Abstract
Background
Few studies show the detrimental effect of canine obesity on cardiopulmonary function (CPF). The 6‐Minute Walk Test (6MWT) is a noninvasive exercise test easy to perform in clinical settings.
Objective
The aim of this study was to investigate the effect of obesity and body weight loss (BWL) on CPF assessed by the 6MWT and arterial blood gas analysis.
Animals
Six experimental Beagles and 9 privately owned obese dogs were enrolled in a diet‐induced BWL program.
Methods
Arterial blood gas analysis and 6MWT were repeated in obese subjects (BCS 8‐9/9), in the middle of BWL (overweight, BCS 6‐7/9), and in lean dogs (BCS 5/9). Heart rate (HRp) and oxygen saturation (SpO2) were measured by pulse oximetry before the 6MWT, at midtest, and during a 5‐minute recovery period.
Results
Twelve dogs completed the BWL program (initial BW, 27.3 ± 2.9 kg; final BW, 20.85 ± 2.9, lsmeans ± SE, P ≤ .001). BWL caused a significant increase in 6MWT walked distance (WD; obese: 509 ± 35 m; overweight: 575 ± 36 m; lean: 589 ± 36 m; P ≤ .05). Resting arterial blood gas results were not influenced by BWL. Including all time points, obese dogs showed higher HRp and lower SpO2 compared to overweight and lean dogs. SpO2 at the end of the walk was significantly lower in obese dogs.
Conclusion and Clinical Importance
Obesity negatively affects 6MWT performances in dogs. The 6MWT may be used to demonstrate the efficacy of BWL to improve CPF and quality of life in obese dogs. Although BWL induced significant improvement of cardiopulmonary parameters before ideal BW, WD improved until the end of the BWL program.
Keywords: Canine, Obesity, Pulmonary function testing
Abbreviations
- 6MWT
6‐minute walk test
- BCS
body condition score
- BE
base excess
- BWL
body weight loss
- CPF
cardiopulmonary function
- DM
dry matter
- HCO3
bicarbonate
- HRc
heart rate based on femoral pulse evaluation
- HRp
heart rate based on pulse oximetry
- mME
measured metabolizable energy
- paCO2
arterial partial pressure of carbon dioxide
- paO2
arterial partial pressure of oxygen
- PAO2
calculated alveolar partial pressure of oxygen
- AaDO2
alveolar‐arterial oxygen tension difference
- pCO2
partial pressure of carbon dioxide
- pO2
partial pressure of oxygen
- RR
respiratory rate
- SaO2
arterial blood oxygen saturation based on arterial blood gas analysis
- SpO2
blood oxygen saturation based on pulse oximetry
- TV
tidal volume
- VO2max
maximal oxygen consumption
- WD
walked distance during a 6MWT
Obesity is a common nutritional problem in canine medicine, occurring in 20–40% of dogs in the general population.1 Consequences of obesity on health have been well documented in dogs and several studies have objectively assessed the effects of obesity on several body systems such as in endocrine2 or cardiovascular.3 Obesity also may subjectively worsen clinical signs in several respiratory disorders, such as brachycephalic airway obstruction syndrome4 or tracheal collapse.5 However, scientific evidence and proper characterization of the effect of obesity on the respiratory system are scarce6, 7 in the veterinary literature.
Development of pulmonary function testing in human medicine8 helped to better describe and understand the effects of obesity on lung function.9, 10 Arterial blood gas analysis is an important noninvasive pulmonary function test extensively used in human pulmonology. Morbidly obese humans have unfavorable arterial blood gas results at rest9, 11 and significant improvement after body weight loss (BWL).12
To our knowledge, only 2 experimental studies described the effect of obesity on basal lung function parameters in dogs, both using plethysmography.6, 7 However, there are no published data about the effect of canine obesity and subsequent BWL on arterial blood gas parameters.
Walking tests have been used in human medicine since the 1960s.13 The 6‐Minute Walk Test (6MWT) is performed by measuring the distance an individual can walk comfortably in 6 min.13 This test directly measures patient's ability to undertake daily activities instead of evaluating exercise capacity. Therefore, it is often used for patients with chronic cardiopulmonary conditions to evaluate the patient's quality of life.14
Recently, the 6MWT has been evaluated in dogs providing a reproducible test1 that is easy to perform in clinical settings and able to discriminate between healthy dogs and dogs with pulmonary disease.15
In human medicine, cardiopulmonary parameters monitored during a walking test or during the recovery period after exercise have been used to provide additional clinical information in obese subjects.10, 16 To our knowledge, monitoring of heart rate and oxygen saturation during and after exercise has not been investigated in obese dogs and might help to better understand and characterize CPF changes associated with canine obesity and subsequent BWL.
