Abstract
Background
Neutrophil gelatinase–associated lipocalin (NGAL) is released from renal tubular cells after injury and serves in humans as a real‐time indicator of active kidney damage, including acute kidney injury (AKI) and chronic kidney disease (CKD). However, NGAL concentrations in dogs with naturally occurring AKI or CKD rarely have been explored in detail.
Hypothesis/Objectives
The goal of this study was to evaluate whether NGAL can serve as a useful biomarker in dogs with naturally occurring renal disease.
Animals
Client‐owned dogs with renal disease (57) and control dogs without any disease (12) were examined.
Methods
Serum NGAL (sNGAL) and urine NGAL (uNGAL) concentrations were measured in each animal by a newly developed ELISA system. Demographic, hematologic, and serum biochemical data were recorded. Survival attributable to AKI and CKD was evaluated at 30 days and 90 days, respectively.
Results
Serum and urine NGAL concentrations in azotemic dogs were significantly higher than in nonazotemic dogs and were highly correlated with serum creatinine concentration (P < .05). Among CKD dogs, death was associated with significantly higher sNGAL and uNGAL concentrations compared with survivors. Receiver‐operating characteristic curve (ROC) analysis showed that sNGAL was better than serum creatinine concentration when predicting clinical outcomes for CKD dogs (P < .05). The best cutoff point for sNGAL was 50.6 ng/mL, which gave a sensitivity and a specificity of 76.9 and 100%, respectively. Furthermore, dogs that had higher concentrations of sNGAL survived for a significantly shorter time.
Conclusion
sNGAL is a useful prognostic marker when evaluating dogs with CKD.
Keywords: Azotemia, Outcome, Renal biomarkers, Renal failure, Survival
Abbreviations
- AKI
acute kidney injury
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- AST
aspartate transaminase
- AUROC
area under the receiver‐operating characteristic curve
- BT
body temperature
- BUN
blood urea nitrogen
- CK
creatine kinase
- CKD
chronic kidney disease
- GFR
glomerular filtration rate
- Hb
hemoglobin
- HRP
horseradish peroxidase
- IgG
immunoglobulin G
- IQR
interquartile ranges
- NGAL
neutrophil gelatinase–associated lipocalin
- OD
optical density
- PBS
phosphate buffered saline
- ROC
receiver‐operating characteristic curve
- SD
standard deviation
- WBC
white blood cell
Patients with either acute kidney injury (AKI) or chronic kidney disease (CKD) can be asymptomatic initially. Several biomarkers can be useful in diagnosing early renal disease, such as detecting urinary protein at an early stage of CKD or measuring the urine gamma‐glutamyl transpeptidase (GGT)‐to‐creatinine ratio to assess aminoglycoside nephro‐toxicity.1 Currently, however, the assessment of both diseases depends mainly on determining the decrease in the glomerular filtration rate (GFR), which is most commonly estimated by an increase in serum creatinine concentration. Unfortunately, serum creatinine concentration only increase when 75% of kidney function has been lost,2 which substantially delays the time when renal insult is identified3 and limits the adoption of early and proper treatment. As a consequence, a poor outcome is common among patients with renal disease. Therefore, identification of more reliable biomarkers for kidney disease is warranted.
Neutrophil gelatinase–associated lipocalin (NGAL), also called lipocalin 2, is a glycoprotein with a molecular weight of 25 kDa and a member of the lipocalin family.4 It was initially purified from neutrophils during infection and inflammation.5 However, NGAL is also expressed in the uterus, prostate gland, salivary glands, bone marrow, stomach, colon, trachea, lungs, liver, and kidneys.6 With its siderophore‐chelating property, NGAL is considered to be a bacteriostatic agent.7 In addition, NGAL has been shown to be involved in the attenuation of apoptosis and the enhancement of the proliferative response.8
Recently, both urine NGAL and serum NGAL have been found to be useful biomarkers for the prediction of AKI in relation to cardiovascular surgery,9, 10 kidney transplantation,11 contrast‐induced AKI,12 critical illness status,13 chronic heart failure with decreased GFR,14 and sepsis.15 In addition, NGAL has been reported to play a role in the prediction of the outcome of AKI after cardiac surgery in pediatric patients,16 in newborns,17 in patients with ST‐segment elevation myocardial infarction18 and after renal replacement therapy,19 as well as with various different grades of renal injury.20 In CKD, NGAL has been demonstrated to be useful as a biomarker for children with nephritis associated with systemic lupus erythematosus,21 in adults with stages 2–422 CKD with nondiabetic disease,23 in diabetes and hypertension,24 and when renal interstitial damage is present.25
In dogs, based on NGAL concentration, AKI can be predicted as early as 12 hours after surgery,26 and NGAL has been shown to increase in X‐linked hereditary nephropathy.27 In dogs with naturally occurring kidney disease, the uNGAL‐to‐creatinine ratio has been used to diagnose AKI.28 However, the prognostic role of NGAL in dogs has not been evaluated yet. The main aim of this study was to examine the diagnostic and prognostic value of urine and serum NGAL concentrations in dogs with a renal disease.
