 boxes in dark gray show all the forelimb SAP measurements obtained first (FL First) as compared with all forelimb measurements obtained second (FL Second). The right 2 boxes in light gray show all the hindlimb SAP measurements obtained first (HL First) as compared with all hindlimb measurements obtained second (HL Second). No systematic difference was evident among any of the 3 comparisons (All First versus All Second, P = .47; FL First versus FL Second, P = .15; HL First versus HL Second, P = .73).
Mean urine specific gravity was 1.038 ± 0.013 with a range of 1.007–1.059; 6 dogs (8%) had a value below 1.020. Urine dipstick results for protein were negative in 2 dogs (3%), trace in 47 dogs (65%), 1+ in 17 dogs (24%), 2+ in 2 dogs (3%), and 3+ in 4 dogs (6%). While urine dipstick results suggested trace or higher proteinuria in most dogs (97%), these results were unsupported by the microalbuminuria and UPC data. By microalbuminuria detection, 56 dogs (78%) were negative, 7 dogs (10%) were low positive, 6 dogs (8%) were medium positive, 2 dogs (3%) were high positive, and 1 dog (1%) was very high positive. Median UPC was 0.09 (IQR: 0.07–0.15; range: 0.05–3.80). Four dogs (6%) had a measured UPC greater than 0.5, and 9 dogs (13%) had a UPC between 0.2 and 0.5. The 4 dogs with a UPC greater than 0.5 were 40, 70, 120, and 120 months of age; 2 of these 4 had a forelimb SAP above 160 mmHg. Of the 9 dogs with an equivocal elevation in UPC (UPC of 0.2–0.5), none had a measured SAP above 160 mmHg. For all dogs, there was no correlation between degree of proteinuria as assessed by UPC and the forelimb SAP (P = .06).
Discussion
This prospective study determined forelimb and hindlimb SAP in a sample of apparently healthy Shetland Sheepdogs as measured by noninvasive Doppler ultrasonography. This study also showed an overall poor correlation between measured forelimb and hindlimb SAP in these dogs.
The mean SAP for this sample, 132 mmHg in the forelimb and 118 mmHg in the hindlimb, is lower than previously reported studies of Doppler SAP in mixed breeds in which SAP values of 145 mmHg16 to 151 mmHg17 were reported. In a single‐breed study of the Kangal dog, the average SAP by Doppler of 146 mmHg was also higher than found in the Shetland Sheepdogs of this study.8 The degree of animal anxiety and resultant lability of SAP may account for some of this variation as all dogs in this study had the owner present and were in a calm, quiet environment. As recently reported in other breeds, the presence of the owner during blood pressure measurement probably results in a lower measured SAP.18 The SAP for the Shetland Sheepdog by Doppler ultrasonography, based upon these data, should be considered lower than what has been reported in other studies for dogs in general and what might be obtained in a typical clinical setting.
The values for SAP reported in the current study are comparable to larger surveys that used oscillometric devices to estimate SAP. In the largest survey to date of 1,267 healthy dogs, average SAP for all dogs was 131 mmHg.6 While the Shetland Sheepdog was not reported as a discrete breed in the study by Bodey and Michell,6 the average SAP of Collies, of which the Shetland Sheepdog shares a genetic lineage, was reported to be 133 mmHg, which is similar to the results reported here.
