Abstract
Background
Treatment of adrenal‐dependent hyperadrenocorticism (ADH) involves either surgical resection of the adrenal tumor or medical therapy. For many years, mitotane has been considered the medical treatment of choice for dogs with ADH.
Objectives
The aim of this study was to determine survival and prognostic factors for dogs with ADH treated with mitotane and trilostane.
Animals
Twenty‐six dogs with ADH were included in the study.
Methods
Fourteen dogs were treated with mitotane and 12 dogs were treated with trilostane. Medical records were reviewed. Epidemiologic factors, signalment, clinicopathologic abnormalities, endocrine test results, and treatment protocols were evaluated to identify potential predictive factors of overall survival time.
Results
Survival times of dogs treated with mitotane (median, 15.6 months) or trilostane (median, 14.0 months) were not significantly different. Using univariate analysis, age and postadrenocorticotropic hormone cortisol concentrations were inversely correlated with survival time. The multivariate model also identified weakness at presentation as a negative prognostic indicator.
Conclusion and Clinical Importance
The type of medical treatment (mitotane versus trilostane) does not influence survival time in dogs with ADH; therefore, trilostane, a drug with less frequent and milder adverse effects, might be used as the primary medical treatment when adrenalectomy cannot be performed.
Keywords: Cushing, Endocrine, Internal medicine
Abbreviations
- 3β‐HSD
3β‐hydroxysteroid dehydrogenase
- ACTH
adrenocorticotropic hormone
- ADH
adrenal‐dependent hyperadrenocorticism
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- CI
confidence interval
- HAC
hyperadrenocorticism
- HR
hazard ratios
- LDDST
low‐dose dexamethasone suppression test
- NSAP
nonselective adrenocorticolysis protocol
- PAP
partial adrenocorticolysis protocol
- PDH
pituitary‐dependent hyperadrenocorticism
- UCCR
urinary corticoid:creatinine ratio
Hyperadrenocorticism (HAC) is one of the most common endocrine disorders in dogs and is characterized by chronically increased circulating cortisol concentrations. This secretion can be the result of exaggerated production of adrenocorticotropic hormone (ACTH) from a pituitary abnormality (pituitary‐dependent HAC or PDH), which accounts for approximately 80–85% of cases or the result of a primary functional adrenocortical tumor (adrenal‐dependent HAC or ADH).1
Treatment of ADH involves either surgical resection of the tumor or medical therapy with mitotane, trilostane, or ketoconazole. For many years, adrenalectomy has been considered the treatment of choice for ADH.1, 2 However, there are no studies directly comparing surgical versus medical treatment for dogs with ADH. A number of factors should be considered before adrenalectomy, including the presence of metastatic lesions and concurrent diseases. In addition, adrenalectomy has reported frequencies of intraoperative and postoperative complications of 15–50% of affected dogs, respectively. Perioperative mortality rate of adrenalectomy is high (5–29%)3, 4, 5, 6 and this could lead to unwillingness of the owner to accept the risk of this procedure. When surgery is not indicated or the owner declines surgery, medical management with the adrenocorticolytic drug mitotane has been recommended as an alternative treatment. The efficacy of mitotane has been described using the partial adrenocorticolysis protocol (PAP)7 in dogs with ADH. Alternatively, it has been proposed that nonselective adrenocorticolysis (NSAP) with mitotane might be useful in dogs with ADH,8 but there are no studies involving a large number of treated dogs. In the last decade, some studies have indicated that trilostane, a competitive inhibitor of 3ß‐hydroxysteroid dehydrogenase (3ß‐HSD), is a good option for the treatment of canine ADH.9, 10 There is only 1 published study that compares the survival time of dogs with ADH treated either with mitotane or trilostane.11 The aims of this study were to compare the long‐term survival of dogs diagnosed with ADH treated with mitotane versus twice daily trilostane and to analyze epidemiologic, clinical and laboratory factors that might be associated with survival time. An additional aim was to identify any factors that might be used to determine the best medical treatment for this disease.
Material and Methods
Clinical Cases
Medical records of dogs evaluated at the Internal Medicine Unit of the Veterinary Teaching Hospital (University Complutense) of Madrid and at the Clínica Veterinaria Atlántico (Las Palmas de Gran Canaria) between September 1994 and July 2009 were reviewed. All dogs medically treated for ADH with mitotane or trilostane were included in the study.
Data obtained from the records included breed, sex, weight, age at diagnosis, clinical signs, concomitant diseases at diagnosis, results of abdominal ultrasound examination and thoracic radiographs, routine clinicopathologic data, and blood pressure measured by a Doppler or oscillometric system. Hypertension was defined as a systolic or diastolic pressure >150 or 95 mmHg, respectively.12 Results of endocrine assays at the time of diagnosis also were recorded.
