Abstract
Background
Maximal aldosterone secretion in healthy dogs occurs 30 minutes postadrenocorticotropin (ACTH; 5 μg/kg IV) stimulation. The effect of trilostane and mitotane on aldosterone at that time is unknown.
Objectives
To assess the effect of trilostane and mitotane in dogs with pituitary‐dependent hyperadrenocorticism on aldosterone secretory reserve. To determine if aldosterone concentration correlates with electrolyte concentrations.
Animals
Serum collected from 79 client‐owned dogs and 33 stored samples.
Methods
Client‐owned dogs had ACTH stimulation tests with cortisol concentrations measured at 0 and 60 minutes and aldosterone concentrations measured at 0, 30, and 60 minutes. Stored samples had aldosterone concentrations measured at 0 and 60 minutes. Ten historical clinically healthy controls were included. All had basal sodium and potassium concentrations measured.
Results
The aldosterone concentrations in the mitotane‐ and trilostane‐treated dogs at 30 and 60 minutes post‐ACTH were significantly lower than in clinically healthy dogs; no significant difference was detected in aldosterone concentration between 30 and 60 minutes in treated dogs. However, a significantly higher percentage of dogs had decreased aldosterone secretory reserve detected at 30 minutes than at 60 minutes. At 30 minutes, decreased secretory reserve was detected in 49% and 78% of trilostane‐ and mitotane‐treated dogs, respectively. No correlation was detected between aldosterone and serum electrolyte concentrations.
Conclusions and Clinical Importance
Decreased aldosterone secretory reserve is common in trilostane‐ and mitotane‐treated dogs; it cannot be predicted by measurement of serum electrolyte concentrations. Aldosterone concentration at 30 minutes post‐ACTH stimulation identifies more dogs with decreased aldosterone secretory reserve than conventional testing at 60 minutes.
Keywords: Cushing's disease, Lysodren, Pituitary‐dependent hyperadrenocorticism, Vetoryl
Abbreviations
- ACTH
adrenocorticotropin
- PDH
pituitary‐dependent hyperadrenocorticism
The 2 most effective medical treatments for pituitary‐dependent hyperadrenocorticism (PDH) are trilostane (Vetoryl) and mitotane (o,p'‐DDD, Lysodren). They appear to be comparable in safety and efficacy.1, 2, 3, 4, 5
Mitotane causes preferential necrosis of the adrenocortical zona fasciculata and zona reticularis, the zones responsible for producing cortisol and sex hormones. It can decrease aldosterone concentrations.6, 7 In 6–10% of dogs receiving mitotane, the zona glomerulosa (site of aldosterone production) is destroyed.4, 8
Trilostane is a reversible, competitive inhibitor of the adrenocortical enzyme 3β‐hydroxysteroid dehydrogenase.9 Its administration effectively blocks cortisol secretion and, to a lesser extent, that of aldosterone.9, 10 Adrenocortical necrosis has been reported in dogs on trilostane treatment11, 12 and in 1 dog hyperkalemia and hyponatremia were observed.11 A potential marker of aldosterone inhibition, hyperkalemia, has been documented in dogs on trilostane treatment,1, 2, 10, 13 but no correlation was detected between serum potassium and basal aldosterone concentrations, even in hyperkalemic dogs.10
Aldosterone deficiency has potentially dangerous consequences, such as hyperkalemia, hyponatremia, severe volume depletion, hypotension, and death. Only serum electrolyte concentrations are routinely monitored to assess for the presence of mineralocorticoid insufficiency during treatment of PDH. By the time serum electrolyte concentration changes occur, some dogs have clinical signs of hypoadrenocorticism. Assessing aldosterone secretory capability may be a better monitoring tool. The adrenocorticotropin (ACTH) stimulation test has been used to evaluate aldosterone secretion in dogs receiving trilostane or mitotane, but stimulated aldosterone concentration was only measured at 60 minutes post‐ACTH administration.7, 9, 10, 14 The optimal sampling time may be at the point of maximal secretion, which occurs in healthy dogs at 30 minutes after a 5 μg/kg dose of cosyntropin (synthetic ACTH) administered IV.1 The 30‐minute time point has never been evaluated, to our knowledge, in dogs receiving trilostane or mitotane. In addition, the effect of mitotane and trilostane on aldosterone secretory ability in dogs with PDH has not been directly compared.
In dogs made hypocortisolemic with mitotane or trilostane, it is impossible to predict if and when adrenal function will recover. Dogs with damage to the zona glomerulosa with resultant hypoaldosteronism may be less likely to recover because the zona glomerulosa contains the progenitor cells for the other layers of the adrenal glands. Thus, measuring aldosterone as well as cortisol may be a more efficient means of evaluating adrenal damage and may allow for prediction of recovery.