The forelimb and hindlimb measurements of SAP in this study showed wide limits of agreement and poor correlation. The 2007 American College of Veterinary Internal Medicine expert consensus statement on systemic hypertension did not address the site of measurement on variation in blood pressure,1 although other studies have reported different cut‐off values for diagnosing SHT depending on whether the measurement was performed on the tail as compared with a limb.4, 5 There is 1 study comparing forelimb and hindlimb Doppler SAP in retired racing Greyhounds.9 That study found that the hindlimb SAP was significantly higher than the forelimb SAP, the opposite effect found in the study reported here, although the hindlimb measurement was consistently performed first in the earlier study of Greyhounds.9 The reason for the difference in forelimb versus hindlimb SAP in this study is not clear. These differences could reflect physiologic, anatomic, or technical factors that differ between the 2 sites. Inasmuch as forelimb and hindlimb measurements in each dog were not simultaneous, both were acquired within minutes of the other and under static conditions. Additionally, the order of measurement (forelimb or hindlimb first, followed by the other) varied, although the randomization procedure failed to create an equal number of forelimb‐ and hindlimb‐first measurements. However, when the groups (forelimb first versus hindlimb first) were compared, there was no difference in SAP found between those that had the forelimb measured first and those that had the hindlimb measured first. It is therefore unlikely that the SAP in these dogs changed substantially between forelimb and hindlimb measurements in a consistent way unless the timing of the measurement inserted a consistent bias. Moreover, results of before and after analysis showed no difference between the order of measurement, suggesting that there was no trend in either the first or second measurement being consistently lower or higher than the other. This discrepancy has clinical consequence and suggests that if Doppler methods are used, the measurement of SAP in dogs should be performed consistently on the same limb if trends in an individual dog's SAP will be monitored over time. These data also provide further evidence that Doppler ultrasonographic measurement of SAP in dogs is suboptimal. As has been shown in other studies, current methods including oscillometric devices and Doppler ultrasonography do not always correlate well with one another, nor with invasive measurements.2, 3, 4, 15, 16, 17
Age was a predictor of forelimb SAP in this population of Shetland Sheepdogs. While the correlation between age and SAP has not proven significant in all studies of canine blood pressure,1 our findings support the study of Bodey and Michell,6 as well as the single‐breed studies of Irish Wolfhounds7 and Kangal dogs,8 which found a significant correlation between age and SAP. In the epidemiologic study of Bodey and Michell, age accounted for 57.5% of the variation in the data.6 An increase in SAP with age is known to occur in humans and is explained by an increase in stiffening of the large arteries with subsequent increases in input impedance and early wave reflection (eg, diminished Windkessel effect).19 Whether arterial stiffness accounts for the increase in SAP with advancing age in dogs is unknown. Additionally, the prevalence of CKD or other disorders associated with hypertension, such as hyperadrenocorticism, might be expected to increase with age.
In addition to age, heart rate was a significant predictor of forelimb SAP, although perceived anxiety score was not. Few studies have evaluated the relationship of heart rate to SAP in dogs, although some have used heart rate as a marker of anxiety level.7 Heart rate has a direct effect on cardiac output and SAP while also serving as a marker of sympathetic tone and therefore systemic vascular resistance, an additional component of SAP. Our results suggest that heart rate was an independent predictor of SAP in these dogs, but it is unclear whether this was because of a direct effect on cardiac output, a marker of elevated sympathetic tone, or both. The study investigating white coat hypertension in retired racing Greyhounds9 found that in acclimatized dogs, heart rate decreased but SAP did not, suggesting that heart rate may not be an accurate indication of stress level in dogs and that the interplay of heart rate, systemic vascular resistance, sympathetic tone, and SAP is complex. Other studies have shown an interaction between temperament (eg, perceived anxiety) and SAP in dogs.6, 7, 9 The reason that our data did not find a significant effect of perceived anxiety on SAP may suggest that our ability to assess actual anxiety level in dogs undergoing blood pressure measurement is poor, or that the categorization of anxiety is too broad to identify changes within the window of SAP recorded here.
Proteinuria, if defined as a UPC greater than 0.5,20 was evident in just a small proportion (6%) of these apparently healthy dogs. Equivocal proteinuria, defined as a UPC between 0.2 and 0.5,20 was present in approximately one‐tenth of the dogs. Proteinuria and SHT share a complex relationship, with SHT known to cause proteinuria by overwhelming the kidney's autoregulatory mechanism and resulting in an increased glomerular capillary pressure. The increased pressure in the glomerular capillaries may then result in mesangial cell hyperplasia, glomerulosclerosis, and finally excessive protein loss through the kidney.21 Conversely, naturally occurring glomerular disease is a known cause of SHT in dogs.22 In this study, no significant correlation was found between SAP and UPC. This probably relates to the small number of dogs in this study as well as the low number of dogs with an elevated SAP. There are insufficient data to identify any relationship between proteinuria and SHT in this sample of healthy dogs. However, it is interesting to note that of the 9 (13%) dogs with SAP > 160 mmHg, only 2 had a UPC greater than 0.2. Whether the elevated SAP in these dogs reflected anxiety, undiagnosed conditions leading to secondary SHT, or idiopathic SHT is unknown. None of the dogs of this study had clinical signs of disease; however, complete diagnostic evaluations to definitively rule out underlying causes of SHT were not performed.
This study supports recent evidence that evaluation of proteinuria in dogs by urine dipstick is questionable and of low positive predictive value.23 Seventy of the 72 dogs (97%) in this study had at least trace proteinuria by urine dipstick analysis, although only 13 (18%) had a UPC greater than 0.2. False positive reactions are known to occur with the colorimetric urine dipstick test,23 a finding confirmed by these data and an important consideration when evaluating the urine of dogs for protein.