Diagnostic Procedures
The diagnosis of HAC was made on the basis of clinical history, physical examination, ultrasonographic findings, and the results of hematology, serum biochemistry, and urinalysis. The diagnosis was confirmed by a combination of adrenal functional tests (at least 1 endocrine test result, ACTH stimulation test, or low‐dose dexamethasone suppression test [LDDST] had to be positive to confirm the diagnosis of HAC). An ACTH stimulation test was carried out in 22 dogs. Plasma cortisol concentrations were measured before and after IM injection of 250 μg tetracosactide.1 , 13 HAC was confirmed by demonstration of an exaggerated cortisol response (post‐ACTH cortisol concentration >20 μg/dL) after ACTH administration.1 A urinary corticoid:creatinine ratio (UCCR) also was performed in 16 dogs. The owner collected 2 consecutive urine samples and the UCCR was determined in both samples. The presence of an increased UCCR in the samples (>60 × 10−6) was considered consistent with HAC.14 The UCCR was measured by chemiluminescence assay validated for use in the dog. A LDDST also was used to confirm the disease in 11 dogs and consisted of measuring cortisol concentrations before, 4 and 8 hours after administration of 0.01 mg/kg dexamethasone2 IV. Failure to suppress circulating cortisol concentration adequately (>1.4 μg/dL) at 8 hours after dexamethasone was considered compatible with HAC.1 This test also was helpful in differentiating dogs with PDH if there was a 4‐hour cortisol concentration <1.4 μg/dL or a 4‐hour cortisol concentration <50% of the basal cortisol concentration, an 8‐hour cortisol concentration <50% of the basal cortisol concentration or both.15
The adrenal origin of the HAC was confirmed on the basis of the ultrasonographic finding of unilateral adrenal enlargement (n = 26) and in 2 of these cases, a high‐dose dexamethasone suppression test also was performed (0.1 mg/kg IV). Nonsuppression was defined as a cortisol concentration >50% of the baseline concentration or >1.4 μg/dL at 4 or 8 hours after injection of dexamethasone.1 An enlarged adrenal gland (width > 7.4 mm) with an abnormal shape and asymmetry with respect to the other gland was considered consistent with an adrenal tumor. To be included in the study, the maximal dorso‐ventral thickness of the contralateral adrenal gland (in dogs with unilateral adrenal gland masses) was ≤5 mm.16
Treatment Protocols
Mitotane Group
Fourteen animals were included in this group. Five dogs were treated with mitotane using the NSAP described by den Hertog et al17 for the treatment of PDH. These animals received a PO initial dosage of mitotane3 of between 75 and 100 mg/kg/day for 25 days. After the third day of treatment, animals were started on lifelong mineralocorticoid supplementation (fludrocortisone, 0.01 mg/kg/day)4 and glucocorticoid supplementation (hydrocortisone, 2 mg/kg/day).5 The dosages of fludrocortisone and glucocorticoid were adjusted based on the clinical response and the results of biochemical evaluation. Nine dogs were treated with mitotane using the PAP as described in the literature.7 These animals received an induction dosage of mitotane of 50–75 mg/kg/day PO for a period of between 9 and 30 days. The aim of this phase was the complete resolution of polyphagia or polydipsia and a post‐ACTH cortisol concentration <4 μg/dL. After successful completion of the induction phase, a maintenance mitotane dosage of 75–100 mg/kg/week PO was provided lifelong. The goal of this phase was to control clinical signs and achieve a post‐ACTH cortisol concentration <4 μg/dL.
Trilostane Group
Twelve animals were included in this group. The initial dosage of trilostane6 was 3 mg/kg PO q12h as described by Pérez Alenza et al.14 The aim of the treatment was resolution of clinical signs and reduction of post‐ACTH cortisol concentrations to <9 μg/dL.14 Two animals in this group underwent adrenalectomy after 11 and 12 months of medical treatment.
Long‐Term Follow‐Up
Mitotane Group
Animals were reviewed at 1st, 3rd, and 6th month after the start of the treatment, and thereafter every 6 months. At each evaluation, history, physical examination, CBC, and a complete biochemical profile (including electrolytes) were done. In addition, in dogs on PAP, an ACTH stimulation test was performed.
Trilostane Group
Dogs were reviewed the 1st week after the start of the treatment and at the 1st, 3rd, and 6th month after treatment, and every 3 months thereafter. At each evaluation, history, physical examination, CBC, complete biochemical profile (including electrolytes), and an ACTH stimulation test were performed 8–12 hours after trilostane administration.14 By testing at this time, trilostane action is evaluated knowing that it is not the peak action time and therefore the ideal range for post‐ACTH cortisol concentration during monitoring will be higher than when it is performed between 4 and 6 hours postpill, but it can be useful as a precautionary measure by assuring that the dog is not at risk of hypocortisolemia just before the next trilostane administration when trilostane is administered q12h.
Statistical Analyses
Results were analyzed using a statistical software package.7 For the descriptive analyses of continuous data, the mean, standard deviation, and the range of values are reported. For categorical data, frequencies and percentages are given. The population characteristics of both groups were compared using the chi‐squared test for sex, breed, concurrent illness, and clinical signs and the Levene t‐test for age and weight.