Therefore, the first goal of our study was to assess and compare the effect of treatment with trilostane and mitotane in dogs with PDH on aldosterone secretory reserve at 30 and 60 minutes post‐ACTH stimulation. The second goal was to determine if changes in aldosterone concentration at the 30‐minute time point, the peak in healthy dogs, correlate with changes in serum sodium and potassium concentrations. We hypothesized that measurement at 30 minutes post‐ACTH would identify greater suppression of aldosterone secretory reserve than would measurement at 60 minutes and that serum electrolyte concentrations would correlate with the 30‐minute aldosterone concentrations. A third goal was to determine if, as we hypothesized, decreased aldosterone secretory reserve was predictive of adrenal recovery.
Materials and Methods
Case Selection
Client‐owned dogs being treated for PDH with either mitotane or trilostane were enrolled. Participants were enrolled at 2 university veterinary teaching hospitals, 1 specialty private practice, and 3 general practices. Diagnosis was made on the basis of consistent history, physical examination findings, and routine laboratory testing along with the results of an ACTH stimulation test, low‐dose dexamethasone suppression test, or both; a urine cortisol:creatinine ratio also may have been determined. An adrenal tumor and PDH were differentiated using a high‐dose dexamethasone suppression test, measurement of endogenous ACTH concentration, or abdominal ultrasound examination.
The dogs could have been treated for any duration before enrollment and could have been in the loading or maintenance phase of mitotane treatment. Dogs could participate in the study up to 4 times. Dogs were excluded if they had pre‐existing renal disease, were receiving any treatment for heart disease (eg, angiotensin‐converting enzyme inhibitors, diuretics, pimobendan), had received repositol glucocorticoid treatment within 60 days, or had received progestin treatment, systemic azole treatment, or anesthesia within 7 days. For inclusion the first time, dogs could not have received glucocorticoid treatment of any form within 30 days. Dogs documented to be hypocortisolemic (“overtreated” group, see below) that had subsequent ACTH stimulation tests for follow‐up were allowed to receive physiologic glucocorticoid treatment at the discretion of the clinician; trilostane and mitotane administration was discontinued until cortisol secretory ability had recovered, as documented by an ACTH stimulation test (basal and ACTH‐stimulated cortisol concentration >1.4 μg/dL [40 nmol/L]). Intact females were included if in diestrus. Ten historical clinically healthy controls were included.1
To ensure that we had a sufficient number of samples from hypocortisolemic dogs, we analyzed stored serum or plasma samples from the Auburn University Endocrine Diagnostic Laboratory that had nondetectable basal cortisol and 60‐minute post‐ACTH cortisol concentrations <1.4 μg/dL (40 nmol/L). The ACTH stimulation test had to have been performed as for the dogs tested prospectively (see below). Samples were collected from November 2008 through October 2010 and stored at −20°C until analysis. Samples were excluded if it was not clear if the patient had PDH or which treatment was being administered.
Data Collection
All dogs had ACTH stimulation tests performed using 5 μg/kg synthetic ACTH.2 For dogs on trilostane, ACTH stimulation tests were initiated 4–6 hours postpill administration as per manufacturer instructions. Blood samples were obtained for hormone analysis just before injection (cortisol and aldosterone), 30 minutes post‐ACTH (aldosterone only), and 60 minutes post‐ACTH (aldosterone and cortisol). All samples were placed into serum or plasma collection tubes and centrifuged for 5 minutes within 1 hour of collection, after being allowed to clot for serum. The serum or plasma was separated and stored at −20°C until analysis. Aldosterone and cortisol concentrations were measured using previously validated radioimmunoassays3 , 15, 16; aldosterone was measured in duplicate, except for a few samples measured singly because of lack of sufficient volume. Cortisol concentrations were measured singly. Sensitivities of the assays were 0.5 μg/dL (14 nmol/L) and 1.2 ng/dL (33 pmol/L) for cortisol and aldosterone, respectively. For statistical purposes, values below the sensitivity of the assay were recorded as 0.25 μg/dL (7 nmol/L) and 0.6 ng/dL (17 pmol/L) for serum cortisol and aldosterone concentrations, respectively. Serum sodium and potassium concentrations were measured in basal samples (0 minute).
For the stored serum or plasma samples from the Auburn University Endocrine Diagnostic service, the time postpill administration of ACTH stimulation testing for trilostane‐treated dogs was unknown in most cases. Samples were analyzed as described above.
Statistical Analysis
The statistics package from SigmaPlot4 was used for statistical analysis. A Kruskal–Wallis one‐way ANOVA on ranks was used to compare aldosterone concentrations between treatment groups and historical clinically healthy controls at baseline, 30 and 60 minutes post‐ACTH; when a difference was detected, post hoc analysis was performed using Dunn's method. A repeated measures ANOVA was used to compare aldosterone concentrations among times (0, 30, 60 minutes) within treatment groups; when a difference was detected, the Tukey test was used for post hoc analysis.
