Abstract
Background
Atrial fibrillation (AF) cycle length (CL) and atrial size have been used in humans to characterize electrical and structural remodeling to predict outcome of cardioversion of AF and risk for AF recurrence (rAF).
Hypothesis
Atrial fibrillation cycle length can be determined in horses with AF, and AFCL and atrial size are related to risk for rAF.
Animals
Eighteen horses with naturally occurring AF that were successfully converted to sinus rhythm (SR) by transvenous electrical cardioversion (TVEC).
Methods
Prospective study. Horses with severe valvular regurgitation, left atrial enlargement, or that required sedation for catheter placement were excluded. In all horses intra‐atrial electrograms were recorded and estimated AF duration and echocardiographic parameters were determined before TVEC. The follow‐up time was 1 year after TVEC.
Results
Atrial fibrillation cycle length could be determined in all horses. The AFCL and the shortest 5th percentile (p5) AFCL in horses with rAF (n = 6 or 33%) were (mean ± SD) 157 ± 28 and 134 ± 24 milliseconds, respectively, and in those maintaining SR (n = 12 or 67%) 166 ± 13 and 141 ± 13 milliseconds, respectively. Significant parameters to predict rAF were (1) the ratios of the p5AFCL to the left atrium (LA) sizes corrected to the size of aorta (AO) and (2) LA sizes corrected to the size of AO.
Conclusions and Clinical Importance
Before TVEC, assessment of LA size and atrial electrophysiologic characteristics might help to identify horses at increased risk for AF recurrence.
Keywords: Atrial effective refractory period, Atrial size, Electrophysiology, Recurrence of atrial fibrillation
Abbreviations
- A
area
- AERP
atrial effective refractory period
- AF
atrial fibrillation
- AFCL
atrial fibrillation cycle length
- AO
aorta
- LA
left atrium
- la
long‐axis
- L
left
- mSR
maintenance of sinus rhythm
- p5AFCL
shortest 5th percentile atrial fibrillation cycle length
- rAF
recurrence of atrial fibrillation
- R
right
- sa
short‐axis
- SR
sinus rhythm
- TVEC
transvenous electrical cardioversion
Atrial fibrillation (AF) is the most important supraventricular dysrhythmia in horses with an impact on athletic performance. AF can be treated pharmacologically using quinidine sulfate (QS) or by transvenous electrical cardioversion (TVEC).1, 2, 3, 4 Currently, there are no studies that compare success rates between the 2 techniques. Recurrence rate after QS treatment is between 15 and 40% and AF duration is a major risk factor.1, 5 Data for the recurrence rate after TVEC are currently lacking, but recurrence rate is probably independent of the treatment technique and therefore is likely to be similar to that reported for pharmacologic conversion of AF.6 Objective criteria that allow prediction of AF recurrence (rAF) after successful TVEC are currently unavailable in horses. In 2 studies on AF in horses, recurrences became more frequent when AF was present for a longer period of time before treatment.5, 7 Atrial size, duration of AF, and atrial fibrillation cycle length (AFCL) are parameters used in human medicine to predict recurrence of AF after successful treatment.8, 9 AFCL is an index of atrial effective refractory period (AERP), an electrophysiologic parameter strongly related to AF susceptibility. AF‐induced electrical remodeling shortens AERP and leads to increased AF sustainability.10, 11 After restoration of sinus rhythm (SR), reverse electrical remodeling to pre‐AF levels takes time, during which the atria are vulnerable to early rAF.2, 10, 12
The purpose of this study was to describe a method to record right intra‐atrial electrograms in horses with AF and to identify possible factors that might be related to rAF after successful TVEC.
Materials and Methods
Study Population
Horses referred to the Department of Large Animal Internal Medicine for treatment of AF were included in this study. Mean age, body weight, and height at the withers were recorded (Table 1). All horses were treated by TVEC. If SR could not be restored by TVEC alone, horses received 5 mg/kg amiodarone IV over 30 minutes and TVEC was repeated. Horses that presented ≥30 atrial premature depolarizations in the first 15 minutes postcardioversion received 5 mg/kg amiodarone IV over 30 minutes before they were allowed to recover from general anesthesia. Inclusion criteria were the availability of a clinical examination, a base‐apex ECG recording, a full echocardiographic examination1 before TVEC, and successful restoration of SR. Horses with severe valvular regurgitation or enlargement of the left atrium (LA) (left parasternal long‐axis view and during peak ventricular systole, >14.5 cm) were excluded from the study. Horses that required sedation for catheter placement, where excluded from the study. AF duration was estimated based upon history and previous examinations (Table 1). Only horses in which AF was determined for the first time were used in this study.