Survival time analysis was performed using a Kaplan–Meier survival curve. Log‐rank, Breslow (Wilcoxon), and Tarone–Ware tests were used to compare the survival curves of the 2 groups. Overall survival was defined as the time from the beginning of treatment to the day of death. Univariate Cox proportional hazard regression analysis was performed to screen potential predictor factors for subsequent inclusion in a multivariate model. Potential predictive variables included epidemiologic factors (age, sex, breed, reproductive status, and weight), clinical signs detected at diagnosis (polyuria, polydipsia, weakness, pulmonary metastases, lethargy, concurrent diseases, and blood pressure), abdominal ultrasonographic findings (adrenal mass size, contralateral adrenal gland size, presence of arterial or venous thrombi), and laboratory findings (hematocrit, hemoglobin, red and white blood cells, glucose, creatinine, alanine aminotransferase, alkaline phosphatase, sodium, potassium, chloride, and urine specific gravity). The results of the endocrine tests at diagnosis (basal and post‐ACTH cortisol concentrations and UCCR) also were analyzed.
To identify the independent impact of variables on survival time of dogs, a multivariate analysis (Cox regression model) was performed. The variables tested for their impact on overall survival were those significantly or nearly significantly correlated at the univariate level. A Wald system was used to establish the parameters significantly related to the survival of the animals. Hazard ratios (HR) with their 95% confidence interval (CI) were calculated. For all the statistical analyses, P ≤ .05 was considered significant.
Results
Descriptive Analysis
Twenty‐six dogs were included in this study. The median age (±SD) at diagnosis was 10.9 ± 2.1 years (range, 7–16 years). The median age for dogs in the mitotane group was 10.9 years and for those in trilostane group was 10.8 years. There were 16 female dogs; 7 were intact and 9 were spayed. There were 10 male dogs; 3 were intact and 7 were neutered. There were 13 different breeds. The most commonly represented were mixed breed (n = 8), Yorkshire Terrier (n = 3), and Labrador Retriever, Fox Terrier, Bobtail, and Samoyed (n = 2 each). There was 1 Dalmatian, West Highland White Terrier, Poodle, Lhasa‐Apso, Miniature Schnauzer, Dachshund, and Scottish Terrier, respectively. In the mitotane group, the dogs ranged in weight from 4.2 to 46 kg (mean, 17.3 ± 11.6 kg; median 14.1 kg). In the trilostane group, the dogs ranged in weight from 8 to 36 kg (mean, 21.0 ± 13.92 kg; median, 19.7 kg). Fourteen dogs had concurrent diseases at presentation (5 dogs in the mitotane group and 9 dogs in the trilostane group); 3 dogs from each group had concurrent diseases classified as severe. The differences between groups regarding age, weight, and presence of concurrent diseases were not statistically significant.
Polyuria and polydipsia were detected in 22 dogs (85%) at diagnosis. Twenty animals (77%) had polyphagia. Six animals (23%) had weakness and 13 (50%) dogs were lethargic. Abdominal distension was found in 50% of the dogs (n = 13). There were no statistically significant differences between the 2 treatment groups for polyuria and polydipsia, polyphagia, weakness, lethargy, and abdominal distension. Dermatologic signs such as alopecia, thin skin, and calcinosis cutis were detected in 7/26 (26%), 6/26 (23%), and 1/26 dogs (4%) respectively; differences between groups were not significant.
There were 23 dogs with unilateral adrenal masses and 3 dogs with bilateral adrenal masses. The maximal dorso‐ventral thickness of the adrenal mass ranged from 10 to 74 mm (mean, 25.32 ± 13.94 mm); the dorso‐ventral thickness of the contralateral adrenal gland (unilateral tumors) ranged between 2 and 5 mm (mean, 3.96 ± 0.81 mm). Right adrenal gland masses were more frequent (n = 14; 54%) than left‐sided masses. Four dogs (2 from each group) had thrombi at diagnosis, all located in the caudal vena cava. Ten dogs (71%) in the mitotane group and 9 dogs (75%) in trilostane group had hepatomegaly at the time of diagnosis. One dog (trilostane group) and 2 dogs (mitotane group) had pulmonary metastasis at diagnosis, respectively. These differences were not statistically significant. One dog had nodular lesions in the liver, histologically confirmed as metastasis from the adrenal tumor.
Hypertension was found in 61% of the dogs in which blood pressure was recorded (8 of 13; 4 dogs in each group). The mean (±SD) systolic arterial pressure was 172 ± 41 mmHg (range, 125–240 mmHg); the mean diastolic pressure was 114 ± 24 mmHg (range, 83–150 mmHg); and the mean of the mean blood pressure was 133 ± 24 mmHg (range, 105–171 mmHg).
Four dogs were positive on the LDDST. Six dogs were positive on the LDDST and negative on the ACTH stimulation test. One dog was positive on the LDDST, positive on the UCCR, and negative on the ACTH stimulation test. Fourteen dogs were positive on the ACTH stimulation test and positive on the UCCR. One dog was positive on the ACTH stimulation test and borderline on the UCCR. Results of the endocrine tests are shown in Table 1.
Table 1.