Materials and Methods
Patients and Sample Collection
Samples were collected from all dogs that were admitted to the Veterinary Teaching Hospital of the National Chung Hsing University (Taichung, Taiwan) from November 2009 to April 2011. The experimental protocol was approved by the Committee on the Ethics of Animal Experiments of National Chung Hsing University (Approval No: 102‐63). Dogs with increased serum creatinine concentration (>1.5 mg/dL) were diagnosed as having azotemia and these animals were recruited to this study; these cases included those with concurrent illness or with prerenal causes. However, cases with urinary obstruction disease were considered postrenal and were excluded. Histopathology was not performed and thus cause of azotemia was not available for the dogs in our study.
Azotemic dogs with a clinical history of ≤7 days of duration and without any signs of CKD (eg, persistent azotemia, polyuria, polydipsia, or small kidneys) were categorized as having AKI. Because acute‐on‐chronic renal disease can be difficult to distinguish from CKD, the remainders of cases in the study were classified as CKD. In addition, 12 healthy client‐owned dogs presented for routine health care were used as a control group. These were confirmed as normal urinalysis as well as serum biochemistry.
All urine and serum samples were collected from patients on the day of treatment. However, urine samples of patients with oliguria or auria could not be collected on the first day, and, in several cases, both urine and serum sample could not be obtained simultaneously. Each patient's information was recorded and included demographic data (age, sex, body weight), hematologic data (hematocrit, white blood cell count, neutrophil count), and serum biochemistry data, including aspartate aminotransferase activity (AST), alanine aminotransferase activity (ALT), alkaline phosphatase activity (ALP), and blood urea nitrogen (BUN), creatinine, total protein, albumin, calcium, phosphorus, sodium, potassium, and chloride concentration. Some variables were not measured in each patient. Survival times were determined at 30 days when the dog was diagnosed with AKI and at 90 days when the dog was diagnosed with CKD. Furthermore, dogs that responded poorly to treatment because of renal‐related progression and were also euthanized were classified as dead. However, dogs that died or were euthanized because of unrelated causes (eg, trauma, gastric dilatation, and volvulus syndrome) or those that were unavailable for follow‐up were all excluded from the study.
ELISA Test for sNGAL and uNGAL
All samples were stored at −80°C before evaluation. Concentrations of NGAL in serum (sNGAL) and NGAL in urine NGAL (uNGAL) were measured by sandwich ELISA and following the procedure described in a previous study.26 Briefly, the capture antibody (rabbit anti‐NGAL polyclonal antibody), tested samples, and detection antibody (mouse anti‐NGAL polyclonal antibody) were diluted 1 : 800, 1 : 20, and 1 : 3,000, respectively. After removal of unbound antibody by washing with PBS, 5,000‐fold diluted HRP‐conjugated goat anti‐mouse IgG antibody was added to each well. After 1 hour of incubation, the result was visualized using a tetramethylbenzidine substrate kit.1 Each sample was tested in triplicate, and the OD of the triplicates was averaged. Samples with an OD value 3X> that of serum obtained from the normal control group were considered positive.