Client‐owned dogs were subdivided based on the level of control according to the stimulated cortisol concentrations. For both medications, dogs with 60‐minute post‐ACTH cortisol concentrations <1.4 μg/dL (40 nmol/L) were considered overtreated. Those with ACTH‐stimulated cortisol concentrations of 1.4–5.4 μg/dL (40–150 nmol/L) were considered in the optimal therapeutic range. Finally, dogs with ACTH‐stimulated cortisol concentrations >5.4 μg/dL (150 nmol/L) were considered undertreated. To compare aldosterone concentrations among overtreated, optimally treated, and undertreated dogs within the client‐owned mitotane treatment group, a Kruskal–Wallis one‐way ANOVA on ranks was used. The Mann–Whitney rank sum test was used to compare aldosterone concentrations between undertreated and optimally treated dogs in the client‐owned trilostane treatment groups. An overtreated trilostane group was not included because of small sample size. To increase sample size in the overtreated groups, stored samples from the Endocrine Diagnostic Laboratory were combined with samples from client‐owned dogs. When the stored samples were included, a Kruskal–Wallis one‐way ANOVA was used to compare aldosterone concentrations in overtreated, optimally treated, undertreated, and clinically healthy dogs for both drugs.
Dogs were considered to have a decreased reserve if their concentration was >2 standard deviations below the mean concentration of the historical, clinically healthy controls at either 30 or 60 minutes. To compare the percentage of dogs with decreased aldosterone secretory reserve between treatment groups, among levels of control, or between times, the Fisher exact test or chi‐square was used.
The Spearman rank order correlation was used to evaluate the relationship between potassium and sodium concentrations and the basal (t = 0) and 30‐min aldosterone concentration; prospectively gathered and stored samples were used. The Spearman rank order correlation also was used to evaluate relationship between 60‐minute cortisol and 30‐minute aldosterone concentrations in the prospectively gathered samples. Results are given as median (range). Significance was set at the P < .05 level for all tests.
Results
Patient Population
Seventy‐nine ACTH stimulation tests were performed on client‐owned dogs with PDH: 42 on mitotane‐treated dogs and 37 on trilostane‐treated dogs. In the mitotane treatment group, 1 dog was enrolled 4 times, 8 dogs were enrolled 3 times, and 3 dogs were enrolled 2 times. In the trilostane treatment group, 4 dogs were enrolled 3 times. The dogs ranged in age from 3 to 14 years with a median of 10 years, and there were 23 castrated males, 1 intact male, and 32 spayed females. Breeds represented more than twice were mixed breed (n = 8), Dachshund (n = 6), Cocker Spaniel (n = 4), Yorkshire Terrier (n = 3), Pomeranian (n = 3), Maltese (n = 3), English Bulldog (n = 3), and Jack Russell Terrier (n = 3). Shih Tzu, Rat Terrier, Miniature Poodle, Miniature Dachshund, and Beagle each were represented twice; Australian Shepherd, Basset Hound, Boxer, Cairn Terrier, Chesapeake Bay Retriever, Border Collie, Labrador Retriever, Miniature Pinscher, Miniature Schnauzer, Springer Spaniel, Petite Basset Griffon Vendeen, Shetland Sheepdog, and unspecified terrier were represented once.
Forty‐three stored samples from 29 dogs were used. The dogs ranged in age from 4 to 16 years with a median of 10 years; 2 dogs were of unknown age. There were 6 castrated males, 1 intact male, 18 spayed females, and 1 intact female; 2 males and 1 female were of unknown neuter status. Breeds represented more than twice were mixed breed (n = 5) and Scottish Terrier (n = 3). Chihuahua, Dachshund, Maltese, Miniature Schnauzer, and Pomeranian were represented twice, whereas Boston Terrier, Boxer, Cocker Spaniel, English Bulldog, Jack Russell Terrier, Labrador Retriever, Miniature Dachshund, Miniature Poodle, Poodle, Rat Terrier, and Rottweiler were each represented once.
ACTH‐Stimulated Aldosterone Concentrations
In the mitotane‐treated dogs, basal (ie, 0‐minute) aldosterone concentrations were 1.8 ng/dL (0.6–55.2) (50 pmol/L [17–1,529]), 30‐minute post‐ACTH aldosterone concentrations were 4.8 ng/dL (0.6–119.5) (133 pmol/L [17–3,310]), and 60‐minute post‐ACTH aldosterone concentrations were 4.5 ng/dL (0.6–108) (125 pmol/L [17–2,986]) (Fig 1). In the trilostane‐treated dogs, basal aldosterone concentrations were 4.8 ng/dL (0.6–53.4) (133 pmol/L [17–1,497]), 30‐minute aldosterone concentrations were 9.2 ng/dL (2.3–81.0) (255 pmol/L [64–2,244]), and 60‐minute aldosterone concentrations were 10.9 ng/dL (0.6–81.2) (302 pmol/L [17–2,249]). In the historical controls,1 basal aldosterone concentrations were 4.9 ng/dL (1.7–14.6) (136 pmol/L [47–404]), 30‐minute aldosterone concentrations were 33.8 ng/dL (17.3–53.1) (936 pmol/L [479–1,471]), and 60‐minute aldosterone concentrations were 29.3 ng/dL (12.6–49.8) (811 pmol/L [349–1,379]).