Many of the studies conducted on obese dogs have used either experimentally induced obese animals,17 fed high‐fat diets to rapidly increase their body weight (BW), or naturally obese privately owned dogs affected by chronic obesity.18, 19 To our knowledge, there is no study investigating the CPF differences between chronic naturally obese subjects and experimentally obese dogs. CPF deterioration is well established in obese human patients,9, 10 but there is no clear information available about the time necessary for CPF deterioration to occur in these patients. Obesity in humans is a progressive chronic condition and some complications may develop gradually. Therefore, CPF differences may exist between a group of dogs artificially and recently made obese and chronic naturally obese patients.
The aim of this study was to investigate the effect of obesity and BWL on pulmonary function assessed by 6MWT and arterial blood gas analysis. Our hypotheses were that (1) obese dogs would have deteriorated pulmonary function, detectable by arterial blood gas analysis and 6MWT performances, (2) BWL would be associated with significant improvement of pulmonary function detectable by both arterial blood gas analysis and 6MWT performances, and (3) dogs with natural chronic obesity would have a different degree of pulmonary function deterioration and would show a different pattern of improvement after BWL compared to dogs with experimentally induced obesity.
Materials and Methods
Animals
Two different groups of dogs were considered in this study: 1 group of dogs with experimentally induced obesity and 1 group of dogs with chronic natural obesity.
The first group included 6 healthy Beagles. Dogs were weighed using an electronic weight scale2 and body condition score (BCS) was assessed on each of them by a 9‐point scale system, as previously described.20 Before being included in the protocol, all dogs underwent a fattening dietary regimen in order to reach a BCS ≥8. To gain weight, the animals were fed a high‐energy commercial extruded diet3 once daily that was formulated to contain 30.5% DM crude protein, 27% DM crude fat, 2.1% DM crude fiber, and 17.7 kJ/g as fed of measured metabolizable energy (mME). The amount of food offered was individually calculated to reach twice the maintenance requirements, estimated as 130 kcal × (BW)0.75, according to the recommendations of the National Research Council (2006).
Once obese, all dogs were clinically evaluated and no clinical sign could be detected. Hematology and biochemical analysis were within normal limits.
The Ethics Committee for Animal Well Being of the University of Liège approved the experimental protocol (No. 921).
In parallel, 9 client‐owned adult dogs with chronic gross obesity (BCS ≥8) but otherwise healthy also were recruited.
Complementary testing including hematology and serum biochemistry, endocrinology testing, diagnostic imaging or ophthalmological examination were performed at the clinicians' discretion when judged necessary after thorough clinical examination. Subjects with any clinical relevant problem that could interfere with the 6MWT performance (eg, orthopedic problems, vision impairment, fever, cardiopulmonary diseases) or any abnormal testing results at any time during the protocol were excluded from the study. Owners were asked to sign an informed consent form before their pet could take part in the study.
The protocol was organized so that no testing would be performed during the summer (between June and September) to avoid high temperature, which could have confounded our results. Room temperature and humidity were considered very stable in the building for the rest of the year.
Weight Loss Program
All obese dogs included in the study were subjected to a diet‐induced BWL program aiming to achieve a BCS of 5/9. A low‐energy‐dense commercial extruded diet was used4 (34.6% DM crude protein, 9% DM crude fat, 13.1% DM crude fiber, 12.6 kJ/g as fed of mME) and the amount of food was individually calculated to allow a weekly BW loss of 2% of the initial BW. Weekly BCS and BW evaluations were planned for each dog using the same scale,1 and the amount of food was adjusted as needed.
Arterial Blood Gas Analysis
Arterial blood gases were evaluated before each 6MWT in all dogs when they were obese (BCS ≥8), in the middle of their BWL program (BCS 6–7), and once they had reached their ideal BW (BCS 5).
Blood was taken from the dorsal metatarsal artery and collected in lyophilized lithium heparin syringes5 using a 22‐gauge needle. Dogs were positioned in lateral recumbency to allow easy access to the artery. Because most dogs were panting and some became excessively stressed, the procedure was canceled if not successful within 10 min. Air bubbles were removed and the syringes were capped as quickly as possible to minimize air contact and before being transported to the automated blood gas analyzer.6 All samples were analyzed within 2 min after blood collection.
Partial pressure of oxygen (paO2), partial pressure of carbon dioxide (paCO2), and blood pH were measured and corrected for current body temperature. Alveolar oxygen partial pressure (PAO2), alveolar‐arterial oxygen tension difference (AaDO2), bicarbonate (HCO3), base excess (BE) in blood, and arterial oxygen saturation (SaO2) were automatically calculated by the blood gas analyzer6 based on standard National Committee for Clinical Laboratory Standards (NCCLS) equations.
Six‐Minute Walk Test
This procedure was performed 3 times for each dog: in the 12 obese dogs (BCS ≥8), in the middle of their BWL program (BCS 6–7), and once they had reached their ideal BW (BCS 5).
Before starting the 6MWT, basal clinical heart rate (HRc) and respiratory rate (RR) were obtained by palpation of the femoral artery and thoracic inspection, respectively.