Table 1.
Detailed information about the patients with atrial fibrillation
| Patient | Estimated AF Duration (months) | MR | TR | AR | PR | Time of rAF (days) |
|---|---|---|---|---|---|---|
| 1 | 9 | — | Moderate | — | — | 2 |
| 2 | 8 | — | Trivial | — | Trivial | 202 |
| 3 | 6 | Mild | Moderate | Mild | — | 72 |
| 4 | 6 | Mild | Trivial | — | — | 354 |
| 5 | 1.5 | Trivial | Trivial | Trivial | Trivial | 25 |
| 6 | 9 | Mild | — | — | — | 355 |
| 7 | 6 | Mild | Trivial | Mild | Moderate | — |
| 8 | 18 | Mild | Mild | — | — | — |
| 9 | 5 | Mild | Mild | Moderate | Trivial | — |
| 10 | 5 | Trivial | Trivial | — | Moderate | — |
| 11 | 5 | Trivial | — | — | — | — |
| 12 | 4 | — | Moderate | Trivial | Trivial | — |
| 13 | 6 | — | Trivial | Trivial | Trivial | — |
| 14 | 0.3 | — | — | — | — | — |
| 15 | 4 | — | — | Mild | — | — |
| 16 | 1.5 | — | — | — | — | — |
| 17 | 6 | — | — | Trivial | — | — |
| 18 | 1 | Trivial | Trivial | Mild | Trivial | — |
MR, mitral valve regurgitation; TR, tricuspid valve regurgitation; AR, aortic valve regurgitation; PR, pulmonic valve regurgitation; rAF, recurrence of atrial fibrillation.
Left Atrial and Aortic Size and Area
The two‐dimensional ultrasonographic measurements from the left (L) and right (R) parasternal long‐axis (la) and short‐axis (sa) view were performed. For the LA, the maximal internal diameter (LA) and area (LAA) were determined at ventricular end‐systole. LA diameters, on the long‐axis views, were measured from the interatrial septum to the atrial free wall, parallel to the mitral annulus (LLA and RLAla).
From a right parasternal short‐axis (sa) view, maximal internal diameter and area (A) of LA (LAsa, LAAsa) and aorta (AOsa, AOAsa) during ventricular systole were obtained.12 For the internal short‐axis diameter of the LA, calipers were placed in a line extending from and parallel to the commissure between the noncoronary and left coronary aortic valve cusps to the distant margin of the LA.12 For the internal short‐axis diameter of the AO at valvular level, calipers were placed along the commissure between the noncoronary and right coronary aortic valve cusps. From a right parasternal long‐axis view the aortic annulus diameter (AOla) was determined by measuring the inner distance between the opened aortic valve leaflets during peak systole.13 From this diameter, aortic area (AOAla) was calculated as π.(Aola/2).2
Left atrium diameters and areas measured from the right long‐ or short‐axis view were indexed to size of the AO in the same view, to obtain a correction for horse size.
AFCL Recording
In the standing unsedated horse, a 14F introducer sheath2 was inserted in the lower half of the right jugular vein. Through this sheath 2 cardioversion catheters3 and 1 bipolar sensing/pacing electrode4 were inserted under echocardiographic guidance. The sensing/pacing catheter was positioned in the right atrium (RA) to record a bipolar intra‐atrial electrogram during 15 minutes whereas cardioversion catheters were positioned in the left pulmonary artery and the RA. Digital electrogram recording was performed with a modified (K. Engel5) ECG recording device (Televet 100 version 5.0.05) that allowed simultaneous recording of 2 independent bipolar traces, the intra‐atrial and surface ECG trace. During off‐line analysis, the time interval between consecutive intra‐atrial depolarizations, which is the AFCL, was manually measured over 500 depolarizations using dedicated software5 (Fig 1). Paper speed and amplitude were 50 mm/s and 10 mm/mV, respectively. From these data a mean AFCL, a 5th percentile (p5) AFCL, and a ratio of p5AFCL to echocardiographic measurements were calculated.