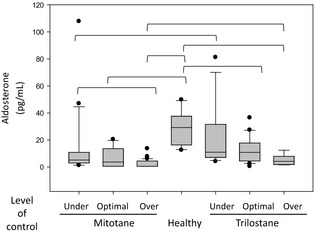
Figure 1.

Box and whisker plot depicting aldosterone concentrations in the treatment groups at 0 (basal) minute and 30 and 60 minutes post‐ACTH. Clinically healthy dogs1 are included for comparison. Values for groups connected by a bracket are significantly different (Kruskal–Wallis one‐way ANOVA on ranks). Each box represents the interquartile (ie, 25–75th percentile) range, the horizontal line within the box represents the median value, the bars represent the 10–90th percentile, and the circles represent outlying datum points.
The aldosterone concentrations in the mitotane‐treated and the trilostane‐treated dogs at both 30 and 60 minutes post‐ACTH were significantly lower than those in clinically healthy dogs (Kruskal–Wallis one‐way ANOVA on ranks, P < .001 for both) (Fig 1). In addition, at all time points, dogs treated with trilostane had significantly higher aldosterone concentrations than dogs treated with mitotane (Kruskal–Wallis one‐way ANOVA on ranks, P = .006 at baseline, P < .001 at 30 and 60 minutes). In treated dogs, aldosterone concentrations at 30 and 60 minutes were significantly higher than basal concentrations (repeated measures ANOVA, P < .001 for both drugs), but no difference was detected between 30‐ and 60‐minute aldosterone concentrations within either treatment group. In comparison, in clinically healthy dogs, aldosterone concentrations were significantly higher at 30 compared with those at 60 minutes.1
Based on 60‐minute post‐ACTH cortisol concentrations in the client‐owned dogs, in the mitotane group, 20 were considered undertreated, 16 optimally treated, and 6 overtreated. In the trilostane group, 13, 21, and 3 were considered undertreated, optimally treated, and overtreated, respectively. The stored samples included 6 tests from trilostane‐treated dogs and 27 from mitotane‐treated dogs; all were considered overtreated based on inclusion criteria for these samples. For client‐owned dogs, no difference was detected among overtreated, optimally treated, or undertreated groups in 30‐ or 60‐minute aldosterone concentrations for either mitotane or trilostane (data not shown). Compared with concentrations in clinically healthy dogs, the 30‐minute aldosterone concentrations were significantly lower in optimally treated dogs for both mitotane and trilostane (Kruskal–Wallis one‐way ANOVA on ranks, P < .001 and P = .005 for mitotane and trilostane, respectively) as well as overtreated and undertreated dogs on mitotane (Kruskal–Wallis one‐way ANOVA on ranks, P < .001 for both) (data not shown). An insufficient number of overtreated dogs on trilostane were available for statistical analysis.
When the client‐owned and stored samples were included, the 60‐minute aldosterone concentrations in the overtreated mitotane group were significantly lower than those in the undertreated group (Kruskal–Wallis one‐way ANOVA on ranks, P = .001). The 60‐minute aldosterone concentrations were significantly lower in optimally treated and overtreated dogs for both mitotane and trilostane when compared with those in clinically healthy dogs (Kruskal–Wallis one‐way ANOVA on ranks, P < .001 for both drugs) (Fig 2). Also, aldosterone concentrations were significantly lower in mitotane‐treated dogs than in trilostane‐treated dogs for the undertreated and overtreated levels of control (Mann–Whitney rank sum test, P = .016 and .010 for undertreated and overtreated dogs, respectively). For the dogs that were optimally treated, aldosterone concentrations in the trilostane‐ and mitotane‐treated dogs were not significantly different (Mann–Whitney rank sum test, P = .053).
Figure 2.
Box and whisker plot depicting the comparison of 60‐minute post‐ACTH aldosterone concentration between treatment groups and clinically healthy dogs, based on level of control (60‐minute post‐ACTH cortisol). Dogs with a 60‐minute post‐ACTH cortisol concentration >5.4 μg/dL (150 nmol/L), 1.4–5.4 μg/dL (40–150 nmol/L), and <1.4 μg/dL (40 nmol/L) were classified as undertreated, optimally treated, and overtreated, respectively. See Figure 1 for further explanation.
Incidence of Decreased Aldosterone Secretory Reserve
For both medications, the number of dogs that had decreased aldosterone secretory reserve at 30 minutes was significantly higher than at 60 minutes (chi‐square; for mitotane P = .015 and for trilostane P = .006) (Fig 3). In addition, the number of dogs receiving mitotane that had decreased aldosterone reserve was higher as compared with the number of dogs receiving trilostane at 30 minutes (chi‐square, P = .014) and 60 minutes (chi‐square, P = .003). At 30 minutes, decreased aldosterone secretory reserve was detected in 49% of trilostane‐treated dogs versus 78% of mitotane‐treated dogs. In contrast, at 60 minutes, a decreased reserve was detected in only 16% and 50% of trilostane‐treated and mitotane‐treated dogs, respectively.
Figure 3.