In addition, a handheld pulse oximeter7 was used to make 3 successive measurements of heart rate (HRp) and oxygen saturation (SpO2) allowing calculation of basal mean values before the walk (T0). The pulse oximeter probe was placed on nonpigmented areas of the buccal mucosa when possible. When it was too pigmented, probe was placed on vaginal or preputial mucosa. In order to be valid, pulse oximeter values had to be recorded within 30 s. If not, the time point was associated with a pulse oximetry failure.
After a few minutes of acclimatization, dogs were allowed to walk at their own pace for 6 min, along an unobstructed 53 m long L‐shaped hallway. Dogs were walked when there were no other persons or animals around that could represent a potential distraction.
After 3 min (T3), dogs were stopped to allow one of the operators to record pulse oximetry midtest values for HRp and SpO2. Time necessary to read a result (2–30 s) was added to the 6MWT. Dogs were stopped again once the walking time allowed to each patient was over. HRp and SpO2 were measured immediately at the end of the walk (T6) and 1, 2, 3, and 5 min after the end of the test (T7, T8, T9, and T11).
The total walked distance was measured and reported in meters.
Statistical Analysis
Parameters related to the 6MWT (WD, HRp, SpO2) and to arterial blood analysis (pH, paO2, paCO2, PAO2, AaDO2, HCO3, BE, SaO2) were assessed as normally distributed (Shapiro–Wilk test >0.90, PROC UNIVARIATE of SAS, 2006) and subsequently analyzed using a repeated mixed linear model (PROC MIXED of SAS, 2006) to detect the fixed effect of the repeated time effect (corresponding to the weight loss effect: obesity versus overweight versus lean condition), the effect of type of obesity (natural chronic obesity versus experimentally induced obesity), and the effect of their interaction. The effect of dog was considered random.
HRp and SpO2 were analyzed by a repeated mixed linear model (PROC MIXED of SAS, 2006) to assess the effect of type of obesity, time (corresponding to the weight loss effect: obesity versus overweight versus lean condition), time points (before the 6MWT [T0], in the middle of the test [T3] and at 0, 1, 2, 3, and 5 min [T6, T7, T8, T9, and T11] posttest) and the interactions between type of obesity and time points and between time and time points. The effect of dog was considered random.
For both analyses, least squares means (LSMEANS) were calculated and standard error (SE) was provided as uncertainty index of the estimation. P values for the multiple comparisons of the interaction effects were adjusted using the Bonferroni method and P < .05 were considered significant.
Results
Animals
Twelve dogs were included in the study including 6 experimental Beagles and 6 of the 9 client‐owned dogs initially recruited, which completed the BWL protocol. The 6 Beagles were all intact females between 5 and 11 years old. As for client‐owned dogs, the final group was composed of 3 intact females, 1 intact male, and 2 castrated males, between 6 and 10 years old. Different breeds were represented in this group including 1 Cocker Spaniel, 1 Cavalier King Charles Spaniel, 1 Labrador Retriever, 1 Weimaraner, 1 Border Collie cross‐breed and 1 Rottweiler cross‐breed.
Type of Obesity
When type of obesity was considered in the statistical analysis, there was no significant difference for any of the parameters analyzed between experimentally induced obese dogs and naturally obese dogs.
Weight Loss Program
The 6 experimental Beagles included in our study gained weight and reached the target obese condition in 16 weeks before successfully completing their BWL program and reaching the targeted ideal BW (BCS=5/9) in a mean time of 22 weeks. Six of 9 enrolled privately owned obese dogs also completed the BWL program but needed a mean time of 32 weeks. The remaining 3 client‐owned dogs dropped out because of a lack of owner compliance. The mean BW decreased progressively and significantly (P < .001) from 27.3 ± 2.86 kg (LSMEANS ± SE) in obese dogs to 23.8 ± 2.86 kg in overweight animals and finally 20.8 ± 2.86 kg in the lean dogs group.
Resting Clinical Examination in All Obese Dogs
Mean resting RR value was 49 ± 4 rpm. Five dogs were already panting at rest before the walk and RR was not measured in these dogs. Mean resting HRc value was 132 ± 7 bpm. HRc was not recorded in 1 obese dog. (Table 1)
Table 1.
Effect of body weight loss on resting respiratory rate (RR pretest) and heart rate (HRc pretest), on resting arterial blood gas parameters, and on 6‐Minute Walk Test (6MWT) walked distance (WD).