Figure 1.
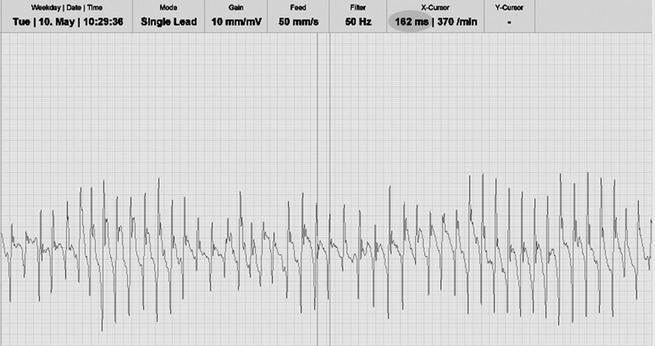
Intra‐atrial electrogram from a horse with atrial fibrillation. The atrial fibrillation cycle length is the time (milliseconds) between 2 successive atrial depolarizations. The current measurement indicates an atrial fibrillation cycle length of 162 milliseconds (gray circle) which corresponds to a fibrillation rate of 370/min.
After TVEC and recovery, surface electrocardiograms were recorded on days 1, 2, 7, and 49 after cardioversion in order to confirm the presence of SR or rAF. The follow‐up period was 1 year after successful cardioversion. The local veterinarian checked the horse, at regular time intervals, for maintenance of SR or rAF by auscultation. If a dysrhythmia was present, an ECG was performed. In all patients, except 1 (recurrence of AF 2 days after TVEC), the recurrence of AF was determined by the local veterinarian (auscultation and ECG) (Table 1).
Statistical Analysis
All variables are reported as mean ± SD. Independent samples t‐tests were performed on all parameters shown in Table 2 between horses maintaining SR (mSR) and those who suffered from rAF. The level of significance was α = 0.05.
Table 2.
Baseline characteristics (mean ± SD) of the horses remaining in SR and those suffering from AF recurrence after transvenous electrical cardioversion
| mSR | rAF | P value | |
|---|---|---|---|
| Number of horses | 12 | 6 | — |
| Age (years) | 10.1 ± 3.0 | 8.7 ± 4.1 | .423 |
| Body weight (kg) | 571 ± 57 | 582 ± 70 | .727 |
| Heigth (cm) | 170 ± 6 | 167 ± 9 | .443 |
| Estimated duration AF (months) | 5.2 ± 4.5 | 6.6 ± 2.8 | .491 |
| LLA (cm) | 13.1 ± 0.7 | 13.6 ± 0.9 | .380 |
| LLAA (cm2) | 91.7 ± 13.2 | 105.9 ± 9.8 | .034 |
| RLAla (cm) | 12.7 ± 1.2 | 13.4 ± 0.7 | .218 |
| RLAla/Aola | 1.68 ± 0.12 | 1.84 ± 0.16 | .022 |
| RLAlaA (cm2) | 95.5 ± 14.6 | 106.7 ± 10.4 | .114 |
| RLAlaA/AOAla | 2.13 ± 0.28 | 2.61 ± 0.47 | .016 |
| LAsa/Aosa | 1.04 ± 0.12 | 1.16 ± 0.11 | .056 |
| LAAsa/AOAsa | 1.65 ± 0.25 | 1.91 ± 0.31 | .073 |
| Mean AFCL (milliseconds) | 166 ± 13 | 157 ± 28 | .351 |
| p5AFCL (milliseconds) | 141 ± 13 | 134 ± 24 | .446 |
| p5AFCL/LLA (ms/cm) | 11.3 ± 1.6 | 10.3 ± 1.8 | .228 |
| p5AFCL/LLAA (ms/cm2) | 1.58 ± 0.33 | 1.29 ± 0.31 | .095 |
| p5AFCL/RLAla (ms/cm) | 11.2 ± 1.5 | 10.1 ± 1.7 | .173 |
| p5AFCL/(RLAla/AOla) (milliseconds) | 126.5 ± 16.5 | 114.4 ± 16.7 | .164 |
| p5AFCL/RLAlaA (ms/cm2) | 1.52 ± 0.31 | 1.28 ± 0.30 | .141 |
| p5AFCL/(RLAlaA/A0Ala) (milliseconds) | 67.7 ± 13.2 | 53.2 ± 13.1 | .043 |
| p5AFCL/(LAsa/A0sa) (milliseconds) | 138.0 ± 19.7 | 116.1 ± 15.5 | .031 |
| p5AFCL/(LAAsa/A0Asa) (milliseconds) | 87.7 ± 16.9 | 71.0 ± 10.9 | .044 |
mSR, maintenance of sinus rhythm; rAF, recurrence of atrial fibrillation; LLA, left atrial diameter or area (LLAA) measured from the left parasternal long‐axis view; RLAla, left atrial diameter or area (RLAlaA) measured from the right parasternal long‐axis view; LAsa, left atrial internal diameter or area (LAAsa) measured from the right parasternal short‐axis view; AOla, aortic diameter or area (AOAla) measured from the right parasternal long‐axis view; AOsa, aortic diameter or area (AOAsa) measured from the right parasternal short‐axis view; AFCL, atrial fibrillation cycle length; p5, shortest 5th percentile; AF, atrial fibrillation.
