Abstract
Background
Inflammatory bowel disease (IBD) and intestinal small cell lymphoma (ISCL) are common diseases in cats. The prevalence of alterations in the serum concentrations of fat soluble vitamins, such as vitamin D, in cats with IBD and ISCL is unknown.
Hypothesis/Objectives
The objective of this study was to measure serum 25 hydroxyvitamin D (25[OH]D) concentrations in cats with IBD or ISCL. Serum 25(OH)D also was measured in healthy cats, and in hospitalized ill cats with nongastrointestinal diseases.
Animals
Eighty‐four cats were included in the study: 23 in the healthy group, 41 in the hospitalized ill group, and 20 in the IBD/ISCL group.
Methods
Retrospective study. Serum samples for vitamin D analysis were frozen at −20°C until serum 25(OH)D was measured by high‐performance liquid chromatography (HPLC).
Results
Although there was overlap in serum 25(OH)D concentrations among the 3 groups, serum 25(OH)D concentrations were significantly lower in the cats with IBD or ISCL compared to healthy cats (P < .0001) and hospitalized ill cats (P = .014). In the IBD/ISCL group, there was a significant moderate positive correlation between serum albumin and 25(OH)D concentrations (r = 0.58, P = .018).
Conclusion and Clinical Importance
The median serum concentration of 25(OH)D was significantly lower in cats with IBD/ISCL than in healthy cats and in hospitalized ill cats. Additional studies are required to elucidate the mechanism of hypovitaminosis D in cats with gastrointestinal diseases, to define the best management strategy to treat this complication, and to investigate its potential prognostic implications.
Keywords: Small cell lymphoma, Inflammatory bowel disease, Vitamin D, Calcium
Abbreviations
- 25[OH]D
25‐hydroxyvitamin D
- BSH
British Shorthair
- DLH
domestic longhair
- DSH
domestic shorthair
- FE
female entire
- FN
female neutered
- HPLC
high‐performance liquid chromatography
- IBD
inflammatory bowel disease
- ISCL
intestinal small cell lymphoma
- ME
male entire
- MN
male neutered
- PLE
protein losing enteropathy
Inflammatory bowel disease (IBD) is a common and debilitating disorder in cats. Diagnosis of IBD in cats relies on extensive diagnostic investigations to rule out systemic diseases, dietary sensitivity, infectious diseases, and alimentary lymphoma.1 Histopathological evaluation of biopsy samples taken from the gastrointestinal tract are required for diagnosis, and the most commonly used techniques are full‐thickness surgical biopsies or partial thickness biopsies obtained endoscopically. However, previous reports have shown that the differentiation of feline IBD and intestinal small cell lymphoma (ISCL) on the basis of endoscopically obtained biopsy samples can be difficult.2, 3, 4
The management of feline IBD and its associated complications can be extremely challenging. Currently, standard approaches include dietary, antibiotic, immunosuppressive, and anti‐inflammatory treatment. However, although many cats with IBD respond favorably to dietary and immunosuppressive treatment, treatment failures may still occur.1 Consequently, there is a need to develop better therapeutic approaches and to understand more about the metabolic complications that develop in gastrointestinal disorders of cats. In particular, there is a need to understand more about the prevalence of disturbances in the metabolism of fat soluble vitamins, such as vitamins D and K, in cats with gastrointestinal diseases. The paucity of data in this area is surprising given that there have been case reports of cats with suspected fat soluble vitamin K deficiency secondary to IBD5 and in light of the strong evidence that the metabolism of fat‐soluble vitamins frequently is deranged in canine and human patients with IBD.6, 7, 8 It is often difficult to make a histological distinction between IBD and ISCL in cats,4, 9, 10 and it is likely that similar derangements in fat‐soluble vitamins occur in both subsets of these patients.
The hypothesis of this study was that cats with IBD or ISCL would have a lower serum 25(OH)D concentration than hospitalized control cats or healthy cats. The aim of this study was to assess the vitamin D status of cats with IBD or ISCL. Vitamin D status was assessed by the measurement of serum concentrations of 25 hydroxyvitamin D (25[OH]D), which is produced by the hydroxylation of vitamin D in the liver. The measurement of serum concentrations of 25(OH)D is the most widely used approach to assess vitamin D status.11 We previously have shown that hospitalized ill cats have decreased serum 25(OH)D concentrations compared to healthy cats,12 and thus serum 25(OH)D concentrations also were measured in control populations of healthy cats and hospitalized ill cats without gastrointestinal diseases.