| Parameter | Mean ± SD | Range | n | |
|---|---|---|---|---|
| Obese | RR pretest (rpm) | 45 ± 12a | 28–64 | 7 |
| HRc pretest (bpm) | 132 ± 20a | 100–164 | 11 | |
| PaO2 (mmHg) | 95.00 ± 16.21 | 70.00–127.00 | 10 | |
| PaCO2 (mmHg) | 37.23 ± 6.06 | 23.80–43.90 | 10 | |
| SaO2 (%) | 90 ± 4 | 84–95 | 10 | |
| HCO3 (mmol/L) | 22.19 ± 3.38 | 15.00–26.20 | 10 | |
| AaDO2 (mmHg) | 12.48 ± 9.53 | 0.00–30.50 | 10 | |
| WD (m) | 509 ± 118a | 238–650 | 12 | |
| Overweight | RR pretest (rpm) | 39 ± 12ab | 20–60 | 9 |
| HRc pretest (bpm) | 105 ± 26b | 60–144 | 11 | |
| PaO2 (mmHg) | 89.26 ± 17.87 | 57.60–117.70 | 11 | |
| PaCO2 (mmHg) | 38.12 ± 4.73 | 29.80–44.90 | 11 | |
| SaO2 (%) | 92 ± 4 | 83–95 | 11 | |
| HCO3 (mmol/L) | 21.90 ± 2.23 | 17.00–26.40 | 11 | |
| AaDO2 (mmHg) | 21.88 ± 19.97 | 0.00–58.20 | 11 | |
| WD (m) | 571 ± 132ab | 344–815 | 12 | |
| Lean | RR pretest (rpm) | 33 ± 6b | 24–40 | 9 |
| HRc pretest (bpm) | 106 ± 22ab | 78–144 | 12 | |
| PaO2 (mmHg) | 88.71 ± 14.61 | 62.30–109.60 | 12 | |
| PaCO2 (mmHg) | 35.49 ± 5.12 | 21.80–40.00 | 12 | |
| SaO2 (%) | 92.36 ± 2.98 | 88.00–96.40 | 12 | |
| HCO3 (mmol/L) | 21.38 ± 2.52 | 15.20–24.70 | 12 | |
| AaDO2 (mmHg) | 21.55 ± 17.08 | 0.00–53.00 | 12 | |
| WD (m) | 611 ± 88b | 452–738 | 12 |
Data are shown as mean values ± SD, n = number of values available.
Different lettersa,b mean statistically significant different values; P < .05.
Arterial Blood Gas Analysis in All Obese Dogs
In 2 obese patients, arterial blood gas values were not available. For the remaining obese dogs, mean values for PaO2, PaCO2, SaO2, HCO3, and AaDO2 are shown in Table 1. Mean values for pH, BE, and PAO2 (data not shown) were within the normal reference range.
6‐MWT Performances in All Obese Dogs
Obese dogs walked a mean distance of 509 ± 35 m (LSMEANS ± SE) during 6 min. None of the patients experienced any complication or syncope during or after exercise. Progressive and moderate exercise intolerance was subjectively reported in all obese dogs during the last half of the required exercise. All obese dogs were panting at the end of the walk and during the 5 min after exercise.
Pulse oximeter mean values for HRp and SpO2 before the test, at midtest, and during 5 min after the end of the walk in obese dogs are presented in Figure 1 (HRp) and Figure 2 (SpO2). Results showed a nonsignificant HRp increase during the exercise before a progressive decrease during the recovery period. SpO2 values showed an opposite trend, decreasing during the exercise and increasing toward resting values after the end of the exercise. Pulse oximetry failure rate was variable in the obese dogs. For example, values were not obtained in only 2 of 12 dogs at T0, but as many as 5 of 12 experienced reading failures at T3.
Figure 1.
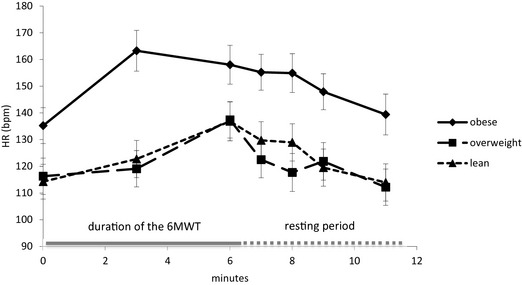
Mean heart rate values recorded with a handheld pulse oximeter before the 6‐Minute Walk Test (6MWT; T0), in the middle of the test (T3), and 0, 1, 2, 3, and 5 min after the end of the walk (T6, T7, T8, T9, and T11) in 12 dogs before (OBESITY), during (OVERWEIGHT), and at the end (LEAN) of the weight loss program.
Figure 2.
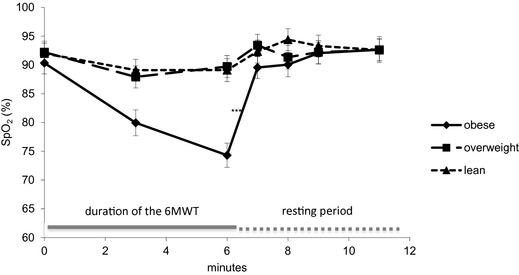
Mean peripheral oxygen saturation values recorded with a handheld pulse oximeter before the 6‐Minute Walk Test (6MWT; T0), in the middle of the test (T3), and 0, 1, 2, 3, and 5 min after the end of the walk (T6, T7, T8, T9, and T11) in 12 dogs before (OBESITY), during (OVERWEIGHT), and at the end (LEAN) of the weight loss program. ***means statistically significant different values in the obese group compared to both other groups; P < .05.