Results
During the study period, 41 horses were presented for AF. Thirteen horses were not treated because the owner denied (n = 6) or because of underlying heart disease (n = 7). Three horses were treated with QS, administered via a nasogastric tube. In 1 horse QS treatment was terminated because of allergic reactions. In 25 horses TVEC was performed and 23 horses successfully converted of which 3 required preoperative amiodarone administration to achieve cardioversion. In all horses, high‐quality intra‐atrial electrograms could be recorded and allowed accurate determination of AFCL. Five horses were sedated for catheter placement and measurements were, therefore, excluded from the study. Eighteen horses (8 geldings, 5 mares, 5 stallions; 15 warmbloods, 1 Friesian, 1 Anglo‐Arabian, 1 trotter horse) met the inclusion criteria. Three horses had ≥30 atrial premature depolarizations within 15 minutes after restoration of SR and received amiodarone IV. None of the horses that received amiodarone for cardioversion showed atrial premature depolarizations immediately postcardioversion. Six horses had rAF which occurred between day 2 and 355 (Table 1). Table 2 shows an overview of all measured parameters.
Values are shown for horses remaining in mSR (n = 12; 67%) versus horses suffering from rAF (n = 6; 33%). There was no significant difference for mean age, body weight, height at the withers, estimated duration of AF, LLA, RLA, RLAA, LAsa/AOsa, LAAsa/AOAsa, mean AFCL, p5AFCL, p5AFCL/LLA, p5AFCL/LLAA, p5AFCL/RLA, p5AFCL/(RLA/AO), and p5AFCL/RLAA between horses who remained in SR and those suffering from rAF. The LLAA, RLAla/AOla, and RLAlaA/AOAla were significantly higher in horses suffering from rAF. P5AFCL/(RLAA/AOAla), p5AFCL/(LAsa/AOsa), and p5AFCL/(LAAsa/AOAsa) were significantly lower in horses suffering from rAF.
Individual results of mean AFCL as a function of estimated duration of AF are shown in Fig 2. There was a significant negative correlation between mean AFCL and estimated duration of AF (Spearman's rank correlation coefficient −0.628; P = .005).
Discussion
This study shows that AFCL can be recorded in standing horses. This study describes risk factors for rAF in horses after electrical cardioversion. The ratios of the p5AFCL to the corrected LA sizes and the corrected LA sizes were associated with rAF after restoration of SR.
Recurrence rate after TVEC of horses (56/63 were racehorses) with a median AF duration of 4 weeks is 15% (11/72).4 Overall recurrence rate after pharmacologic treatment of racehorses is 30% but appeared 15% for AF of <1 month duration, 14% for AF of <4 months duration, and 62% for horses with AF of more than 4 months.5 The recurrence rate in our study (33%), with most horses showing long‐lasting AF, was comparable with pharmacologic treatment of AF. The high prevalence of chronic AF in our horse population was probably related to our horse population (16/18 were warmbloods) and the associated sport discipline (most horses used for jumping or dressage). Indeed, compared to warmbloods, racehorses with AF are generally younger and AF is often of shorter duration because clinical signs are usually more obvious in racehorses compared to jumping or dressage horses.