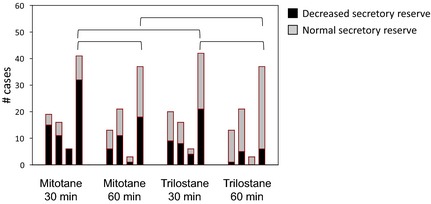
Number of dogs with decreased aldosterone secretory reserve versus those with normal reserve according to level of control. Aldosterone concentrations measured at 60 minutes post‐ACTH. Black‐shaded area represents dogs with decreased aldosterone secretory reserve and gray‐shaded area represents dogs with normal reserves. In each group, the first bar represents the undertreated dogs, the second bar the optimally treated, the third bar the overtreated, and the fourth bar the 3 former groups combined. To compare the number of dogs with decreased aldosterone secretory reserve between treatment groups, among levels of control, or between times, the Fisher exact test or chi‐square was used. Groups connected by a bracket are significantly different.
The number of dogs that had decreased aldosterone secretory reserve varied by level of control for trilostane (chi‐square, P = .004), but not for mitotane (chi‐square, P = .077). For trilostane, the percentages of dogs with decreased reserve detected at 60 minutes in the undertreated, optimally treated, and overtreated groups were 8, 24, and 36%, respectively. For mitotane, the percentages of dogs with decreased reserve detected at 60 minutes in the undertreated, optimally treated, and overtreated groups were 45, 50, and 74%, respectively. At 30 minutes post‐ACTH, a decreased aldosterone secretory reserve was detected in 50% of well‐controlled dogs on trilostane and 69% of well‐controlled dogs on mitotane.
Relationship between Aldosterone, Cortisol, and Electrolyte Concentrations
A significant, but weak, relationship was detected between 30‐minute aldosterone and 60‐minute cortisol concentrations for mitotane‐treated dogs (Spearman rank order correlation; r = 0.38, P = .015) (Fig 4A). In comparison, no relationship was detected in trilostane‐treated dogs (Spearman rank order correlation; r = 0.13, P = .44) (Fig 4B). No relationship was detected between basal aldosterone and potassium concentrations for dogs receiving mitotane (Spearman rank order correlation; P = .26, Fig 5A) or trilostane (Spearman rank order correlation; P = .81, Fig 5B) or between basal aldosterone and sodium concentrations in either treatment group (data not shown). In addition, no relationship was detected between the 30‐minute aldosterone concentration and sodium or potassium concentration for either drug (data not shown). For either medication, the presence of decreased aldosterone secretory reserve was not significantly related to the number of patients with hyponatremia (Table 1; Fisher exact test for mitotane and trilostane, P = .43 and P = 1.0, respectively) or hyperkalemia (Table 2; Fisher exact test for mitotane and trilostane, P = .064 and P = .61, respectively).
Figure 4.

Thirty‐minute post‐ACTH aldosterone concentrations versus 60‐minute post‐ACTH cortisol concentrations. (A) Mitotane‐treated dogs (r = 0.38, P = .015, Spearman rank order correlation). (B) Trilostane‐treated dogs (r = 0.13, P = .44, Spearman rank order correlation).
Figure 5.
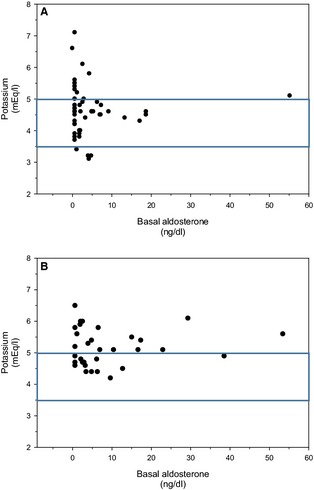
Basal aldosterone versus potassium concentrations. (A) Mitotane‐treated dogs (r = −0.15, P = .26, Spearman rank order correlation). (B) Trilostane‐treated dogs (r = −0.044, P = .81, Spearman rank order correlation). The area within the box represents the reference range for serum potassium concentration.
Table 1.
Dogs in each treatment group that had hyponatremia with or without decreased aldosterone secretory reserve at 30 and/or 60 minutes post‐ACTH.
| Hyponatremic | Normonatremic | |
|---|---|---|
| Mitotane‐treated | ||
| Decreased aldosterone secretory reserve | 9a (28.1%) | 23 (71.9%) |
| Normal aldosterone secretory reserve | 4 (44.4%) | 5 (55.6%) |
| Trilostane‐treated | ||
| Decreased aldosterone secretory reserve | 8 (42.1%) | 11 (57.9) |
| Normal aldosterone secretory reserve | 8 (50.0%) | 8 (50.0%) |
Data presented as n (%).
Table 2.