Results of the endocrine tests performed on the 26 dogs at diagnosis.
| Endocrine Tests | Mean ± SD | Minimum Value | Maximum Value | Reference Laboratory Values | Number of Dogs Positive for the Test |
|---|---|---|---|---|---|
| ACTH stimulation test (n = 22) | |||||
| Cortisol pre (μg/dL) | 6.1 ± 5.1 | 1.4 | 26.2 | 1.5–5 | 15 |
| Cortisol post (μg/dL) | 25.8 ± 13.7 | 5.6 | 64 | 5–19 | |
| LDDST (n = 11) | |||||
| Cortisol 0 hours (μg/dL) | 4.3 ± 2.6 | 1.4 | 9.8 | 1.4–5 | 11 |
| Cortisol 4 hours (μg/dL) | 3.5 ± 2.9 | 0.8 | 9.6 | >1.4 | |
| Cortisol 8 hours (μg/dL) | 3.8 ± 2.4 | 1.4 | 8.2 | >1.4 | |
| UCCR (n = 16) | 290 ± 282 | 57.6 | 1011 | <60 | 15 |
ACTH, adrenocorticotropic hormone; LDDST, low‐dose dexamethasone suppression test; UCCR, urinary corticoid:creatinine ratio.
Histopathologic evaluation of the adrenal tumors was performed in 11 dogs at necropsy. Of these, 10 were adrenocortical carcinomas (5 of them in the group treated with trilostane and 5 in the group treated with mitotane; 4 with PAP and 1 with NSAP). One tumor was diagnosed as an adenoma (from a dog treated with mitotane).
In the mitotane group, 3 dogs in the NSAP in each re‐evaluation, respectively, had a good clinical response. After 15 months of treatment, 1 dog needed to be retreated with mitotane. Of the dogs receiving the PAP, at 1‐month evaluation, 7 had post‐ACTH cortisol concentrations above the reference range and 3 of them had signs of HAC. At the 3‐month re‐evaluation, 3 dogs had post‐ACTH cortisol concentrations above the reference range and 2 of them had signs of HAC. At the 6‐month re‐evaluation, 4 dogs had post‐ACTH cortisol concentrations above the reference range and 2 of them had signs of HAC. At the 12‐month re‐evaluation, 2 dogs had post‐ACTH cortisol concentrations above the reference range and signs of HAC. On each occasion, all of these dogs were reinduced with mitotane and the maintenance dosage was increased.
In the trilostane group, after 1 month of treatment, 4 dogs had post‐ACTH cortisol concentrations above the reference range and signs of HAC and the dosage of trilostane was increased. At each of the 3‐, 6‐, and 12‐month re‐evaluations, 3 dogs had post‐ACTH cortisol concentrations above the reference range and signs of HAC and the dosage of trilostane was increased.
Adverse effects were observed in 8 dogs (57.1%) in mitotane group; 4 dogs on PAP developed mild effects (eg, anorexia, lethargy, and vomiting) and signs were considered severe in 1 dog that developed hypocortisolism. Two animals on NSAP developed mild adverse effects (eg, anorexia, lethargy, and vomiting) and 1 dog developed severe adverse effects (vomiting and neurologic signs). Four animals in the trilostane group (33.3%) developed adverse effects. These signs were mild in 3 cases (eg, anorexia, vomiting, and diarrhea) and severe in 1 dog that developed prolonged hypocortisolemia.
Survival Analysis
At date of censorship, 24 dogs were dead, 1 dog in mitotane group was alive, and another dog from trilostane group was lost to follow‐up. Among the dogs treated with mitotane, 4 died because of unrelated diseases (renal failure [n = 1], hemangiosarcoma [n = 1], congestive heart failure [n = 1], and pulmonary edema [n = 1]). In 4 dogs, the reason for death or euthanasia was uncertain; the recorded reasons were progressive deterioration (n = 2), advanced age (n = 1), and weakness and gastrointestinal signs without laboratory evidence of hypoadrenocorticism (n = 1). In 2 additional dogs, the reason for death was attributable to the evolution of the disease or because of treatment (presence of renal metastases and invasion of vena cava in 1 and failure to manage vomiting and anorexia in the other dog). In another 3 dogs, the reason for death was not recorded.
The specific cause of death (inflammatory carcinoma, lymphoma, and anal sac carcinoma) in 3 dogs treated with trilostane was not directly attributable to the disease. These causes included neoplastic disease. There were 2 animals in which the reason for death or euthanasia was vague (progressive deterioration and advanced age, 1 dog each, respectively); 4 dogs died because of reasons attributable to the disease or its treatment (vascular and renal invasion in 2, pulmonary metastasis and pulmonary and hepatic metastasis in 1 dog, respectively). In another 2 cases, after 11 and 12 months of treatment, the dogs underwent adrenalectomy because of a lack of response to treatment and both died in the postoperative period.