Materials and Methods
Study Population
The study population consisted of 3 groups of cats that were referred to the Feline Centre, Langford Veterinary Services, University of Bristol or the Hospital for Small Animals of the University of Edinburgh for further investigations. The first group consisted of cats with IBD or ISCL. A diagnosis of IBD or ISCL was made if the cat had both:
Compatible clinical signs which included ≥1 of the following clinical signs: vomiting, diarrhea, weight loss, increased or decreased appetite, and abdominal discomfort for at least 3 weeks, and
Gastric biopsies, intestinal biopsies or both, which were collected endoscopically or surgically and evaluated histologically. Cats with evidence of an inflammatory gastric or intestinal infiltrate for which no cause could be identified based on fecal examination for parasites, fecal culture, serum feline pancreatic lipase immunoreactivity testing, and abdominal ultrasound examination were diagnosed with IBD. The diagnosis of ISCL was made based upon the histological identification of infiltration with characteristic monomorphous sheets of neoplastic small lymphocytes in the biopsy sections.
At the time of initial investigations, blood samples were collected from each patient for hematological and serum biochemical analysis. Serum samples for 25(OH)D measurement also were collected and within 2 hours of collection were frozen at −20°C until analysis. Samples were frozen for up to 5 years and serum 25(OH)D concentrations have been shown to be stable under these conditions.13
The second group consisted of cats that were hospitalized and underwent investigations for systemic diseases without any gastrointestinal tract involvement (hospitalized ill group). Cats with historical complaints of vomiting or diarrhea or any abnormalities in the gastrointestinal tract on abdominal ultrasound examination were excluded.
The third group consisted of healthy cats that were presented for wellness visits and whose owners reported no health complaints or clinical signs (healthy cat group). The healthy cats did not have any clinically relevant abnormalities on physical examination or on hematology or serum biochemistry, which was performed as part of their standard health care program.
Because corticosteroids can alter calcium homeostasis,14, 15 all cats that had been treated with corticosteroids in the 2 weeks before referral were excluded from the study. In addition, cats that had received other medications or supplements that could have interfered with calcium homeostasis also were excluded.
Laboratory Measurements
Total calcium, phosphate, albumin, and total protein were measured on an automated wet chemistry analyzer.1
Serum concentrations of 25(OH)D were measured as previously described.16 Briefly, samples were extracted with acetonitrile and applied to C18 Silica Sep‐paks. Metabolites were separated by straight phase HPLC2 using a Hewlett‐Packard Zorbax‐Sil Column3 eluted with hexane : propan‐2‐ol : methanol (92 : 4 : 4). Serum 25(OH)D was measured by application to a second Zorbax_Sil Column eluted with hexane : propan‐2‐ol (98 : 2) and quantified by UV absorbance at 265 nm and corrected for recovery (sensitivity 2 ng/mL with intra‐ and interassay coefficients of variation of 3.0 and 4.2%, respectively).16 The assay laboratory was accredited to ISO 9001:2008 and ISO 13485:2003 and is now CPA certified (0865) and participates successfully in the Vitamin D quality assurance scheme (DEQAS).
Statistics
A Mann–Whitney U‐test was performed to compare median 25(OH)D concentrations in the IBD and ISCL group with the hospitalized ill cats with nongastrointestinal disease and the healthy cats. A Spearman Rank correlation test was used to investigate for the presence of correlations between serum albumin, calcium and phosphate concentrations and 25(OH)D concentrations in the IBD and ISCL group. Statistical analyses were performed with a commercial software package4 and P < .05 was considered significant. The study was approved by the University of Edinburgh's Veterinary Ethical Review Committee.
Results
Eighty‐four cats were included in the study: 23 in the healthy group, 41 in the hospitalized ill group, and 20 in the IBD and ISCL group. In the gastrointestinal disease group, 14 cats were diagnosed with IBD and 6 were diagnosed with ISCL. For cats with endoscopic biopsies, gastroduodenoscopy and ileocolonoscopy was performed in 10 cats, gastroduodenoscopy alone was performed in 5 cats, and ileocolonoscopy alone was performed in 1 cat. Surgical biopsy samples were collected from 4 cats. The histopathological diagnosis was lymphoplasmocytic infiltrate in 9 cases, eosinophilic infiltrate in 1 case, neutrophilic infiltrate in 1 case, and combined lymphoplasmocytic and eosinophilic infiltrate in 2 cases, and combined lymphoplasmocytic and neutrophilic infiltrate in 1 case.