Dogs in each treatment group that had hyperkalemia with or without decreased aldosterone secretory reserve at 30 and/or 60 minutes post‐ACTH.
| Hyperkalemic | Normokalemic | |
|---|---|---|
| Mitotane‐treated | ||
| Decreased aldosterone secretory reserve | 0 (0%) | 31 (100%) |
| Normal aldosterone secretory reserve | 1 (11.1%) | 8 (88.9%) |
| Trilostane‐treated | ||
| Decreased aldosterone secretory reserve | 3 (17.7%) | 14 (82.3%) |
| Normal aldosterone secretory reserve | 1 (7.1%) | 13 (92.9%) |
Follow‐Up of Dogs with Decreased Aldosterone Secretory Reserve
Eighteen dogs with low cortisol concentrations (nondetectable at baseline and <1.4 μg/dL [<40 nmol/L] at 60 minutes) had follow‐up ACTH stimulation tests performed. At the time hypocortisolemia was documented, 15 dogs had 60‐minute aldosterone concentrations that were undetectable or below the reference range established in clinically healthy dogs (1.7–19.4 ng/dL [46–536 pmol/L]).1 Nine of the 15 dogs with decreased aldosterone secretory reserve (6 mitotane‐treated and 3 trilostane‐treated dogs) had follow‐up ACTH stimulation tests that documented adrenocortical recovery with 60‐minute cortisol concentrations >1.4 μg/dL (>40 nmol/L) within 0.5–5 months. Six of the 15 dogs (4 mitotane‐treated and 2 trilostane‐treated) did not have documented adrenocortical recovery during a follow‐up period of 1–7 months. At the time hypocortisolemia was initially detected, 3 dogs had 60‐minute aldosterone concentrations within the reference range. Of the 3 dogs, 2 had documented recovery of cortisol secretory ability 2 and 5 months later, but the third dog (on mitotane) remained hypocortisolemic through the end of the follow‐up at 21 months.
Discussion
Our results document that treatment of canine PDH with either mitotane or trilostane causes decreased aldosterone secretory reserve in a relatively high percentage of dogs. The dogs in this study were considered to have decreased aldosterone secretory reserve if their concentration was more than 2 standard deviations below the mean concentration of historical, clinically healthy controls at either 30 or 60 minutes. Our findings are similar to previous studies6, 7, 9, 10, 11 that showed that both medications can decrease serum aldosterone concentrations at 60 minutes post‐ACTH stimulation; 2 studies found no difference in basal aldosterone concentrations after trilostane treatment.10, 14 Unlike the former studies, which evaluated aldosterone concentrations at only 60 minutes post‐ACTH, the current study is the first to identify decreased aldosterone secretory reserve by evaluating aldosterone concentrations at 30 minutes post‐ACTH stimulation. Indeed, by evaluating 30‐minute post‐ACTH aldosterone secretion, we detected a significantly larger percentage of dogs treated with either drug that had decreased aldosterone secretory reserve. By measuring aldosterone at 30 minutes, 49% and 78% of trilostane‐treated and mitotane‐treated dogs, respectively, were identified to have a decreased reserve as compared to only 16% and 50% of trilostane‐treated and mitotane‐treated dogs identified, respectively, at 60 minutes.
Aldosterone concentrations measured at 30 and 60 minutes posttreatment were not significantly different in either treatment group. In comparison, in clinically healthy dogs, the aldosterone concentrations 30 minutes post‐ACTH were significantly higher than at 60 minutes.1 The difference between treated and healthy dogs could be attributable to the effect of the medications. However, as hypothesized, significantly more dogs were identified with a decreased aldosterone secretory reserve at 30 minutes compared with 60 minutes post‐ACTH stimulation in both treatment groups. Therefore, measuring aldosterone concentration at 30 minutes is important for detection of decreased aldosterone secretory reserve; if it were only measured at 60 minutes, some dogs with aldosterone suppression would be missed.
Decreased aldosterone secretory reserve caused by mitotane treatment appears to be much more common than the 6–10% prevalence previously reported;4, 8 however, the rate previously had been inferred from measurement of serum electrolyte concentrations. Even though we hypothesized that electrolyte and aldosterone concentrations would be correlated at the 30‐minute time point, we did not detect a significant relationship. In addition, we did not detect a significant relationship between basal aldosterone concentration and sodium and potassium concentrations, consistent with the findings of another study.10 Similarly, in dogs with spontaneous hypoadrenocorticism, serum electrolyte concentrations do not correlate with serum aldosterone concentrations.17 In our study, for both drugs combined, 67% and 94% of dogs with decreased aldosterone secretory reserve were normonatremic and normokalemic, respectively. Conversely, a number of dogs with normal secretory reserve were either hyponatremic (n = 12) or hyperkalemic (n = 2). Therefore, hyperkalemia and hyponatremia are insensitive markers of aldosterone deficiency. Accordingly, aldosterone concentrations should be measured in patients with normal serum electrolyte concentrations that have clinical signs suggestive of, or are at risk for, decreased aldosterone secretory reserve. Detection of decreased reserve can help predict which patients may need mineralocorticoid supplementation routinely or during times that aldosterone secretory reserve is important (eg, dehydration, hypovolemia).