The median (±SD) survival time of the dogs included in this study was 15.6 ± 1.8 months. The population characteristics and survival time for dogs treated with mitotane using PAP (mean ± SD, 15.4 ± 4.3 months; median, 12.2 ± 6.2 months) were not statistically different (P = .662) to those of dogs using NSAP (mean ± SD 15.9 ± 3.9 months; median, 19.2 ± 7.3 months). Therefore, the data from all of these dogs were pooled together in the same group (mitotane group) for the survival analysis. When taken together including all of the dogs treated with mitotane (PAP and NSAP), the mean survival time was 15.4 ± 3.2 months (range, 2.0–37.0 months; median, 15.6 months). The 6‐month and 1–3 year survival fractions were 65, 54, 15, and 7%, respectively. The mean survival time (±SD) for animals treated with trilostane was 17.7 ± 4.2 months (range, 3.3–55.0 months; median, 14.0 months). The 6‐month and 1–3 year survival fractions were 81, 63, 18, and 9%, respectively. There was no significant difference between the median survival time for animals treated with mitotane and those treated with trilostane (P values: log‐rank, .742; Wilcoxon, .713; Tarone–Ware, .765).
When censoring the 2 dogs in the trilostane group that underwent adrenalectomy, the differences in the survival time of dogs treated with mitotane compared with those treated with trilostane were not statistically significant (15.42 ± 3.2 months for mitotane group and 20.10 ± 5.3 months for trilostane group; Fig 1).
Figure 1.
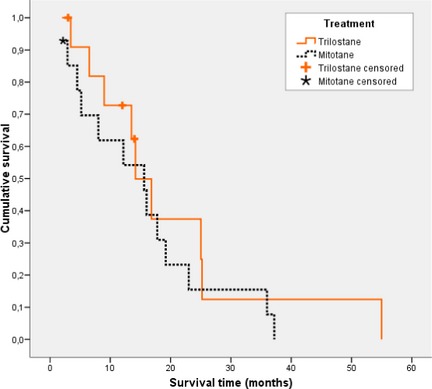
Kaplan–Meier survival curve for animals with adrenal‐dependent hyperadrenocorticism treated with mitotane and trilostane. Dogs alive at the completion of the study, dogs lost to follow‐up, and dogs in trilostane group that underwent adrenalectomy were censored.
When calculations were performed assuming a 5% level of significance and considering 60 dogs in each group, the proportion of dogs that would survive at 24 months was 0.685 (trilostane group) and 0.540 (mitotane group) (HR 0.614 and power of 37%). In order to achieve 80% power, 178 animals in each group would have been necessary.
Epidemiologic, clinical, and laboratory variables and endocrine function tests at diagnosis were examined for a potential influence on survival time. In the univariate analysis, factors negatively correlated with survival time were age (P = .040; coefficient, 0.218) and serum post‐ACTH cortisol concentrations at diagnosis (P = .006; coefficient, 0.056), both being inversely correlated with survival.
In the multivariable model, at the first step, age was selected as a parameter negatively correlated with survival (P = .006; HR, 1.3; 95% CI, 1.05–1.61) followed by serum post‐ACTH cortisol concentration (P = .000; HR, 1.1; 95% CI, 1.04–1.14) and weakness (P = .007; HR, 6.2; 95% CI, 1.62–23.65) in the following steps.
Discussion
This study evaluated survival time and potential prognostic factors in dogs diagnosed with ADH and treated with either mitotane or trilostane. The results suggest that the choice of trilostane or mitotane treatment does not influence the survival time of the dogs with ADH.
Adrenal‐dependent HAC is not a common condition, and it accounts only for approximately 15% of dogs with HAC. Dogs in this study exhibited clinical signs commonly seen in dogs with HAC. Ultrasonographic evaluation of the adrenal glands demonstrated unilateral masses in most cases and bilateral masses in 3 (12%). Similar findings have been previously reported.18 Tumor thrombi also were detected in a similar proportion of dogs (15%) to that previously reported.3
The median survival time of the dogs included in this study was 15.6 months. This survival time is lower compared to dogs in which adrenalectomy is performed (23–31.8 months).5, 6 When surgery is performed and the tumor is removed, the risk of tumor spreading is minimized (ie, growth of the tumor, invasion of blood vessels), the treatment can be curative in most of the patients, and also the adverse effects of lifelong hypercortisolism are prevented. However, the risk of potential perioperative complications and the mortality rates of this procedure should be considered,3, 19, 20 and individual assessment in each case is encouraged.