Statistical Analysis
Analysis was performed using commercial software.2 Variable data were first assessed by the Shapiro‐Wilk test. Normally distributed data were reported as means and standard deviation (SD) and the Student's t‐test was used to compare the difference between 2 groups. Non‐normally distributed data were expressed as medians with interquartile range (IQR) and were statistically analyzed by the Mann‐Whitney U‐test. Categorical data were reported as proportions and the difference between groups was evaluated by the Pearson chi‐square test or Fisher's exact test as appropriate. Pearson correlation was adopted to evaluate the correlation between factors related to death in dogs with azotemia. Univariate logistic regression analysis was applied to determine whether NGAL was related to death associated with renal disease. Finally, when predicting prognosis, the area under the receiver‐operating characteristic curve (AUROC) was used to compare the performances of serum creatinine concentration, serum NGAL, and urine NGAL in the presence of renal disease.
According to the best cutoff of the ROC, patients with values ≥ and < the number were classified into a high value group and a low value group, respectively. The Kaplan‐Meier method was used to assess the 30‐day survival curve of the AKI subgroup and the 90‐day survival curve of the CKD subgroup. The differences between the 2 groups were simultaneously evaluated by the log‐rank test. A P value of <.05 was considered as significant.
Results
In total, 57 cases with azotemia were collected. Seventeen cases were classified as AKI and 40 cases were classified as CKD. Compared with the dogs in the control group, the azotemic dogs were significantly older and had higher concentrations of serum creatinine, sNGAL, and uNGAL (P < .05; Table 1).
Table 1.
Characteristics, serum creatinine, sNGAL, and uNGAL in the azotemic and control groups.
| Variable | Azotemia | Control | P c |
|---|---|---|---|
| Sex (male) | 53% (30/57) | 33% (4/12) | .34 |
| Age (years)b | 8.5 (7.8; n = 56 | 2 (2); n = 12 | <.01 |
| Body weight (kg)b | 9.5 (10.3; n = 55 | 6.8 (14); n = 12 | .42 |
| Serum creatinine concentration (mg/dL)a | 7.3 (0.7); n = 57 | 1.0 (0.1); n = 12 | <.01 |
| sNGAL (ng/mL)a | 49.4 (13.4); n = 51 | 12.7 (9.2); n = 12 | <.01 |
| uNGAL (ng/mL)b | 56.3 (22.9); n = 51 | 7.6 (7.0); n = 12 | <.01 |
Mean (SE).
Median (interquartile range).
P < .05 is considered significant.
For all patients, serum creatinine concentration was significantly correlated with both sNGAL and uNGAL concentrations. In addition, sNGAL concentration was also significantly correlated with uNGAL concentration. However, the number of white blood cell count was not associated with the sNGAL concentration. When the dogs were classified into the azotemia and CKD groups, serum creatinine was also highly correlated with sNGAL concentration. However, creatinine, sNGAL, and uNGAL showed no significant correlation with each other in the AKI group (Table 2).
Table 2.
The correlations among creatinine, uNGAL, sNGAL, and white blood cell count (WBC).
| Variable | Pearson Correlation | P a | |
|---|---|---|---|
| All | Creatinine:sNGAL | 0.507 | <.01 |
| Creatinine:uNGAL | 0.341 | .01 | |
| sNGAL:uNGAL | 0.734 | <.01 | |
| WBC:sNGAL | 0.136 | .35 | |
| Azotemia | Creatinine:sNGAL | 0.308 | .02 |
| Creatinine:uNGAL | 0.172 | .41 | |
| sNGAL:uNGAL | 0.208 | .17 | |
| WBC:sNGAL | 0.1 | .51 | |
| AKI | Creatinine:sNGAL | 0.141 | .61 |
| Creatinine:uNGAL | 0.046 | .87 | |
| sNGAL:uNGAL | 0.021 | .94 | |
| WBC:sNGAL | 0.118 | .73 | |
| CKD | Creatinine:sNGAL | 0.377 | .02 |
| Creatinine:uNGAL | 0.188 | .27 | |
| sNGAL:uNGAL | 0.281 | .11 | |
| WBC:sNGAL | 0.105 | .56 |
Correlation is significant at P < .05 (2‐tailed).