This is the first study to directly compare the effect of the 2 drugs on aldosterone suppression. Aldosterone concentrations at 30 and 60 minutes post‐ACTH were significantly lower in dogs receiving mitotane treatment compared with those in dogs receiving trilostane treatment regardless of the level of control. Furthermore, aldosterone concentrations would not be expected to change during the day in dogs treated with mitotane as compared with dogs treated with trilostane, where cortisol concentrations are suppressed for a shorter period of time.18 In other words, aldosterone suppression in trilostane‐treated dogs may be present only transiently. Whether or not we evaluated the dogs at the time of maximal trilostane‐induced inhibition of aldosterone secretion is unknown, because the effect of trilostane waxes and wanes and timing is patient‐dependent. Because aldosterone deficiency is more immediately life‐threatening than cortisol deficiency, the effect of mitotane and trilostane on aldosterone secretion should be considered.
A limitation of this study was combining prospectively collected samples from client‐owned dogs with samples stored by the Endocrine Diagnostic Service, because the former met with stricter criteria for inclusion. However, inclusion of stored samples allowed for evaluation of a greater number of dogs that were completely cortisol‐deficient, a specific population not previously evaluated. As we hypothesized, aldosterone suppression was common in dogs overtreated for PDH. Although decreased aldosterone secretory reserve did vary significantly with level of control for trilostane‐treated dogs, it did not for mitotane‐treated dogs. Interestingly, aldosterone suppression was common in well‐controlled dogs on either treatment. Fifty percent of well‐controlled dogs on trilostane and 69% of well‐controlled dogs on mitotane had decreased aldosterone secretory reserve detected at 30 minutes post‐ACTH. The findings contradict the belief that destruction of the zona glomerulosa by mitotane is the end‐stage result after complete destruction of the zona fasciculata and zona reticularis. On the other hand, our data support the hypothesis that in some dogs trilostane may more effectively block the synthesis of mineralocorticoids than of glucocorticoids18; this could explain why some dogs treated with trilostane develop clinical signs of hypoadrenocorticism, such as hypovolemia and hypotension, but have optimal basal and ACTH‐stimulated cortisol concentrations. Deficiency of 1 hormone cannot be predicted by concentrations of the other.
Of the 18 hypocortisolemic dogs with follow‐up ACTH stimulation test results, 15 initially had undetectable or low 60‐minute aldosterone concentrations; 9 of the 15 had documented adrenocortical recovery. Therefore, the presence of decreased aldosterone secretory reserve cannot be used to predict permanent hypoadrenocorticism. Adrenocortical function may have recovered in even more dogs if the follow‐up period had been longer. One dog on trilostane treatment remained hypocortisolemic for at least 3.5 months after drug administration had been discontinued, indicating that the dog probably experienced adrenal necrosis. Interestingly, aldosterone (1.5 ng/dL or 42 pmol/L) concentrations were only slightly below the reference range when the hypocortisolism was first documented. In addition, 1 of the 3 dogs with hypocortisolism, but a 60‐minute aldosterone concentration within the reference range, remained hypocortisolemic for at least 21 months. Therefore, an aldosterone concentration within the reference range may not predict rapid recovery from hypocortisolism.
In conclusion, decreased aldosterone secretory reserve as a sequela of treatment with mitotane and trilostane for PDH is much more common than previously recognized and is more common with mitotane than with trilostane. Decreased aldosterone secretory reserve may not be apparent from serum electrolyte concentrations or from basal or ACTH‐stimulated cortisol concentrations. Conversely, hyponatremia, hyperkalemia, or both may be detected without apparent changes in aldosterone secretory reserve. Measurement of serum aldosterone concentrations at both 30 and 60 minutes post‐ACTH stimulation is necessary to identify more cases of decreased aldosterone secretory reserve than conventional testing at 60 minutes. Aldosterone should be measured in mitotane‐ or trilostane‐treated dogs that are at risk for, or have signs of, hypoaldosteronism even if cortisol concentrations are within ideal ranges for dogs on treatment. Lastly, the presence of aldosterone suppression does not predict reversibility of adrenocortical insufficiency.
Acknowledgments
The authors thank Drs Barrett Mann and Mark Miller of Cahaba Valley Animal Hospital, Dr Dan Whitlow of Helton Plaza Animal Hospital, and Drs Tabitha Tanis, Anthony Bruno, and Scott Reinold of AMC Lawrenceville Animal Hospital for procuring samples. The authors also thank Ann Busch and Kristin Larrimore for their technical assistance. This study was supported by a grant from the ACVIM Foundation.
Conflict of Interest Declaration: The authors disclose no conflict of interest.
Analysis of data and manuscript preparation were performed at Auburn University, Auburn, AL.