The healthy control cats consisted of 15 male neutered (MN), 2 male entire (ME), 5 female neutered (FN), and 1 female entire (FE) cats. There were 17 domestic shorthair (DSH), 3 domestic longhair (DLH), 2 British Blues and 1 Maine Coon cats. The median age of the healthy cats was 6 years (range, 2–14 years).
The hospitalized ill control cats consisted of 26 MN, 14 FN and 1 FE. There were 24 DSH, 8 DLH, 4 British Shorthair (BSH), 2 Ragdolls, 1 Siamese, 1 Sphynx, and 1 Maine Coon. Diseases that were diagnosed in these cats were neoplasia in 7 (1 bronchoalveolar carcinoma, 2 fibrosarcoma, 1 cutaneous small cell lymphoma, 1 osteosarcoma, 1 salivary adenocarcinoma, 1 hepatic carcinoma), metabolic disease in 2 (diabetes mellitus, hyperaldosteronism), cardiorespiratory disease in 9 (3 congestive heart failure, 1 undefined cardiac disease, 3 pneumonia, 2 chylothorax), urogenital disease in 11 (6 chronic kidney disease, 2 urolithiasis, 3 idiopathic feline cystitis), infectious disease in 4 (2 feline immunodeficiency virus, 2 feline herpesvirus‐associated conjunctivitis), skin disease in 1 (dermatitis), ear disease in 1 (otitis media and interna), liver disease in 1 (chronic lymphocytic hepatitis), other categories in 6 (1 dysautonomia, 1 abdominal mass of unknown cause, 1 oral mass of unknown origin, 1 chronic sinusitis, 1 cognitive dysfunction). The median age in the hospitalized ill control cats was 8 years (range, 2–21.5 years).
The GI disease group consisted of 10 MN and 10 FN cats. There were 8 DSH, 2 DLH 2 Bengals, 2 Tonkinese, 2 Siamese, 1 Maine Coon, 1 Persian, 1 British Shorthair, and 1 Burmese. The median age of the GI disease group was 8 years (range, 1–20 years). There was no significant difference in age among the 3 groups.
The median serum 25(OH)D concentration was significantly lower in cats with IBD or ISCL (12.7 ng/mL) compared to the healthy cats (45.1 ng/mL, P < .0001). The median serum 25(OH)D concentration in cats with IBD or ISCL also was significantly lower than in the hospitalized ill cats with nongastrointestinal disease (33.8 ng/mL, P = .014) (Fig 1). In the healthy feline group, serum 25(OH)D concentrations ranged from 30.4 to 61.0 ng/mL; in the ISCL group, the serum 25(OH)D concentrations ranged from 8.5 to 32.2 ng/mL; and, in the IBD group, the serum 25(OH)D concentrations ranged from 2 to 83.1 ng/mL (Fig 1). Thirteen of the 20 cats (65%) in the IBD and ISCL group had serum 25(OH)D concentrations below 30.4 ng/mL compared to 18 of 41 cats (44%) in the hospitalized ill group.
Figure 1.
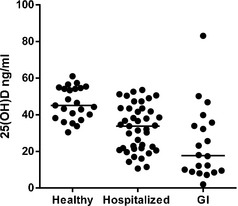
Serum 25(OH)D concentrations in healthy cats, hospitalized ill cats and cats with IBD or ISCL.
Serum calcium concentration was measured in 16 of the 20 cats with IBD or ISCL. Serum total hypocalcemia was only found in 2 cats, both in the IBD group. Concurrent hypoalbuminemia was documented in 1 of the cats. The median albumin concentration in the IBD/ISCL group was 29 g/L (range, 17.7–31.1 g/L [reference range, 24–35 g/L]). The median calcium concentration was 2.44 mmol/L (range, 1.89–2.71 mmol/L; reference range, 2.3–2.5 mmol/L). The median phosphate concentration was 1.19 mmol/L (range, 0.71–1.69 mmol/L; reference range, 0.95–1.55 mmol/L). There was a moderate positive correlation between serum albumin concentration and 25(OH)D concentration (r = 0.58; 95% confidence interval, 0.11–0.84; P = .018; Fig 2). There were no significant correlations between either calcium or phosphate with 25(OH)D concentration. There was no significant difference in serum 25(OH)D concentrations in cats with ISCL (median, 14.3 ng/mL) compared to cats with IBD (median, 20.6 ng/mL; P = .43).
Figure 2.
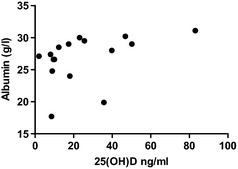
Scatter plot comparing serum albumin concentration and 25(OH)D concentrations.