The 30‐day case fatality rate for dogs with AKI was 64.7% (11/17). Most variables (sex, age, body weight, white blood cell count, PCV, AST, ALT, ALP, total protein, Albumin, BUN, Calcium, Phosphorus, Sodium, Potassium) were not significantly different between the survival and nonsurvival groups except for serum creatinine concentration (64.1 ng/mL in dead vs. 41.7 ng/mL in the survivor group, P < .01). The 90‐day case fatality rate for dogs with CKD was 72.5% (29/40). The nonsurvivors had significantly higher concentrations of sNGAL (64.1 ng/mL in dead versus 41.7 ng/mL in the survivor group, P = .002), uNGAL (52.5 ng/mL in dead versus 42.8 ng/mL in survivor group, P = .02), and serum creatinine concentration (8.7 mg/dL in dead versus 4.9 mg/dL in the survivor group, P = .02), as well as higher white blood cell counts (19,600 cells/μL in dead versus 10,750 cells/μL in the survivor group, P < .01).
The results of the univariate logistic analysis indicated that an increase in both sNGAL and serum creatinine concentration significantly increase the odds ratio for death among CKD dogs. However, an increase in uNGAL concentration did not significantly increase the odds ratio for death among CKD dogs (Table 3). However, for the AKI dogs, all 3 variables were not significantly associated with an increase in odds ratio for the death (data not shown). In terms of ROC analysis, the AUROC of the sNGAL concentration (0.843) was the highest followed by serum creatinine concentration (0.767) and uNGAL concentration (0.745) among dogs with CKD (Fig 1).
Table 3.
Results of univariate logistic analysis for sNGAL, uNGAL, and creatinine in dogs with CKD.
| Variable | Odds Ratio | 95% CI | P valuea |
|---|---|---|---|
| SNGAL | 0.983 | 0.971–0.996 | <.01 |
| uNGAL | 0.997 | 0.993–1.001 | .09 |
| Creatinine | 0.769 | 0.603–0.980 | .03 |
P < .05 as significant
Figure 1.
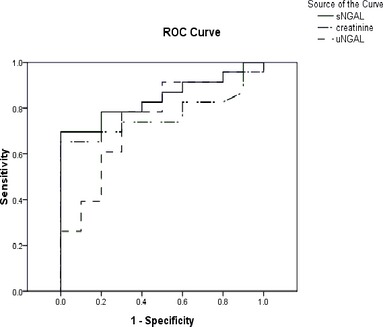
Receiver‐operating characteristic (ROC) curve analysis for sNGAL, serum creatinine concentration, and uNGAL. The areas under the ROC (AUROC) for the sNGAL, uNGAL, and serum creatinine concentration curves are 0.843, 0.745, and 0.767, respectively (P < .05). Using a cutoff point of 50.6 ng/mL for sNGAL, the sensitivity was 69.6% and the specificity was 100%. When a cutoff for uNGAL of 51.4 ng/mL was used, the sensitivity was 78.3% and the specificity was 70%. For creatinine, the best cutoff point was 6.95, which had a sensitivity of 65.2% and a specificity of 90%.
Based on a cutoff value of 50.6 ng/mL for sNGAL and 51.9 ng/mLfor uNGAL, dogs with CKD were divided into high‐value and low‐value groups. These consisted of those patients with values ≥ to the cutoff, and those patients with values < the cutoff value, respectively. For dogs with CKD (as shown in Fig 2), the cumulative 90‐day survival rates of the high‐value group and low‐value group were significantly different by log‐rank test for both the sNGAL and the uNGAL groups (P < .05). Median survival time was 7 days for the high‐value group and 35 days for the low‐value group using the sNGAL concentration. For dogs with AKI, the survival of the high‐value group and low‐value group using sNGAL and uNGAL concentrations were not significantly different (data not shown).
Figure 2.
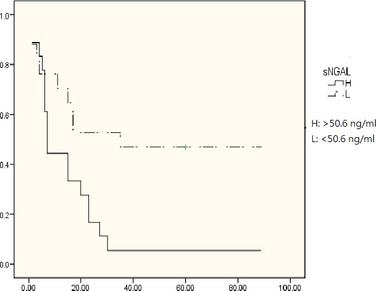
Comparison of the Kaplan‐Meier survival rate for urinary neutrophil gelatinase–associated lipocalin (sNGAL) concentrations. The correlation of survival rate with sNGAL concentration in the groups above and below the cutoff concentration (50.6 ng/mL, log‐rank test, P < .05) was analyzed using Kaplan‐Meier survival curves.