Preliminary results of this study were presented as a research abstract at the 2011 ACVIM Forum, Denver, CO
Footnotes
Carlson KJ, Behrend EN, Martin LG, Kemppainen RJ. Optimization of a test protocol to assess aldosterone secretory capacity in dogs. J Vet Int Med 2010;24:685 (abstract)
Cortrosyn; Amphastar Pharmaceuticals, Inc, Rancho Cucamonga, CA
Coat‐A‐Count assays; Diagnostic Products Corporation, Los Angeles, CA
SigmaPlot 11; Systat Software, Inc, Chicago, IL
References
- 1. Neiger R, Ramsey IK, O'Conner J, et al. Trilostane treatment of 78 dogs with pituitary‐dependent hyperadrenocorticism. Vet Rec 2002;150:799–804. [DOI] [PubMed] [Google Scholar]
- 2. Ruckstuhl NS, Nett CS, Reusch C. Results of clinical examinations, laboratory tests, and ultrasonography in dogs with pituitary‐dependent hyperadrenocorticism treated with trilostane. Am J Vet Res 2002;63:506–512. [DOI] [PubMed] [Google Scholar]
- 3. Braddock JA, Church DB, Robertson ID, Watson ADJ. Trilostane treatment in dogs with pituitary‐dependent hyperadrenocorticism. Aust Vet J 2003;81:600–607. [DOI] [PubMed] [Google Scholar]
- 4. Kintzer PP, Peterson ME. Mitotane (o, p'‐DDD) treatment of 200 dogs with pituitary‐dependent hyperadrenocorticism. J Vet Int Med 1991;5:182–190. [DOI] [PubMed] [Google Scholar]
- 5. Feldman EC, Feldman MS, Farver TB. Comparison of mitotane treatment for adrenal tumor versus pituitary‐dependent hyperadrenocorticism in dogs. J Am Vet Med Assoc 1992;200:1642–1647. [PubMed] [Google Scholar]
- 6. Goy‐Thollot I, Pechereau D, Keroack S, et al. Investigation of the role of aldosterone in hypertension associated with spontaneous pituitary‐dependent hyperadrenocorticism in dogs. J Small Anim Pract 2002;43:489–492. [DOI] [PubMed] [Google Scholar]
- 7. Golden DL, Lothrop CD. A retrospective study of aldosterone secretion in normal and adrenopathic dogs. J Vet Int Med 1988;2:121–125. [DOI] [PubMed] [Google Scholar]
- 8. Kintzer PP, Peterson ME. Mitotane treatment of 32 dogs with cortisol‐secreting adrenocortical neoplasms. J Am Vet Med Assoc 1994;225:54–61. [PubMed] [Google Scholar]
- 9. Sieber‐Ruckstuhl NS, Boretti FS, Wenger M, et al. Cortisol, aldosterone, cortisol precursor, androgen and endogenous ACTH concentrations in dogs with pituitary‐dependent hyperadrenocorticism treated with trilostane. Domest Anim Endocrinol 2006;31:63–75. [DOI] [PubMed] [Google Scholar]
- 10. Wenger M, Sieber‐Ruckstuhl NS, Müller C, Reusch C. Effect of trilostane on serum concentrations of aldosterone, cortisol, and potassium in dogs with pituitary‐dependent hyperadrenocorticism. Am J Vet Res 2004;65:1245–1250. [DOI] [PubMed] [Google Scholar]
- 11. Chapman PS, Kelly DF, Archer J, et al. Adrenal necrosis in a dog receiving trilostane for the treatment of hyperadrenocorticism. J Small Anim Pract 2004;45:307–310. [DOI] [PubMed] [Google Scholar]
- 12. Reusch CE, Sieber‐Ruckstuhl N, Wenger M, et al. Histological evaluation of the adrenal glands of seven dogs with hyperadrenocorticism treated with trilostane. Vet Rec 2007;160:219–224. [DOI] [PubMed] [Google Scholar]
- 13. Alenza DP, Arenas C, Lopez ML, Melian C. Long‐term efficacy of trilostane administered twice daily in dogs with pituitary‐dependent hyperadrenocorticism. J Am Anim Hosp Assoc 2006;42:269–276. [DOI] [PubMed] [Google Scholar]
- 14. Galac S, Buijtels JJCWM, Mol JA, Kooistra HS. Effects of trilostane on the pituitary‐adrenocortical and renin‐aldosterone axis in dogs with pituitary‐dependent hypercortisolism. Vet J 2010;183:75–80. [DOI] [PubMed] [Google Scholar]
- 15. Behrend EN, Weigand CM, Whitley EM, et al. Corticosterone‐ and aldosterone‐secreting adrenocortical tumor in a dog. J Am Vet Med Assoc 2004;226:1662–1666. [DOI] [PubMed] [Google Scholar]
- 16. Kemppainen RJ, Thompson FN, Lorenz MD. Use of a low dose synthetic ACTH challenge test in normal and prednisone‐treated dogs. Res Vet Sci 1983;35:240–242. [PubMed] [Google Scholar]
- 17. Muller C, Boretti FS, Wenger M, et al. Investigation on the aldosterone concentration before and after ACTH application in 44 dogs with hypoadrenocorticism. Kleintierpraxis 2007;52:216–224. [Google Scholar]
- 18. Vaughn MA, Feldman EC, Hoar BR, Nelson RW. Evaluation of twice‐daily, low‐dose trilostane treatment administered orally in dogs with naturally occurring hyperadrenocorticism. J Am Vet Med Assoc 2008;232:1321–1328. [DOI] [PubMed] [Google Scholar]