Discussion
In this study, cats with gastrointestinal disease had significantly lower 25(OH)D concentrations (median, 12.7 ng/mL) than hospitalized ill cats without gastrointestinal disease (median, 33.8 ng/mL). This observation is consistent with a previous study in dogs that found that dogs with protein‐losing enteropathies (PLE) had marked hypovitaminosis D.6 The finding that albumin concentrations correlated with 25(OH)D concentrations also is consistent with the previous study in dogs.6 Importantly, we have demonstrated that the decrease in 25(OH)D concentration in cats with IBD or ISCL is more marked than the nonspecific decrease that occurs in hospitalized ill cats suffering from other diseases.12
It is well established that hypovitaminosis D is common in humans with IBD.17, 18, 19 The underlying cause has not been elucidated, and it is likely that several factors are involved.17, 19 Decreased oral intake of vitamin D in humans with IBD has been proposed. Support for decreased appetite being an important mediator of hypovitaminosis D in companion animals with IBD comes from the previous finding that serum 25(OH)D concentrations in dogs with IBD and moderately or severely decreased appetite were significantly lower than dogs with IBD that had normal appetite. Other potential causes of hypovitaminosis D in humans with chronic enteropathies include decreased intestinal absorption of vitamin D and increased loss of vitamin D through the gastrointestinal tract.
A significant correlation between 25(OH)D and albumin concentrations also has been reported in humans with IBD. The cause of this association remains unclear. One possible explanation that also would offer a possible explanation for the development of hypovitaminosis D is protein loss through the diseased intestine leading to loss of both albumin and vitamin D that is bound to vitamin D‐binding protein (DBP), which is structurally similar to albumin and is the predominant transport protein for vitamin D metabolites.
Decreased hepatic production of DBP secondary to chronic inflammation also has been suggested as a possible cause for hypovitaminosis D in people with IBD. However, a chronic inflammatory state also would be expected in the hospitalized ill cats, and as such this is unlikely to be the primary mechanism for hypovitaminosis D. It is unlikely that decreased exposure to sunlight, which may play a role in the development of hypovitaminosis D in humans, is involved in its development in cats because it has been shown that cats cannot effectively synthesize vitamin D in their skin.
Evidence is accumulating from experimental models of IBD to suggest that hypovitaminosis D may predispose to the development of IBD rather than simply be a secondary consequence of the condition. Numerous studies have shown that the most biologically active form of vitamin D, 1,25 dihydroxyvitamin D (1,25[OH]2D), can have a marked effect on the innate and adaptive immune system. These effects include downregulating the expression of proinflammatory cytokines such as tumor necrosis factor‐α and interferon‐γ as well as impairing the ability of dendritic cells to prime T cells.20
Although the majority of cats with IBD or ISCL had serum 25(OH)D concentrations below the lowest 25(OH)D concentration in the healthy feline group, this rarely resulted in hypocalcemia. Serum total hypocalcemia only was found in 2 cats, and in both cases, the cats were diagnosed with IBD. Concurrent hypoalbuminemia was documented in 1 of the cats. Total and ionized hypocalcemia also has been documented in dogs with IBD and concurrent hypoalbuminemia. Hypoalbuminemia attributable to gastrointestinal disease in cats, however, is rare as also was found in this study.1
In summary, this study showed that, similar to human patients and dogs with IBD, cats with IBD and ISCL frequently have decreased serum 25(OH)D concentrations. The pathophysiology of hypovitaminosis D in canine and feline gastrointestinal disease is not clear. It remains to be clarified whether it is a consequence of gastrointestinal disease or whether it plays a role in the development of the disease. Additional studies are needed to make this distinction, to investigate its potential prognostic implications, and to define the most appropriate therapeutic approaches to reverse hypovitaminosis D.
Acknowledgments
Stephanie Lalor's and Lara Boland's residencies were supported by International Cat Care (previously the Feline Advisory Bureau). We thank all of the veterinarians and owners involved in the care of the cats in the study.
Funding: This study was funded by the RCVS Trust Fund.
Conflict of Interest Declaration: The authors disclose no conflict of interest.