There are few studies evaluating the survival time of dogs with medically treated ADH.7, 11 All of these studies had reported a similar survival time to that observed in this study, and they demonstrate that the survival time of dogs with ADH is lower than that of dogs with PDH.21, 22 This result is not unexpected because PDH generally is a benign condition; most of these dogs have a pituitary adenoma, which ultimately is responsible for the excessive secretion of cortisol by the adrenal glands. In contrast, dogs with ADH have a higher reported incidence of carcinomas16, 23 and also are at higher risk of thromboembolism, which may lead to the development of thrombi in the caudal vena cava or other blood vessels than dogs with PDH. Finally, dogs with ADH tend to be more difficult to manage with mitotane compared to dogs with PDH.1, 7, 24
In this study, the median survival time for dogs with ADH treated with mitotane was similar to that of dogs treated with trilostane as previously described.11 Mitotane significantly decreases the adrenal gland size of dogs with PDH25 and it also is effective in dogs with ADH.6, 22, 25 Unfortunately, there are no studies evaluating the progression of ultrasonographic features of adrenal tumors in dogs with ADH treated with mitotane; only case reports are documented, describing similar effects in those dogs with PDH.22 Therefore, if mitotane could prevent tumor growth, decrease adrenal gland tumor size, or even induce complete necrosis of the neoplasm, it would be reasonable to expect a higher survival time for these dogs, and logically mitotane should be a better option for dogs with adrenal tumors. Dogs treated with mitotane, however, do not have a longer expected survival time and they are exposed to more adverse effects. In contrast, adrenal glands of dogs with PDH treated with trilostane have a tendency to grow.26 The evolution of adrenal glands in dogs with ADH after trilostane treatment has not been evaluated. If these glands continued to grow, as do the glands of dogs with PDH, it might contribute to the complications of these oncologic patients. Approximately 60% of dogs treated with mitotane have clinical adverse effects7 whereas the percentage of dogs treated with trilostane with adverse effects is approximately 23%.22 Because dogs with ADH treated with trilostane have a similar survival time and fewer adverse effects than dogs treated with mitotane, trilostane also could be used as a primary medical treatment for dogs with ADH. However, after 6 months of treatment, dogs in the trilostane group had been reviewed more frequently than dogs in mitotane group. This may have led to better control of the consequences of ADH in dogs in the trilostane group and could have had a positive impact on the survival time of these dogs.
The median survival time of dogs treated with mitotane in this study (15.6 months) is similar to that observed by other authors,7 using PAP. In this study, we included dogs treated with both PAP and NSAP. In this study, animals treated with both protocols were analyzed together. However one might expect dogs treated with NSAP to survive longer because NSAP could lead to complete necrosis of the adrenal tumor, in this study survival times were similar, and the incidence of adverse effects was higher in the NSAP group. Perhaps this result was influenced by the proportion of dogs with carcinomas or adenomas in both groups. However, histologic evaluation of tumors could not be evaluated in all cases, and thus this influence could not be evaluated. Likewise, in dogs with PDH treated with mitotane, similar survival times also have been observed using either NSAP or PAP.21, 22
The survival time of dogs treated with trilostane in this study (14 months) is similar to that described before (11.5 months).11 However, Helm et al included a larger population of dogs (22 versus 12 dogs) and also the protocol of trilostane administered in both studies was different (q24h in the previous study and q12h in the present one). Therefore, on the basis of these results, it is not possible to determine which protocol of treatment with trilostane might be more beneficial for dogs with ADH. On the other hand, trilostane also has been used in dogs with ADH that had metastases, with survival times of 3.6 and 17 months.10, 27 Based on these findings, trilostane may be considered a good option for dogs with ADH when adrenalectomy is not an option, being a safer alternative treatment than mitotane.
In a previous study, the prognosis of dogs with ADH managed medically was analyzed.11 Helm et al evaluated several parameters including epidemiologic factors and the presence of distant metastases and invasion into vessels as potential prognostic factors and the presence of metastases was found as the unique prognostic factor related to survival for dogs with ADH.11
In this study, an analysis of a large number of different parameters that could influence the survival of dogs, including epidemiologic data, clinical signs, laboratory results, and endocrine tests was performed. Factors associated with a poorer prognosis were age at diagnosis, increased post‐ACTH cortisol concentration, and the presence of weakness at diagnosis. Age also has been described as a negative prognostic factor for dogs with PDH.22 Regarding the ACTH stimulation test results at diagnosis and observed relationship with survival, this test indicates adrenal cortisol reserve, and animals with higher concentrations of cortisol in this test are those with increased chronic exposure to hypercortisolemia, leading to more complications because of excess chronic cortisol. Also, it has been described that animals with adrenal carcinomas have higher post‐ACTH cortisol concentrations than do animals with adenomas.28 The ACTH stimulation test at diagnosis might be used as another tool for assessing the prognosis in dogs with ADH because in dogs an increased post‐ACTH cortisol concentration might be associated with shorter survival.
Another factor associated with a poor prognosis in this study was the presence of weakness, which increased the risk of mortality 6.2‐fold. Weakness also has been described by other authors as a negative factor for dogs with adrenal tumors.5 Weakness could be the result of increased concentrations of cortisol because although this clinical sign is not specific, glucocorticoids are known to produce severe muscular atrophy caused by destruction of the muscular myofibrils29 and weakness also has been observed as a clinical sign in animals with caudal vena cava invasion,30 which could explain the lower survival time in some of these dogs.