Weight Loss Effect on Resting Clinical Examination
Mean pretest RR decreased progressively as subjects lost weight. RR in lean subjects was significantly lower compared to RR measured in the obese group (Table 1). Fewer dogs (3 versus 5) were panting at rest in both overweight and lean subjects compared to obese dogs.
Mean pretest HRc was significantly higher in obese dogs compared to overweight dogs. However, no difference was detected between the obese dogs and the lean dogs. HRc was not available in 1 overweight dog (Table 1).
Weight Loss Effect on Arterial Blood Gas Analysis
An arterial blood sample was not obtained in 2 overweight dogs. BWL had no significant effect on any of the measured arterial blood gas parameters. Mean values for PaO2, PaCO2, SaO2, HCO3, and AaDO2 in the 3 groups are shown in Table 1.
Weight Loss Effect on 6‐MWT Performances
Body weight loss caused a progressive increase in WD with a significantly longer WD in lean subjects compared to obese dogs (Table 1). Subjective improvement in the demeanor of all dogs during the test was also noted. There was no more exercise intolerance reported in any of the lean dogs. However, all overweight and lean patients still were panting at the end of the exercise and during the 5‐min recovery period.
The impact of BWL on HRp and SpO2 during and after exercise is presented in Figure 1 (HRp) and Figure 2 (SpO2). Including all time points, obese dogs showed significantly higher mean HRp values and significantly lower mean SpO2 values than overweight and lean dogs (HRp, bpm: obese, 150.6 ± 4.5; overweight, 121 ± 4.5; lean, 123.7 ± 4.4, means ± SE, P < .001; SpO2, %: obese, 87.0 ± 0.88; overweight, 91.3 ± 0.90; lean, 91.8 ± 0.84, means ± SE, P < .001).
The trend described in obese dogs for HRp (Fig 1) and SpO2 (Fig 2) during and after exercise still was visible in overweight and lean dogs. However, differences between time points were more modest in these groups for both parameters. Statistical analysis failed to show a significant effect of BWL on HRp among the different time points (Fig 1). SpO2 in obese dogs was significantly lower at the end of the walk (T6) compared to overweight and lean patients (Fig 2).
Pulse oximetry failure rate was still very variable in overweight and lean patients. Values were not obtained in 1 of the 12 lean dogs at T0 and as many as 3 reading failures were reported at several time points posttest in overweight or lean dogs.
Discussion
By investigation of 2 sets of obese dogs (naturally obese versus experimentally induced), we confirmed a significant impact of obesity on CPF and quality of life in dogs. Subsequent BWL was associated with significant improvement in the 6MWT WD and several cardiopulmonary parameters before, during, and after the 6MWT. However, we failed to show any significant effect of obesity or subsequent BWL on arterial blood gas parameters.
One of our hypotheses was that the type of obesity (natural chronic obesity versus experimentally induced obesity) would influence the degree of CPF deterioration in severely obese dogs and possibly the pattern of improvement observed during BWL. However, none of the CPF parameters analyzed during this study were significantly affected by the type of obesity. Therefore, all dogs used in this study were regrouped and discussed as only 1 group of obese dogs.
The only difference observed between these 2 groups of dogs (privately owned dogs versus experimental Beagles) was the mean time necessary to reach the targeted BW (32 weeks versus 22 weeks, respectively), especially during the second half of the BWL program. This was probably because of decreased owner compliance, which also was the cause of drop‐out of 3 dogs that did not complete the BWL program. The reasons why 3 owners abandoned the study were behavioral problems in the dog, the dog's hunger, or the owner's health problem.
When basic cardiopulmonary parameters were investigated in obese dogs, a clear tendency to increased resting RR was seen, which subsequently improved during BWL. Obese dogs also were panting more often in comparison to overweight or lean dogs. Therefore, even though RR values were not recorded in panting dogs, differences would have been even more accentuated if panting values had been included. This consequence of obesity is well described in human patients. Tidal volumes often are decreased in severely obese subjects, and breathing follows a rapid and shallow pattern to try to maintain a constant minute ventilation.9 Global stiffening of the respiratory system with decreased compliance of lungs and chest walls is believed to be involved.9 However, many possible mechanisms by which excess body fat might influence ventilatory function are discussed in the literature.21, 22
Several veterinary studies using different techniques to evaluate lung function (barometric whole body plethysmography,7 spirometry, or head‐out plethysmography6) showed similar results in dogs. Decreased compliance has been suspected in obese dogs to explain this increased resting RR.7
Another interesting finding was a decrease in resting HR after significant BWL. Similar results have been reported in obese human patients.16 Higher energy output required because of increased total body mass in conjunction with modified ventilatory function9 could cause a relative oxygenation deficit and a compensatory increased heart rate in obese patients.16
Unexpectedly, arterial blood gas parameters were within normal limits23 in our group of obese dogs. We also failed to demonstrate any significant effect of BWL on arterial blood gas parameters in our study as all parameters remained within normal limits during the study.