There are several limitations of this study. As these were client‐owned and otherwise healthy dogs, noninvasive SAP measurement was performed rather than direct arterial puncture. Noninvasive measurement of blood pressure in dogs is imperfect, with wide coefficients of variation and poor agreement between and within devices.3 However, this remains the accepted clinical standard in conscious dogs and both Doppler and oscillometric techniques are used widely in veterinary medicine. Furthermore, we chose a cut‐off value for suspected SHT of 160 mmHg based on current guidelines; however, it is unknown if this is an accurate cut‐off value for SHT in this particular breed. Additionally, and as noted above, the forelimb and hindlimb SAP measurements were not simultaneous nor effectively randomized; however, close temporal proximity, variable order of collection, and the static conditions during measurement make it unlikely that substantial changes in SAP occurred between each measurement. An additional limitation is the single time point of measurement in these dogs. Blood pressure is a labile physiologic parameter, affected by anxiety level and hydration status. Conclusive diagnosis of SHT should be based on either evidence of target organ damage, repeatable and consistently elevated measurements of SAP above a known reference interval, or both. Last, the dogs of this study did not undergo exhaustive testing for secondary diseases known to be associated with SHT (eg, renal disease, adrenal disease, other endocrine disorders). However, the population studied represents a real‐world cross‐section of apparently healthy Shetland Sheepdogs as might be encountered in practice.
In summary, the prevalence of SHT in this population of Shetland Sheepdogs was determined to be 13% using a cut‐off value of 160 mmHg. Few of the animals with elevated SAP displayed proteinuria and forelimb SAP was not correlated with the degree of proteinuria. Positive reactions for dipstick protein also did not predict elevated UPC. Mean values for SAP were determined in Shetland Sheepdogs obtained by Doppler ultrasonography and were in agreement, although slightly lower than those reported in previous canine studies. Importantly, the forelimb and hindlimb SAP measurements showed significant bias and wide limits of agreement, making it impossible to use forelimb and hindlimb measures interchangeably. Accordingly, serial measurements of SAP for an individual dog should be performed on the same limb. Further studies are necessary to define any association between SHT and proteinuria in Shetland Sheepdogs.
Acknowledgments
This study was supported by a gift from John and Leota Folsom.
Conflict of Interest: The digital refractometer used in this study was donated by the company to DJ Chew. DJ Chew serves on the Nestle Purina advisory board and the IDEXX renal advisory board. The other authors declare no conflict relative to the material contained in this manuscript.
This work was performed at the Ohio State University, Columbus, Ohio.
Footnotes
Model 811‐B Doppler Ultrasonic Flow Detector, Parks Medical Electronics, Inc, Aloha, OR
VETMED01 Clinical Veterinary Refractometer, MISCO Refractometer, Cleveland, OH
Chemstrip 7, Roche Diagnostics, Indianapolis, IN
Canine E.R.D.‐HealthScreen Urine Test, HESKA Corporation, Loveland, CO
Roche Hitachi cobas c 501, Roche Diagnostics
GraphPad Prism 6, GraphPad Software, Inc, La Jolla, CA
SYSTAT 13, Systat Software, Inc, Chicago, IL
References
- 1. Brown S, Atkins C, Bagley R, et al. Guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. J Vet Intern Med 2007;21:542–558. [DOI] [PubMed] [Google Scholar]
- 2. Hsiang TY, Lien YH, Huang HP. Indirect measurement of systemic blood pressure in conscious dogs in a clinical setting. J Vet Med Sci 2008;70:449–453. [DOI] [PubMed] [Google Scholar]
- 3. Wernick MB, Hopfner RM, Francey T, et al. Comparison of arterial blood pressure measurements and hypertension scores obtained by use of three indirect measurement devices in hospitalized dogs. J Am Vet Med Assoc 2012;240:962–968. [DOI] [PubMed] [Google Scholar]