This study has some limitations most important of which is the low number of dogs evaluated. The low prevalence of ADH and the fact that many dogs are treated surgically made it difficult to conduct a prospective study. This limitation led to the necessity of including dogs diagnosed over a large period of time to collect enough dogs for statistical analysis. The use of trilostane to control hypercortisolism in dogs began in the last decade and therefore dogs included in this group are more recent cases. Despite all of these factors, there were no significant differences regarding population attributes of dogs in both groups (trilostane and mitotane). Another limitation of this study is the lack of histologic data in most cases because differentiating adrenocortical adenoma from carcinoma may affect survival times in these dogs. Despite these limitations, the clinical data obtained in this study may help when choosing a medical treatment for dogs with ADH.
In summary, in dogs with ADH that are older, presented with weakness or with high post‐ACTH cortisol concentrations at the time of diagnosis may have shorter survival times. The choice of medical treatment (mitotane or trilostane) does not affect survival time in dogs with ADH. Because trilostane is less toxic than mitotane, trilostane could be recommended as the first option when medical treatment for dogs with ADH is selected.
Acknowledgments
This study was not supported by a grant or other financial support. The authors thank Dr Pedro Cuesta for the statistical support.
Conflict of Interest Declaration: The authors disclose no conflict of interest.
Part of this paper has been presented at the ECVIM Congress, 2010, Seville, Spain
Footnotes
Nuvacthen depot; Novartis Farmaceútica, Barcelona, Spain31
Caliercortin; Calier Laboratories, Barcelona, Spain
Lysodren; Bristol‐Myers, Montreal, QC, Canada
Astonín Merck; Merck Farma Química, Madrid, Spain
Hidroaltesona; Alter, Madrid, Spain
Vetoryl; Dechra Pharmaceuticals, Shrewsbury, Shropshire, UK
SPSS 17.0 for Windows; SPSS Inc, Chicago, IL
References
- 1. Melián C, Pérez‐Alenza MD, Peterson ME. Hyperadrenocorticism in dogs In: Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Internal Medicine, 7th ed St. Louis, MO: Saunders‐Elsevier; 2010:1816–1840. [Google Scholar]
- 2. Rijnberk A, Kooistra HS. Glucocorticoid excess In: Schlütersche , ed. Clinical Endocrinology of Dogs and Cats. Hannover: Schlütersche Verlagsgesellschaft mbH & Co; 2010:110–134. [Google Scholar]
- 3. Kyles AE, Feldman EC, De Cock HE, et al. Surgical management of adrenal gland tumors with and without associated tumor thrombi in dogs: 40 cases (1994–2001). J Am Vet Med Assoc 2003;223:654–662. [DOI] [PubMed] [Google Scholar]
- 4. Jiménez Peláez M, Bouvy BM, Dupre GP. Laparoscopic adrenalectomy for treatment of unilateral adrenocortical carcinomas: Technique, complications, and results in seven dogs. Vet Surg 2008;37:444–453. [DOI] [PubMed] [Google Scholar]
- 5. Schwartz P, Kovak JR, Koprowski A, et al. Evaluation of prognostic factors in the surgical treatment of adrenal gland tumors in dogs: 41 cases (1999–2005). J Am Vet Med Assoc 2008;232:77–84. [DOI] [PubMed] [Google Scholar]
- 6. Massari F, Nicoli S, Romanelli G, et al. Adrenalectomy in dogs with adrenal gland tumors: 52 cases (2002–2008). J Am Vet Med Assoc 2011;239:216–221. [DOI] [PubMed] [Google Scholar]
- 7. Kintzer PP, Peterson ME. Mitotane treatment of 32 dogs with cortisol‐secreting adrenocortical neoplasms. J Am Vet Med Assoc 1994;205:54–61. [PubMed] [Google Scholar]
- 8. Keiser M, Fluckiger M, Gardelle O, et al. [Mitotane treatment in a dog with a recurring adrenocortical carcinoma—A case report]. Schweiz Arch Tierheilkd 1999;141:279–285. [PubMed] [Google Scholar]
- 9. Eastwood JM, Elwood CM, Hurley KJ. Trilostane treatment of a dog with functional adrenocortical neoplasia. J Small Anim Pract 2003;44:126–131. [DOI] [PubMed] [Google Scholar]
- 10. Benchekroun G, de Fornel‐Thibaud P, Lafarge S, et al. Trilostane therapy for hyperadrenocorticism in three dogs with adrenocortical metastasis. Vet Rec 2008;163:190–192. [DOI] [PubMed] [Google Scholar]
- 11. Helm JR, McLauchlan G, Boden LA, et al. A comparison of factors that influence survival in dogs with adrenal‐dependent hyperadrenocorticism treated with mitotane or trilostane. J Vet Intern Med 2011;25:251–260. [DOI] [PubMed] [Google Scholar]