Our findings differ from those observed in humans. Indeed, some severely obese subjects showed lower arterial oxygen saturation as a possible consequence of decreased expiratory reserve volume and abnormal ventilation distribution.9 In another study,12 an extensive BWL of 45 kg (BMI = −13 kg/m2) over a period of 18 months was associated with improvement in PaO2 (by 10 mmHg, range 1–23) and a decrease in PaCO2 (by −3 mmHg, range 3 to −14) in a group of morbidly obese human patients.
This discrepancy between arterial blood gas values in obese dogs and humans might be related to the low number of dogs included in the present study and some lack of statistical power. It was challenging to follow up client‐owned dogs until an ideal BCS within an appropriate timeframe, and some dogs unfortunately were dropped from the protocol.
The technique used to collect the arterial samples in our patient also may be responsible for some increased variability and preanalytical errors in blood gas parameters. Occasional low values of PaO2 may have resulted from venous contamination of some arterial samples. However, we wanted to evaluate CPF in a clinical setting and more invasive techniques (eg arterial catheterization) were not deemed appropriate and could have interfered with the dogs' ability to walk normally.
Another possible explanation may be related to extreme obesity and extensive BWL observed in humans making changes in arterial blood gases more severe. This kind of obesity appears difficult to reproduce in dogs.
Furthermore, some of our patients, especially client‐owned dogs, were not familiar with the veterinary hospital and appeared to experience stress as soon as they entered the premises. Firm handling was sometimes necessary to obtain the arterial blood sample, which probably amplified existing stress. Panting and some degree of hyperventilation in these dogs also may have influenced our results to some extent and could explain the observed discrepancy with human patients.
The natural tendency for panting in dogs in general also may be part of the explanation because panting was frequently observed in our group of obese dogs. This canine behavior could be compared to techniques of high frequency ventilation used in human pediatric medicine to improve blood gas exchange efficiency.24
Finally, specific anatomical differences in dogs may be implicated. In humans, increased abdominal mass because of fat tissue accumulation may decrease the downward movement of the diaphragm limiting lung expansion and inflation.9 The quadruped posture of dogs may limit this ventilatory effect in obese subjects.
As previously reported,15 the 6MWT was easy and safe to perform for all dogs in the study. It required very little time for dogs to acclimate to this noninvasive procedure. The 6MWT was found to be very safe in several other studies in which dogs with severe pulmonary disease15 or induced cardiac failure25 also were tested without complications.
Mean 6MWT WD in our group of obese dogs was 509 ± 35 m, which was slightly lower than what has been reported in previous studies involving healthy nonobese dogs (522.7 ± 52.4 m15 and 573 ± 85.5 m25). BWL was significantly associated with longer 6MWT WD in our study.
Increased BW in humans is known to negatively influence the 6MWT WD.16 6MWT WD also has been shown to correlate with maximal oxygen consumption (VO2max)26 and health‐related quality of life in human subjects.27 A recent publication indicates that the 6MWT may be used in clinical practice to evaluate intervention success beyond kilogram weight loss in obese patients undergoing a weight reduction program.10
Recently, some detrimental changes in pulmonary function have been described in obese dogs,7 but VO2max and quality of life were not evaluated in these dogs. Shorter 6MWT WD in obese dogs in our study may reflect these detrimental pulmonary function changes and may be associated with decreased respiratory well‐being and decreased quality of life. Ideal BW was necessary to reach an optimal and significant improvement of WD.
However, obesity also seems to have an important impact on cardiac function in dogs3 and human beings.28 In addition, 6MWT WD was significantly decreased in dogs with heart dysfunction in 1 experimental study.25 Therefore, our results should be interpreted with caution because improvement in 6MWT performance observed during BWL in our study also could be related to improvement of cardiac function. Echocardiography was not performed in our study to confirm this hypothesis. Consequences of obesity on cardiac function still remain unclear in human medicine, and some researchers have observed normal cardiac function in obese individuals.29
In our study, BWL also was associated with improvement in heart rate and oxygen saturation during and after the 6MWT. However, these results should be interpreted cautiously because high and variable pulse oximetry failure rates were observed in our study, especially in obese dogs.
Most studies evaluating pulse oximetry accuracy in dogs recorded SpO2 relatively accurately over a wide range of normal saturation values.30 However, these studies were performed on anesthetized dogs. In our study, dogs were not anesthetized. Many dogs were panting at midtest, during the recovery period or at both times. Therefore, motion artifact and lack of cooperation from the dog may explain our results and could be associated with underestimation of SpO2 and inaccurate HRp. Furthermore, despite care to avoid pigmented mucous membranes in the buccal cavity, some dogs were very pigmented, and this factor seemed to be associated with increased failure rates.