Discussion
Studies have demonstrated that NGAL is a useful biomarker in humans with renal disease,3 but the use of NGAL as a marker in dogs with naturally occurring kidney disease has only recently been explored in AKI.28 The results of this study indicated that NGAL is a promising renal biomarker when serum and urine NGAL concentrations in azotemic dogs, especially those with CKD, are evaluated. The serum and urine NGAL concentrations of these animals are significantly higher than those of healthy dogs. Moreover, sNGAL concentration is a better prognostic indicator than serum creatinine concentration based on ROC analysis, which is consistent with studies in humans.24, 29 These findings indicate that the sNGAL concentration may be a useful prognostic indicator for dogs with CKD.
Although studies in human patients have indicated that sNGAL concentration shows good predictive ability with AKI,20 in this study, an increase in sNGAL and uNGAL were not associated with serum creatinine concentration or death in AKI patients. There may be several reasons for this observation. First, most reported studies have evaluated disease progression based on an increase in serum creatinine concentration rather than using death. NGAL can dramatically increase at an early stage of damage, namely when the renal insult occurs, but then gradually decreases with time. Because most of the cases explored here were referrals, the peak concentration of NGAL may have been missed by the time a sample was collected. Second, the case numbers for AKI in our study were much lower than for CKD, and this limited statistical analysis. Third, the separation between AKI and CKD can be difficult. In our study, days of clinical signs were used as the arbitrary cutoff between AKI and CKD. However, dogs with acute‐on‐chronic kidney disease may have been grouped into the CKD disease group. More accurate diagnosis of diseases has been helpful in grouping cases appropriately. Similarly, NGAL in our study was not significantly related to azotemia. This result is not in agreement with a recent study28 in which the uNGAL‐to‐urine creatinine ratio was evaluated as a biomarker for AKI. Consequently, a larger AKI sample size and a serial evaluation are needed during any follow‐up study. Comparison of single uNGAL concentration to uNGAL‐to‐creatinine ratios should also be evaluated.
Serum NGAL has been reported to be a valuable factor when predicting progression in humans with CKD,22 and a similar conclusion may be drawn for dogs from our study. In addition, the results indicated that sNGAL can play an important role in predicting progression in CKD, not only because sNGAL increases in parallel with serum creatinine concentration but also because it has better capability of predicting death than does creatinine. Because sNGAL can have from different origins6 and release is not limited to the kidneys, concurrent diseases may contribute to the increase in sNGAL and this might lead to prediction of a poor prognosis. However, because of the small sample size and the lack of urine protein‐to‐creatinine ratio in this study, it is difficult to categorize the CKD cases into smaller groups such as glomerular disease and tubular disease; additional investigations are required to evaluate the clinical relevance of NGAL in various types of CKD.
Although NGAL has been reported to be secreted by neutrophils, the sNGAL concentrations of the dogs in this study were not found to be significantly correlated with their leukocyte counts by linear regression. Despite NGAL being expressed in many organs,6 sNGAL seems to serve as an inflammatory marker; a more severe inflammatory reaction is likely to lead to a poorer prognosis. Therefore, even if the increase in sNGAL concentration is not attributable to the renal insult, its measurement seems to perform well when predicting outcome.
The median age of onset in the azotemic dogs was 8.5 years, whereas the median age of the healthy control group was 2 years. This discrepancy may have influenced on the results because CKD is an age‐related disease in dogs. However, in the CKD group, the survivors, despite having lower sNGAL and uNGAL concentrations than the nonsurvivors, were not significantly younger than the nonsurvivors. This observation suggests that age is not related to the increase in NGAL.
Although previous studies have indicated that uNGAL concentrations can be correlated with the presence of acute damage during AKI,3 uNGAL concentration does not seem to be associated with death. To explain the role of NGAL in renal injury, some authors have proposed the “forest fire theory”.30 In this analogy, nephrons represent the trees in the forest and NGAL represents the fire. After damage, the remaining nephrons re‐establish renal function, which is evaluated as the glomerular filtration rate (GFR) and is commonly evaluated as the serum creatinine concentration. In this model, NGAL indicates the active lesions during renal damage. Although active damage increases in parallel with the decrease in renal function, theoretically, NGAL and serum creatinine concentration represent different functionalities. Urine NGAL has been reported to be more specific for renal damage than sNGAL. However, during renal disease, renal damage cannot totally account for death and other factors that are associated with systemic failure must also be involved. Therefore, an increase in uNGAL concentration is unlikely to be able to fully explain a patient's death. On this basis, increases in either the sNGAL or uNGAL concentration are not equivalent to an increase in serum creatinine concentration and cannot replace it when making a diagnosis and establishing a prognosis for patients with renal disease.