The inducibility and sustainability of AF depends on the ability of the atria to maintain a critical number of re‐entry circuits.14 Identified factors in favor of AF are large atrial size, short AERP, and atrial structural disease.10, 11 In horses, there is no gold standard for determination of the most accurate LA diameter. Therefore, LA sizes from left and right parasternal views were obtained. Atrial sizes were indexed to the aortic size to obtain body size independent parameters.15 Similarly as previously reported, larger atria seemed to predispose to rAF,5 especially when atrial size was corrected for aortic size.
Since the length of a wavelet circulating in the atria is the product of conduction velocity and AERP, a decrease in either conduction velocity or AERP will lead to an increase in the maximum number of wavelets that can coexist in the atria.16 AERP can be determined in horses during SR by programmed electrical stimulation using temporary or implanted pacing devices.17, 18, 19 However, during AF this technique cannot be used. AFCL and minimum AFCL (5th percentile of AFCL, p5AFCL) have been shown to be an index of AERP.20, 21 From reports investigating the effects of AF on atrial electrophysiology it has been concluded that AFCL and AERP shorten with maintenance of AF, and prolong progressively after medical treatment or ablation.10, 22, 23, 24 An experimentally induced AF model in ponies and horses showed that the shortening of AFCL occurs mainly in the first weeks after AF induction.12, 18, 25 Our study in horses with naturally occurring AF confirmed that increasing AF duration was significantly correlated with shortening of AFCL (Fig 2) (P = .005).
Figure 2.
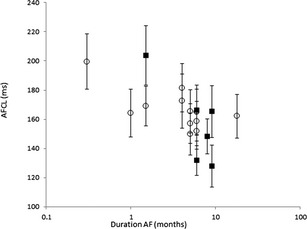
Scatterplot of atrial fibrillation cycle length (AFCL; milliseconds; mean ± SD) as a function of estimated atrial fibrillation duration on a logarithmic scale of horses with recurrence of atrial fibrillation (■) and those remaining in sinus rhythm (○) after successful transvenous electrical cardioversion. There is a significant negative correlation between mean AFCL and estimated duration of AF (Spearman's rank correlation coefficient −0.628; P = .005).
In humans, AF duration, left atrial size, and AFCL are the most important parameters to predict mSR after cardioversion.8, 9, 26, 27, 28 In 2 large studies in horses with AF, AF duration was related to recurrence rate.5, 7 Our study did not identify AF duration as a significant risk factor for rAF (Table 2). This might be explained by the low number of horses in our study, especially of horses with short AF duration before TVEC.
We obtained AFCL from the RA. In humans, left atrial electrophysiologic measurements are performed by introducing a catheter in the LA through a transseptal puncture, a procedure that has not yet been done in horses. Besides electrical remodeling, atrial size, and AF duration other factors should be considered in determining the risk of rAF. Documented additional factors potentially triggering and promoting AF are ectopic atrial foci, rotors, and structural remodeling of the atria.29, 30 Changes in the distribution of the protein connexin 40, myolysis characterized by disruption of the sarcoplasmatic reticulum, accumulation of glycogen and the presence of apoptosis or fibrosis have been shown to influence the outcome of cardioversion and rAF in human patients.31, 32, 33 Also in horses, atrial fibrosis has been associated with AF.34 Currently no additional information is available about the importance of these mechanisms in horses.
Our study included a number of limitations. A first limitation of our study is that baseline AERP (AERP during normal SR) was not measured. We measured AFCL which represents the AERP after electrical remodeling because of AF. AF duration but also individual degree of AF‐induced remodeling may differ between horses. In addition, the relation between baseline AERP and AERP in the remodeled atrium may show individual differences. A second limitation of this study was that the presence of atrial structural lesion could not be established. In a number of horses, date of diagnosis of AF by the local veterinarian was used to estimate AF duration. However, as clinical signs of AF are less obvious in warmbloods compared to racehorses, real AF duration was probably even longer.
In conclusion, RA AFCL can be determined in standing horses. Despite the small number of horses, this study shows that recurrence of AF is associated with differences in left atrial size and electrophysiologic characteristics. Pretreatment measurement of these parameters might provide a risk assessment for rAF.
Acknowledgments
Grant support: The work was not supported by a grant.
Conflict of Interest Declaration: Authors disclose no conflict of interest.