Despite these limitations, our results are similar to what has been reported in human medicine. Indeed, normal weight human subjects have been shown to have a significantly better cardiopulmonary response during a treadmill 6MWT compared to a group of severely obese patients.16 This observation may be explained by a blunted hyperventilation response during exercise, which has been well described in obese human subjects.12 Such subjects do not increase their RR proportionally to their higher absolute peak VO2.16 A combination of some degree of ventilatory deficit and high‐energy output requirement in obese patients9 could be responsible for a relative blood oxygenation deficit and a compensatory increased heart rate.
Similar ventilatory deficits recently have been suspected in obese Beagles7 and might explain a relative oxygenation deficit in our obese dogs and the subsequent cardiovascular response observed during and after the walk. In our study, however, 50% of targeted BWL was sufficient to observe adequate mean HRp and SpO2 as compared to overweight and lean dogs.
Finally, HRp and SpO2 showed different recovery patterns in obese dogs in our study. A previous study15 showed similar postexercise heart rate in healthy dogs and dogs with pulmonary disease, and there was no significant difference in HRp at any time point during the recovery period among obese, overweight and lean dogs. As for SpO2, although it was lower in obese dogs at the end of the walk, a very quick recovery was observed with optimal values already seen 1 min postexercise (T7).
Practically, monitoring SpO2 after a 6MWT may be useful to monitor the effect of BWL on CPF but only within 1 min after exercise because of the quick recovery. On the other hand, heart rate monitoring at a specific time point within 5 min after exercise does not seem to be helpful, and only mean heart rate including all time points may be used.
Conclusion
In conclusion, our results confirm that obesity in dogs negatively and significantly affects cardiopulmonary function as assessed by the 6MWT including heart rate and blood oxygen saturation monitoring during and after the walk.
Partial weight loss induces significant improvement in cardiopulmonary function even before dogs achieve their targeted ideal BW. However, 6MWT WD continued to improve until ideal body condition (BCS 5/9) was achieved. Therefore, results of the present study allow us to strongly recommend owners of obese dogs to target an ideal BW (BCS 5/9) for their dogs.
The 6MWT including heart rate and blood oxygen saturation monitoring, but not arterial blood gas analysis, was an efficient tool to demonstrate the efficacy of BWL to improve cardiopulmonary function and quality of life in dogs.
Acknowledgments
The study was not supported by any grant.
Conflict of Interest: The authors disclose no conflict of interest.
Preliminary results were presented during the ECVIM congress in September 2012 in Maastricht, The Netherlands
Footnotes
Gault S, et al. Six‐Minute Walk Test in Healthy Dogs/Reproducibility, Effect of Age and Breeds. In: ECVIM Congress, Toulouse, France, 2010
Soehnle Professional GmbH & Co KG, Backnang, Germany
Hill's Science Plan™ Canine Adult Performance, Hill's Pet Nutrition Inc, Topeka, KS
Hill's Prescription Diet™ r/d™ Canine, Hill's Pet Nutrition Inc
Blood Gas Monovette®, Sarstedt AG & Co, Nümbrecht, Germany
AVL Omni 3, Roche Diagnostics®, Vilvoorde, Belgium
Handheld pulse oximeter N‐20PA™, Nellcor Puritan Bennett Inc, Boulder, CO
References
- 1. Gossellin J, Wren JA, Sunderland SJ. Canine obesity – an overview. J Vet Pharmacol Ther 2007;30:1–10. [DOI] [PubMed] [Google Scholar]
- 2. Daminet S, Jeusette I, Duchateau L, et al. Evaluation of thyroid function in obese dogs and in dogs undergoing a weight loss protocol. J Vet Med A Physiol Pathol Clin Med 2003;50:213–218. [DOI] [PubMed] [Google Scholar]
- 3. Mehlman E, Bright JM, Jeckel K, et al. Echocardiographic evidence of left ventricular hypertrophy in obese dogs. J Vet Intern Med 2013;27:62–68. [DOI] [PubMed] [Google Scholar]
- 4. Hendricks J. Brachycephalic airway syndrome In: King LG, ed. Textbook of Respiratory Disease in Dogs and Cats. Philadelphia, PA: WB Saunders Company; 2004:310–317. [Google Scholar]
- 5. Spaulding GL. Medical management considerations for upper airway disease. Probl Vet Med 1992;4:419–428. [PubMed] [Google Scholar]
- 6. Bach JF, Rozanski EA, Bedenice D, et al. Association of expiratory airway dysfunction with marked obesity in healthy adult dogs. Am J Vet Res 2007;68:670–675. [DOI] [PubMed] [Google Scholar]