In conclusion, NGAL concentrations in urine and in serum can be used clinically in dogs as biomarkers to indicate a state of impaired renal function. Furthermore, sNAGL seems to have better prognostic relevance in dogs with CKD than those with AKI.
Acknowledgment
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Footnotes
Tetramethylbenzidine substrate kit, Clinical Science Laboratory, Inc, Mansfield, MA
SPSS 16.0 for Windows, SPSS inc, Chicago, IL
References
- 1. Rivers BJ, Walter PA, O'Brien TD, et al. Evaluation of urine gamma‐glutamyl transpeptidase‐to‐creatinine ratio as a diagnostic tool in an experimental model of aminoglycoside‐induced acute renal failure in the dog. J Am Anim Hosp Assoc 1996;32:323–336. [DOI] [PubMed] [Google Scholar]
- 2. Finco DR, Brown SA, Vaden SL, et al. Relationship between plasma creatinine concentration and glomerular filtration rate in dogs. J Vet Pharmacol Ther 1995;18:418–421. [DOI] [PubMed] [Google Scholar]
- 3. Devarajan P. Review: Neutrophil gelatinase‐associated lipocalin: A troponin‐like biomarker for human acute kidney injury. Nephrology (Carlton) 2010;15:419–428. [DOI] [PubMed] [Google Scholar]
- 4. Xu SY, Carlson M, Engstrom A, et al. Purification and characterization of a human neutrophil lipocalin (HNL) from the secondary granules of human neutrophils. Scand J Clin Lab Invest 1994;54:365–376. [DOI] [PubMed] [Google Scholar]
- 5. Kjeldsen L, Johnsen AH, Sengelov H, et al. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J Biol Chem 1993;268:10425–10432. [PubMed] [Google Scholar]
- 6. Cowland JB, Borregaard N. Molecular characterization and pattern of tissue expression of the gene for neutrophil gelatinase‐associated lipocalin from humans. Genomics 1997;45:17–23. [DOI] [PubMed] [Google Scholar]
- 7. Schmidt‐Ott KM, Mori K, Li JY, et al. Dual action of neutrophil gelatinase‐associated lipocalin. J Am Soc Nephrol 2007;18:407–413. [DOI] [PubMed] [Google Scholar]
- 8. Mishra J, Mori K, Ma Q, et al. Amelioration of ischemic acute renal injury by neutrophil gelatinase‐associated lipocalin. J Am Soc Nephrol 2004;15:3073–3082. [DOI] [PubMed] [Google Scholar]
- 9. Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase‐associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 2005;365:1231–1238. [DOI] [PubMed] [Google Scholar]
- 10. Wagener G, Jan M, Kim M, et al. Association between increases in urinary neutrophil gelatinase‐associated lipocalin and acute renal dysfunction after adult cardiac surgery. Anesthesiology 2006;105:485–491. [DOI] [PubMed] [Google Scholar]
- 11. Mishra J, Ma Q, Kelly C, et al. Kidney NGAL is a novel early marker of acute injury following transplantation. Pediatr Nephrol 2006;21:856–863. [DOI] [PubMed] [Google Scholar]
- 12. Hirsch R, Dent C, Pfriem H, et al. NGAL is an early predictive biomarker of contrast‐induced nephropathy in children. Pediatr Nephrol 2007;22:2089–2095. [DOI] [PubMed] [Google Scholar]
- 13. Constantin JM, Futier E, Perbet S, et al. Plasma neutrophil gelatinase‐associated lipocalin is an early marker of acute kidney injury in adult critically ill patients: A prospective study. J Crit Care 2010;25:176 e171–176. [DOI] [PubMed] [Google Scholar]
- 14. Poniatowski B, Malyszko J, Bachorzewska‐Gajewska H, et al. Serum neutrophil gelatinase‐associated lipocalin as a marker of renal function in patients with chronic heart failure and coronary artery disease. Kidney Blood Press Res 2009;32:77–80. [DOI] [PubMed] [Google Scholar]