- 12. Carr AP. Treatment of hypertension In: Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Internal Medicine, 7th ed St. Louis, MO: Saunders‐Elsevier; 2010:582–585. [Google Scholar]
- 13. Ginel PJ, Sileo MT, Blanco B, et al. Evaluation of serum concentrations of cortisol and sex hormones of adrenal gland origin after stimulation with two synthetic ACTH preparations in clinically normal dogs. Am J Vet Res 2012;73:237–241. [DOI] [PubMed] [Google Scholar]
- 14. Pérez Alenza DP, Arenas C, López ML, et al. Long‐term efficacy of trilostane administered twice daily in dogs with pituitary‐dependent hyperadrenocorticism. J Am Anim Hosp Assoc 2006;42:269–276. [DOI] [PubMed] [Google Scholar]
- 15. Feldman EC, Nelson RW, Feldman MS. Use of low‐ and high‐dose dexamethasone tests for distinguishing pituitary‐dependent from adrenal tumor hyperadrenocorticism in dogs. J Am Vet Med Assoc 1996;209:772–775. [PubMed] [Google Scholar]
- 16. Benchekroun G, de Fornel‐Thibaud P, Rodriguez Piñeiro MI, et al. Ultrasonography criteria for differentiating ACTH dependency from ACTH independency in 47 dogs with hyperadrenocorticism and equivocal adrenal asymmetry. J Vet Intern Med 2010;24:1077–1085. [DOI] [PubMed] [Google Scholar]
- 17. den Hertog E, Braakman JC, Teske E, et al. Results of non‐selective adrenocorticolysis by o, p'‐DDD in 129 dogs with pituitary‐dependent hyperadrenocorticism. Vet Rec 1999;144:12–17. [DOI] [PubMed] [Google Scholar]
- 18. Hoerauf A, Reusch C. Ultrasonographic characteristics of both adrenal glands in 15 dogs with functional adrenocortical tumors. J Am Anim Hosp Assoc 1999;35:193–199. [DOI] [PubMed] [Google Scholar]
- 19. Anderson CR, Birchard SJ, Powers BE, et al. Surgical treatment of adrenocortical tumors: 21 cases (1990–1996). J Am Anim Hosp Assoc 2001;37:93–97. [DOI] [PubMed] [Google Scholar]
- 20. Lang JM, Schertel E, Kennedy S, et al. Elective and emergency surgical management of adrenal gland tumors: 60 cases (1999–2006). J Am Anim Hosp Assoc 2011;47:428–435. [DOI] [PubMed] [Google Scholar]
- 21. Barker EN, Campbell S, Tebb AJ, et al. A comparison of the survival times of dogs treated with mitotane or trilostane for pituitary‐dependent hyperadrenocorticism. J Vet Intern Med 2005;19:810–815. [DOI] [PubMed] [Google Scholar]
- 22. Clemente M, De Andrés PJ, Arenas C, et al. Comparison of non‐selective adrenocorticolysis with mitotane or trilostane for the treatment of dogs with pituitary‐dependent hyperadrenocorticism. Vet Rec 2007;161:805–809. [PubMed] [Google Scholar]
- 23. Bailey CS, Page RL. Tumors of the endocrine system In: Withrow SJ, Vail DM, eds. Withrow and MacEwen's Small Animal Clinical Oncology, 4th ed Edinburgh: Elsevier Saunders; 2007:583–609. [Google Scholar]
- 24. Feldman EC, Nelson RW, Feldman MS, et al. Comparison of mitotane treatment for adrenal tumor versus pituitary‐dependent hyperadrenocorticism in dogs. J Am Vet Med Assoc 1992;200:1642–1647. [PubMed] [Google Scholar]
- 25. Horauf A, Reusch C. [Effects of mitotane therapy in dogs with pituitary dependent Cushing syndrome on the adrenal gland size—An ultrasonographic study]. Schweiz Arch Tierheilkd 1999;141:239–245. [PubMed] [Google Scholar]
- 26. Mantis P, Lamb CR, Witt AL, et al. Changes in ultrasonographic appearance of adrenal glands in dogs with pituitary‐dependent hyperadrenocorticism treated with trilostane. Vet Radiol Ultrasound 2003;44:682–685. [DOI] [PubMed] [Google Scholar]
- 27. Machida T, Uchida E, Matsuda K, et al. Aldosterone‐, corticosterone‐ and cortisol‐secreting adrenocortical carcinoma in a dog: Case report. J Vet Med Sci 2008;70:317–320. [DOI] [PubMed] [Google Scholar]
- 28. Peterson ME, Gilbertson SR, Drucker WD. Plasma cortisol response to exogenous ACTH in 22 dogs with hyperadrenocorticism caused by adrenocortical neoplasia. J Am Vet Med Assoc 1982;180:542–544. [PubMed] [Google Scholar]
- 29. Platt SR. Neuromuscular complications in endocrine and metabolic disorders. Vet Clin North Am Small Anim Pract 2002;32:125–146. [DOI] [PubMed] [Google Scholar]
- 30. Culvenor J. Surgery in the treatment of canine hyperadrenocorticism. Adrenalectomy. Aust Vet J 2003;81:34–35. [DOI] [PubMed] [Google Scholar]
- 31. Ginel PJ, Sileo MT, Blanco B, et al. Evaluation of serum concentrations of cortisol and sex hormones of adrenal gland origin after stimulation with two synthetic ACTH preparations in clinically normal dogs. Am J Vet Res 2011;73:237–241. [DOI] [PubMed] [Google Scholar]