- 7. Manens J, Bolognin M, Bernaerts F, et al. Effects of obesity on lung function and airway reactivity in healthy dogs. Vet J 2012;193:217–221. [DOI] [PubMed] [Google Scholar]
- 8. Crapo RO. Pulmonary‐function testing. N Engl J Med 1994;331:25–30. [DOI] [PubMed] [Google Scholar]
- 9. Salome CM, King GG, Berend N. Physiology of obesity and effects on lung function. J Appl Physiol 2010;108:206–211. [DOI] [PubMed] [Google Scholar]
- 10. Ekman MJ, Klintenberg M, Bjorck U, et al. Six‐minute walk test before and after a weight reduction program in obese subjects. Obesity 2013;21:E236–E243. [DOI] [PubMed] [Google Scholar]
- 11. Gabrielsen AM, Lund MB, Kongerud J, et al. The relationship between anthropometric measures, blood gases, and lung function in morbidly obese white subjects. Obes Surg 2011;21:485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zavorsky GS, Hoffman SL. Pulmonary gas exchange in the morbidly obese. Obes Rev 2008;9:326–339. [DOI] [PubMed] [Google Scholar]
- 13. Enright PL. The six‐minute walk test. Respir Care 2003;48:783–785. [PubMed] [Google Scholar]
- 14. Guyatt GH, Sullivan MJ, Thompson PJ, et al. The 6‐minute walk: A new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J 1985;132:919–923. [PMC free article] [PubMed] [Google Scholar]
- 15. Swimmer RA, Rozanski EA. Evaluation of the 6‐minute walk test in pet dogs. J Vet Intern Med 2011;25:405–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Di Thommazo‐Luporini L, Jurgensen SP, Castello‐Simoes V, et al. Metabolic and clinical comparative analysis of treadmill six‐minute walking test and cardiopulmonary exercise testing in obese and eutrophic women. Revista brasileira de fisioterapia 2012;16:469–478. [DOI] [PubMed] [Google Scholar]
- 17. Tvarijonaviciute A, Martinez S, Gutierrez A, et al. Serum acute phase proteins concentrations in dogs during experimentally short‐term induced overweight. A preliminary study. Res Vet Sci 2011;90:31–34. [DOI] [PubMed] [Google Scholar]
- 18. German AJ, Hervera M, Hunter L, et al. Improvement in insulin resistance and reduction in plasma inflammatory adipokines after weight loss in obese dogs. Domest Anim Endocrinol 2009;37:214–226. [DOI] [PubMed] [Google Scholar]
- 19. Wakshlag JJ, Struble AM, Levine CB, et al. The effects of weight loss on adipokines and markers of inflammation in dogs. Br J Nutr 2011;106(Suppl. 1):S11–S14. [DOI] [PubMed] [Google Scholar]
- 20. Laflamme DP, Kuhlman G, Lawler DF. Evaluation of weight loss protocols for dogs. J Am Anim Hosp Assoc 1997;33:253–259. [DOI] [PubMed] [Google Scholar]
- 21. Lazarus R, Sparrow D, Weiss ST. Effects of obesity and fat distribution on ventilatory function: The normative aging study. Chest 1997;111:891–898. [DOI] [PubMed] [Google Scholar]
- 22. Conway B, Rene A. Obesity as a disease: No lightweight matter. Obes Rev 2004;5:145–151. [DOI] [PubMed] [Google Scholar]
- 23. Haskins S, Pascoe PJ, Ilkiw JE, et al. Reference cardiopulmonary values in normal dogs. Comp Med 2005;55:156–161. [PubMed] [Google Scholar]
- 24. Kneyber MC, van Heerde M, Markhorst DG. Reflections on pediatric high‐frequency oscillatory ventilation from a physiologic perspective. Respir Care 2012;57:1496–1504. [DOI] [PubMed] [Google Scholar]
- 25. Boddy KN, Roche BM, Schwartz DS, et al. Evaluation of the six‐minute walk test in dogs. Am J Vet Res 2004;65:311–313. [DOI] [PubMed] [Google Scholar]
- 26. Cahalin LP, Mathier MA, Semigran MJ, et al. The six‐minute walk test predicts peak oxygen uptake and survival in patients with advanced heart failure. Chest 1996;110:325–332. [DOI] [PubMed] [Google Scholar]
- 27. Guyatt GH, Townsend M, Keller J, et al. Measuring functional status in chronic lung disease: conclusions from a randomized control trial. Respir Med 1991;85(Suppl. B):17–21; discussion 33–17. [PubMed] [Google Scholar]
- 28. Pascual M, Pascual DA, Soria F, et al. Effects of isolated obesity on systolic and diastolic left ventricular function. Heart 2003;89:1152–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chockalingam A, Linden MA, Dellsperger KC, et al. Correlation of normal diastolic cardiac function with VO in the metabolic syndrome. Prev Cardiol 2009;12:163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Burns PM, Driessen B, Boston R, et al. Accuracy of a third (Dolphin Voyager) versus first generation pulse oximeter (Nellcor N‐180) in predicting arterial oxygen saturation and pulse rate in the anesthetized dog. Vet Anaesth Analg 2006;33:281–295. [DOI] [PubMed] [Google Scholar]