- 15. Shapiro NI, Trzeciak S, Hollander JE, et al. The diagnostic accuracy of plasma neutrophil gelatinase‐associated lipocalin in the prediction of acute kidney injury in emergency department patients with suspected sepsis. Ann Emerg Med 2010;56:52–59, e51. [DOI] [PubMed] [Google Scholar]
- 16. Dent CL, Ma Q, Dastrala S, et al. Plasma neutrophil gelatinase‐associated lipocalin predicts acute kidney injury, morbidity and mortality after pediatric cardiac surgery: A prospective uncontrolled cohort study. Crit Care 2007;11:R127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Argyri I, Xanthos T, Varsami M, et al. The role of novel biomarkers in early diagnosis and prognosis of acute kidney injury in newborns. Am J Perinatol 2013;30:347–352. [DOI] [PubMed] [Google Scholar]
- 18. Akcay AB, Ozlu MF, Sen N, et al. Prognostic significance of neutrophil gelatinase‐associated lipocalin in ST‐segment elevation myocardial infarction. J Investig Med 2012;60:508–513. [DOI] [PubMed] [Google Scholar]
- 19. Kumpers P, Hafer C, Lukasz A, et al. Serum neutrophil gelatinase‐associated lipocalin at inception of renal replacement therapy predicts survival in critically ill patients with acute kidney injury. Crit Care 2010;14:R9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Haase‐Fielitz A, Bellomo R, Devarajan P, et al. The predictive performance of plasma neutrophil gelatinase‐associated lipocalin (NGAL) increases with grade of acute kidney injury. Nephrol Dial Transplant 2009;24:3349–3354. [DOI] [PubMed] [Google Scholar]
- 21. Brunner HI, Mueller M, Rutherford C, et al. Urinary neutrophil gelatinase‐associated lipocalin as a biomarker of nephritis in childhood‐onset systemic lupus erythematosus. Arthritis Rheum 2006;54:2577–2584. [DOI] [PubMed] [Google Scholar]
- 22. Mitsnefes MM, Kathman TS, Mishra J, et al. Serum neutrophil gelatinase‐associated lipocalin as a marker of renal function in children with chronic kidney disease. Pediatr Nephrol 2007;22:101–108. [DOI] [PubMed] [Google Scholar]
- 23. Malyszko J, Bachorzewska‐Gajewska H, Sitniewska E, et al. Serum neutrophil gelatinase‐associated lipocalin as a marker of renal function in non‐diabetic patients with stage 2‐4 chronic kidney disease. Ren Fail 2008;30:625–628. [DOI] [PubMed] [Google Scholar]
- 24. Chaudhary K, Phadke G, Nistala R, et al. The emerging role of biomarkers in diabetic and hypertensive chronic kidney disease. Curr Diab Rep 2010;10:37–42. [DOI] [PubMed] [Google Scholar]
- 25. Nickolas TL, Forster CS, Sise ME, et al. NGAL (Lcn2) monomer is associated with tubulointerstitial damage in chronic kidney disease. Kidney Int 2012;82:718–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee YJ, Hu YY, Lin YS, et al. Urine neutrophil gelatinase‐associated lipocalin (NGAL) as a biomarker for acute canine kidney injury. BMC Vet Res 2012;8:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nabity MB, Lees GE, Cianciolo R, et al. Urinary biomarkers of renal disease in dogs with X‐linked hereditary nephropathy. J Vet Intern Med 2012;26:282–293. [DOI] [PubMed] [Google Scholar]
- 28. Segev G, Palm C, Leroy B, et al. Evaluation of neutrophil gelatinase‐associated lipocalin as a marker of kidney injury in dogs. J Vet Intern Med 2013;27:1362–1367. [DOI] [PubMed] [Google Scholar]
- 29. Lalanne A, Beaudeux JL, Bernard MA. NGAL: A biomarker of acute and chronic renal dysfunction. Ann Biol Clin (Paris) 2011;69:629–636. [DOI] [PubMed] [Google Scholar]
- 30. Mori K, Nakao K. Neutrophil gelatinase‐associated lipocalin as the real‐time indicator of active kidney damage. Kidney Int 2007;71:967–970. [DOI] [PubMed] [Google Scholar]