- 4. Stepien RL, Rapoport GS, Henik RA, et al. Comparative diagnostic test characteristics of oscillometric and Doppler ultrasonographic methods in the detection of systolic hypertension in dogs. J Vet Intern Med 2003;17:65–72. [DOI] [PubMed] [Google Scholar]
- 5. Bodey AR, Young LE, Bartram DH, et al. A comparison of direct and indirect (oscillometric) measurements of arterial blood pressure in anaesthetised dogs, using tail and limb cuffs. Res Vet Sci 1994;57:265–269. [DOI] [PubMed] [Google Scholar]
- 6. Bodey AR, Michell AR. Epidemiological study of blood pressure in domestic dogs. J Small Anim Pract 1996;37:116–125. [DOI] [PubMed] [Google Scholar]
- 7. Bright JM, Dentino M. Indirect arterial blood pressure measurement in nonsedated Irish Wolfhounds: Reference values for the breed. J Am Anim Hosp Assoc 2002;38:521–526. [DOI] [PubMed] [Google Scholar]
- 8. Sanan TB, Arslan M. Determination of reference arterial blood pressure values by indirect methods for Kangal dogs. J Small Anim Pract 2007;48:638–642. [DOI] [PubMed] [Google Scholar]
- 9. Marino CL, Cober RE, Iazbik MC, et al. White‐coat effect on systemic blood pressure in retired racing Greyhounds. J Vet Intern Med 2011;25:861–865. [DOI] [PubMed] [Google Scholar]
- 10. Wehner A, Hartmann K, Hirschberger J. Associations between proteinuria, systemic hypertension and glomerular filtration rate in dogs with renal and non‐renal diseases. Vet Rec 2008;162:141–147. [DOI] [PubMed] [Google Scholar]
- 11. Jacob F, Polzin DJ, Osborne CA, et al. Association between initial systolic blood pressure and risk of developing a uremic crisis or of dying in dogs with chronic renal failure. J Am Vet Med Assoc 2003;222:322–329. [DOI] [PubMed] [Google Scholar]
- 12. Parker VJ, Freeman LM. Association between body condition and survival in dogs with acquired chronic kidney disease. J Vet Intern Med 2011;25:1306–1311. [DOI] [PubMed] [Google Scholar]
- 13. Brownie CF, Tess MW, Prasad RD. Bilateral renal agenesis in two litters of Shetland Sheepdogs. Vet Hum Toxicol 1988;30:483–485. [PubMed] [Google Scholar]
- 14. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307–310. [PubMed] [Google Scholar]
- 15. Bodey AR, Michell AR, Bovee KC, et al. Comparison of direct and indirect (oscillometric) measurements of arterial blood pressure in conscious dogs. Res Vet Sci 1996;61:17–21. [DOI] [PubMed] [Google Scholar]
- 16. Chalifoux A, Dallaire A, Blais D, et al. Evaluation of the arterial blood pressure of dogs by two noninvasive methods. Can J Comp Med 1985;49:419–423. [PMC free article] [PubMed] [Google Scholar]
- 17. Stepien RL, Rapoport GS. Clinical comparison of three methods to measure blood pressure in nonsedated dogs. J Am Vet Med Assoc 1999;215:1623–1628. [PubMed] [Google Scholar]
- 18. Hoglund K, Hanas S, Carnabuci C, et al. Blood pressure, heart rate, and urinary catecholamines in healthy dogs subjected to different clinical settings. J Vet Intern Med 2012;26:1300–1308. [DOI] [PubMed] [Google Scholar]
- 19. Franklin SS, Gustin WT, Wong ND, et al. Hemodynamic patterns of age‐related changes in blood pressure. The Framingham Heart Study. Circulation 1997;96:308–315. [DOI] [PubMed] [Google Scholar]
- 20. Lees GE, Brown SA, Elliott J, et al. Assessment and management of proteinuria in dogs and cats: 2004 ACVIM Forum Consensus Statement (small animal). J Vet Intern Med 2005;19:377–385. [DOI] [PubMed] [Google Scholar]
- 21. Ljutic D, Kes P. The role of arterial hypertension in the progression of non‐diabetic glomerular diseases. Nephrol Dial Transplant 2003;18(Suppl 5):v28–v30. [DOI] [PubMed] [Google Scholar]
- 22. Cook AK, Cowgill LD. Clinical and pathological features of protein‐losing glomerular disease in the dog: A review of 137 cases (1985‐1992). J Am Anim Hosp Assoc 1996;32:313–322. [DOI] [PubMed] [Google Scholar]
- 23. Lyon SD, Sanderson MW, Vaden SL, et al. Comparison of urine dipstick, sulfosalicylic acid, urine protein‐to‐creatinine ratio, and species‐specific ELISA methods for detection of albumin in urine samples of cats and dogs. J Am Vet Med Assoc 2010;236:874–879. [DOI] [PubMed] [Google Scholar]