Footnotes
KoneLab 60i; Thermo Clinical Labsystems, Vantaa, Finland
Waters Associates, Milford, MA
Hichrom, Reading, UK
GraphPad Prism 5, La Jolla, CA
References
- 1. Jergens AE. Feline idiopathic inflammatory bowel disease: What we know and what remains to be unraveled. J Feline Med Surg 2012;14:445–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kleinschmidt S, Harder J, Nolte I, et al. Chronic inflammatory and non‐inflammatory diseases of the gastrointestinal tract in cats: Diagnostic advantages of full‐thickness intestinal and extraintestinal biopsies. J Feline Med Surg 2010;12:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Evans SE, Bonczynski JJ, Broussard JD, et al. Comparison of endoscopic and full‐thickness biopsy specimens for diagnosis of inflammatory bowel disease and alimentary tract lymphoma in cats. J Am Vet Med Assoc 2006;229:1447–1450. [DOI] [PubMed] [Google Scholar]
- 4. Kiupel M, Smedley RC, Pfent C, et al. Diagnostic algorithm to differentiate lymphoma from inflammation in feline small intestinal biopsy samples. Vet Pathol 2011;48:212–222. [DOI] [PubMed] [Google Scholar]
- 5. Edwards DF, Russell RG. Probable vitamin K‐deficient bleeding in two cats with malabsorption syndrome secondary to lymphocytic‐plasmacytic enteritis. J Vet Intern Med 1987;1:97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gow AG, Else R, Evans H, et al. Hypovitaminosis D in dogs with inflammatory bowel disease and hypoalbuminaemia. J Small Anim Pract 2011;52:411–418. [DOI] [PubMed] [Google Scholar]
- 7. Dhupa N, Proulx J. Hypocalcemia and hypomagnesemia. Vet Clin North Am Small Anim Pract 1998;28:587–608. [DOI] [PubMed] [Google Scholar]
- 8. Alkhouri RH, Hashmi H, Baker RD, et al. Vitamin and mineral status in patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2013;56:89–92. [DOI] [PubMed] [Google Scholar]
- 9. Louwerens M, London CA, Pedersen NC, et al. Feline lymphoma in the post‐feline leukemia virus era. J Vet Intern Med 2005;19:329–335. [DOI] [PubMed] [Google Scholar]
- 10. Gieger T. Alimentary lymphoma in cats and dogs. Vet Clin North Am Small Anim Pract 2011;41:419–432. [DOI] [PubMed] [Google Scholar]
- 11. Zerwekh JE. Blood biomarkers of vitamin D status. Am J Clin Nutr 2008;87:1087S–1091S. [DOI] [PubMed] [Google Scholar]
- 12. Lalor SM, Mellanby RJ, Friend EJ, et al. Domesticated cats with active mycobacteria infections have low serum vitamin D (25(OH)D) concentrations. Transbound Emerg Dis 2012;59:279–281. [DOI] [PubMed] [Google Scholar]
- 13. Agborsangaya C, Toriola AT, Grankvist K, et al. The effects of storage time and sampling season on the stability of serum 25‐hydroxy vitamin D and androstenedione. Nutr Cancer 2010;62:51–57. [DOI] [PubMed] [Google Scholar]
- 14. Ramsey IK, Tebb A, Harris E, et al. Hyperparathyroidism in dogs with hyperadrenocorticism. J Small Anim Pract 2005;46:531–536. [DOI] [PubMed] [Google Scholar]
- 15. Tebb AJ, Arteaga A, Evans H, et al. Canine hyperadrenocorticism: Effects of trilostane on parathyroid hormone, calcium and phosphate concentrations. J Small Anim Pract 2005;46:537–542. [DOI] [PubMed] [Google Scholar]
- 16. Berry JL, Martin J, Mawer EB. 25‐Hydroxyvitamin D assay kits: Speed at the cost of accuracy? In: Norman AW, Bouillion R, Thomasset M, eds. Vitamin D Endocrine System: Structural, Biological, Genetic and Clinical Aspects. Riverside,CA: University of California, 2000:797–800. [Google Scholar]
- 17. Garg M, Lubel JS, Sparrow MP, et al. Review article: Vitamin D and inflammatory bowel disease—Established concepts and future directions. Aliment Pharmacol Ther 2012;36:324–344. [DOI] [PubMed] [Google Scholar]
- 18. Ananthakrishnan AN, Khalili H, Higuchi LM, et al. Higher predicted vitamin D status is associated with reduced risk of Crohn's disease. Gastroenterology 2012;142:482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pappa HM, Mitchell PD, Jiang H, et al. Treatment of vitamin D insufficiency in children and adolescents with inflammatory bowel disease: A randomized clinical trial comparing three regimens. J Clin Endocrinol Metab 2012;97:2134–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mora JR, Iwata M, Von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol 2008;8:685–698. [DOI] [PMC free article] [PubMed] [Google Scholar]