This study was carried out at the Department of Large Animal Internal Medicine, Faculty of Veterinary Medicine, Ghent University
Footnotes
GE Vivid 7 Dimension, GE Healthcare, Horten, Norway and a 3S Phased Array Transducer, GE Healthcare
Triport; Mansfield EP, Watertown, MA
Custom catheter; Rhythm Technologies Inc, Irvine, CA
Bipolar intracardiac electrode, USCI; C. R. Bard, Inc, Lowell, MA
Engel Engineering Services GmbH, Offenbach an Main, Germany
References
- 1. Goltz A, Gehlen H, Rohn K, et al. Therapy of atrial fibrillation with class‐1A and class‐1C antiarrhythmic agents and ACE inhibitors. Pferdeheilkunde 2009;25:220–227. [Google Scholar]
- 2. De Clercq D, van Loon G, Schauvliege S, et al. Transvenous electrical cardioversion of atrial fibrillation in six horses using custom made cardioversion catheters. Vet J 2008;177:198–204. [DOI] [PubMed] [Google Scholar]
- 3. McGurrin MK, Physick‐Sheard PW, Kenney DG. How to perform transvenous electrical cardioversion in horses with atrial fibrillation. J Vet Cardiol 2005;7:109–119. [DOI] [PubMed] [Google Scholar]
- 4. McGurrin MKJ, Physick‐Sheard PW, Kenney DG. Transvenous electrical cardioversion of equine atrial fibrillation: Patient factors and clinical results in 72 treatment episodes. J Vet Intern Med 2008;22:609–615. [DOI] [PubMed] [Google Scholar]
- 5. Reef VB, Levitan CW, Spencer PA. Factors affecting prognosis and conversion in equine atrial fibrillation. J Vet Intern Med 1988;2:1–6. [DOI] [PubMed] [Google Scholar]
- 6. van Loon G. Dysrhythmias: cardiac pacing and electrical cardioversion In: Marr CM, Bowen M, eds. Cardiology of the Horse, 2nd ed Edinburgh: Saunders; 2010:179–192. [Google Scholar]
- 7. Morris DD, Fregin GF. Atrial fibrillation in horses: Factors associated with response to quinidine sulfate in 77 clinical cases. Cornell Vet 1982;72:339–349. [PubMed] [Google Scholar]
- 8. Meurling CJ, Roijer A, Waktare JE, et al. Prediction of sinus rhythm maintenance following DC‐cardioversion of persistent atrial fibrillation—The role of atrial cycle length. BMC Cardiovasc Disord 2006;6:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Holmqvist F, Stridh M, Waktare JE, et al. Atrial fibrillatory rate and sinus rhythm maintenance in patients undergoing cardioversion of persistent atrial fibrillation. Eur Heart J 2006;27:2201–2207. [DOI] [PubMed] [Google Scholar]
- 10. Wijffels M, Kirchhof C, Dorland R, et al. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation 1995;92:1954–1968. [DOI] [PubMed] [Google Scholar]
- 11. Everett TH, Li H, Mangrum JM, et al. Electrical, morphological, and ultrastructural remodeling and reverse remodeling in a canine model of chronic atrial fibrillation. Circulation 2000;102:1454–1460. [DOI] [PubMed] [Google Scholar]
- 12. De Clercq D, van Loon G, Tavernier R, et al. Atrial and ventricular electrical and contractile remodeling and reverse remodeling owing to short‐term pacing‐induced atrial fibrillation in horses. J Vet Intern Med 2008;22:1353–1359. [DOI] [PubMed] [Google Scholar]
- 13. Patteson MW, Gibbs C, Wotton PR. Echocardiographic measurements of cardiac dimensions and indices of cardiac function in normal adult thoroughbred horses. Equine Vet J 1995; supplement 19:18–27. [DOI] [PubMed] [Google Scholar]
- 14. Moe GK. On the multiple wavelet hypothesis of atrial fibrillation. Arch Int Pharmacodyn 1962;140:183–188. [Google Scholar]
- 15. Young L. Diseases of the heart and vessels In: Hinchcliff KW, Kaneps AJ, Geor RJ, eds. Equine Sports Medicine and Surgery. Edinburgh: Saunders; 2004:728–767. [Google Scholar]
- 16. Allessie MA, Lammers WJ, FI B. Experimental evaluation of Moe's multipele wavelet hypothesis of atrial fibrillation In: Zipes DP, Jalife J, eds. Cardiac Electrophysiology and Arrythmias. New York: Grune and Stratton Inc; 1985:26–275. [Google Scholar]
- 17. De Clercq D, van Loon G, Baert K, et al. Intravenous amiodarone treatment in horses with chronic atrial fibrillation. Vet J 2006;172:129–134. [DOI] [PubMed] [Google Scholar]
- 18. van Loon G. Atrial Pacing and Experimental Atrial Fibrillation in Equine. Ghent: Ghent University, Faculty Veterinary Medicine; 2001:1–253. [Google Scholar]
- 19. van Loon G, Duytschaever M, Tavernier R, et al. An equine model of chronic atrial fibrillation: Methodology. Vet J 2002;164:142–150. [DOI] [PubMed] [Google Scholar]
- 20. Kim IB, Rodefeld MD, Schuessler RB, et al. Relationship between local atrial fibrillation interval and refractory period in the isolated canine atrium. Circulation 1996;94:2961–2967. [DOI] [PubMed] [Google Scholar]
- 21. Duytschaever M, Mast F, Killian M, et al. Methods for determining the refractory period and excitable gap during persistent atrial fibrillation in the goat. Circulation 2001;104:957–962. [DOI] [PubMed] [Google Scholar]
- 22. Haissaguerre M, Sanders P, Hocini M, et al. Changes in atrial fibrillation cycle length and inducibility during catheter ablation and their relation to outcome. Circulation 2004;109:3007–3013. [DOI] [PubMed] [Google Scholar]
- 23. Matsuo S, Lellouche N, Wright M, et al. Clinical predictors of termination and clinical outcome of catheter ablation for persistent atrial fibrillation. J Am Coll Cardiol 2009;54:788–795. [DOI] [PubMed] [Google Scholar]
- 24. Goette A, Honeycutt C, Langberg JJ. Electrical remodeling in atrial fibrillation. Time course and mechanisms. Circulation 1996;94:2968–2974. [DOI] [PubMed] [Google Scholar]
- 25. De Clercq D. Pathophysiology and Treatment of Atrial Fibrillation in Horses. Ghent: Ghent University, Faculty Veterinary Medicine; 2008:1–233. [Google Scholar]
- 26. Frick M, Frykman V, Jensen‐Urstad M, et al. Factors predicting success rate and recurrence of atrial fibrillation after first electrical cardioversion in patients with persistent atrial fibrillation. Clin Cardiol 2001;24:238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Biffi M, Boriani G, Bartolotti M, et al. Atrial fibrillation recurrence after internal cardioversion: Prognostic importance of electrophysiological parameters. Heart 2002;87:443–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bollmann A, Husser D, Steinert R, et al. Echocardiographic and electrocardiographic predictors for atrial fibrillation recurrence following cardioversion. J Cardiovasc Electrophysiol 2003;14:S162–S165. [DOI] [PubMed] [Google Scholar]
- 29. Jais P, Haissaguerre M, Shah DC, et al. A focal source of atrial fibrillation treated by discrete radiofrequency ablation. Circulation 1997;95:572–576. [DOI] [PubMed] [Google Scholar]
- 30. Mandapati R, Skanes A, Chen J, et al. Stable microreentrant sources as a mechanism of atrial fibrillation in the isolated sheep heart. Circulation 2000;101:194–199. [DOI] [PubMed] [Google Scholar]
- 31. Li D, Fareh S, Leung TK, et al. Promotion of atrial fibrillation by heart failure in dogs: Atrial remodeling of a different sort. Circulation 1999;100:87–95. [DOI] [PubMed] [Google Scholar]
- 32. van der Velden HMW, van Zijverden M, van Kempen MJA, et al. Abnormal expression of the gap junction protein connexin 40 during chronic atrial fibrillation in the goat. Circulation 1996;94(Supp.1):l–593. [Google Scholar]
- 33. Ausma J, Wijffels M, Thone F, et al. Structural changes of atrial myocardium due to sustained atrial fibrillation in the goat. Circulation 1997;96:3157–3163. [DOI] [PubMed] [Google Scholar]
- 34. Else RW, Holmes JR. Pathological changes in atrial fibrillation in the horse. Equine Vet J 1971;3:56–64. [DOI] [PubMed] [Google Scholar]


