Abstract
Background
Diabetes mellitus (DM) is a common endocrinopathy in cats. Most affected cats suffer from a type of diabetes similar to type 2 diabetes in humans. An increasing prevalence has been described in cats, as in humans, related to obesity and other lifestyle factors.
Objectives
To describe the incidence of DM in insured Swedish cats and the association of DM with demographic risk factors, such as age, breed and sex.
Animals
A cohort of 504,688 individual cats accounting for 1,229,699 cat‐years at risk (CYAR) insured by a Swedish insurance company from 2009 to 2013.
Methods
We used reimbursed insurance claims for the diagnosis of DM. Overall incidence rates and incidence rates stratified on year, age, breed, and sex were estimated.
Results
The overall incidence rate of DM in the cohort was 11.6 cases (95% confidence interval [CI], 11.0–12.2) per 10,000 CYAR. Male cats had twice as high incidence rate (15.4; 95% CI, 14.4–16.4) as females (7.6; 95% CI, 6.9–8.3). Domestic cats were at higher risk compared to purebred cats. A significant association with breed was seen, with the Burmese, Russian Blue, Norwegian Forest cat, and Abyssinian breeds at a higher risk compared to other cats. No sex predisposition was found among Burmese cats. Several breeds with a lower risk of DM were identified.
Conclusions and clinical importance
Our results verify that the Burmese breed is at increased risk of developing DM. We also identified several previously unreported breeds with increased or decreased risk of DM.
Keywords: Burmese, Epidemiology, Feline, Type 2 diabetes
Abbreviations
- CI
confidence interval
- CYAR
cat‐years at risk
- DM
diabetes mellitus
- IR
incidence rate
- IRR
incidence rate ratio
- SD
standard deviation
Diabetes mellitus (DM) is a common endocrinopathy in cats, and the prevalence reportedly has increased during the last few decades,1 comparable to the globally increasing prevalence described in humans.2 Most cats suffer from a type of diabetes similar to type 2 diabetes in humans, characterized by a relative deficiency of insulin combined with insulin resistance, in contrast to the primary beta cell deficiency seen in human type 1 diabetes. Similarities between cats and humans include common risk factors that cause insulin resistance, such as obesity, increasing age, and physical inactivity, as well as similar pathologic findings with beta cell loss and deposition of pancreatic islet amyloid.3, 4, 5, 6, 7 Several studies have reported breed predispositions for DM in cats; in particular, the Burmese breed seems to be overrepresented.8, 9, 10 In humans, ethnic differences in the incidence of type 2 diabetes are well documented.11
Current estimates of the worldwide occurrence of DM in cats are scarce. Published data are based on hospital records,1, 6, 10, 12 submissions to laboratory services9, or insurance data.8 An increase in hospital prevalence from 8 cases per 10,000 cats in 1970 to 124 per 10,000 in 1999 was reported using tertiary care data in the United States.1
Insurance data have been used previously to estimate the prevalence of DM.8 A benefit of using insurance databases for estimating disease frequency is that the population at risk is known, thus allowing estimation of disease incidence, which is comparable among populations. Moreover, the considerable size of the insurance database usually allows stratifications by age, breed, and sex.13 The database held by Agria Pet Insurance has been used previously to describe patterns of mortality and morbidity in cats, and it also has been validated for this use.13, 14, 15 Approximately 36% of the Swedish cat population (an estimated 1,160,000 individuals) has veterinary care insurance,16 and Agria Pet Insurance1 is the largest insurance company for pets in Sweden.
The aim of this study was to provide population‐based information on the occurrence of DM in cats in a large Swedish population of insured cats, including incidence rates (IR) in the whole cohort and stratified by year, age, breed, and sex.
Materials and Methods
Study population
All cats with veterinary care insurance by Agria Pet Insurance at any time during the years 2009–2013 were included in the study. With the veterinary care insurance, the pet owner is reimbursed for costs exceeding the deductible for veterinary treatment in case of disease or accident. Although we had access to data from 2008, we decided to exclude cats with a claim for DM during that year to decrease the risk of including prevalent cases of DM in the analysis. Cats were at risk from either January 1, 2009 or the start date of insurance (if later), and until the first date of diagnosis of DM, death, or withdrawal from insurance.
Breeds were classified according to 41 breed codes. Breeds that were considered closely related were combined as follows: Abyssinians and Somalis were combined to “Abyssinian”; Burmese and Burmilla to “Burmese”; Cornish and German Rex to “Cornish/German Rex”; Devon Rex and Sphynx to “Devon Rex/Sphynx”; Persians and Exotic shorthairs to “Persian/Exotic”; Siamese, Balinese, Foreign White, Javanese, Oriental shorthair, and Seychellois to “Oriental breeds”; and Siberian cat and Neva Masquerade to “Siberian”. “Domestic cats” included domestic shorthair and longhair cats.
Sex was recorded as either male or female, because reliable information on neutering status was not available in the database. Cats were assigned to their age category on January 1st of each year (0–1, 1–2 years, etc.).
Diagnostic classification
The diagnosis underlying each insurance claim was assigned by the attending veterinarian based on a standardized system with approximately 8,000 diagnostic codes.17 We included 4 diagnostic codes used for cases of DM (diabetes mellitus, diabetes mellitus without complication, diabetes mellitus with complication, and diabetes mellitus with ketoacidosis). The date of the first claim of DM was used as a proxy for DM onset. Cases, thus were only counted once.
Data analysis
Variables used in this study included birth date, breed, sex, date of start, and renewal of insurance, date of withdrawal from insurance, and diagnostic codes for veterinary care insurance claims. Incidence rate calculations were performed using the first event of DM as the numerator, and the exact time at risk as the denominator. Standard errors multiplied by a factor of 1.96 yielded 95% confidence intervals (CI). Incidence rate ratios (IRR) were used to compare the incidence rates between groups, mainly comparing breed‐ and sex‐specific rates. Each breed was compared to all the cats minus the breed of interest. For comparisons between the purebred cats, each breed was compared to all purebred cats minus the breed of interest. Confidence intervals for the IRRs were calculated using Eq. (1) where the straight lines denote the lower and upper limits of the IRR and the hats denote the point estimates of IRR and standard deviation (SD):18
| (1) |
A 95% CI for the IRRs not including the null value of 1 was considered evidence to conclude that the groups were statistically significantly different from other cats. Age in the population at risk was calculated as mean age ± SD per breed and in the whole cohort as of January 1st of each study year and during the whole study period. Age at the first identified event was described using mean age ± SD. In addition, age‐standardized rates per breed were constructed to adjust for age differences among breeds. Breed‐specific incidence rates and age‐standardized rates were ranked from high to low, and the ranking orders were compared. To what extent insurance was continued after DM diagnosis was expressed as median time and quartiles, and the percentage with continued insurance for ≥4 years after diagnosis of DM in the year 2009 was calculated. Data handling was performed using SAS (version 9.3).2
Results
Study population
During 2009–2013, the total number of insured cats was 504,688 contributing 1,229,699 CYAR. Purebred cats contributed 282,340 CYAR (23% of total CYAR), domestic cats 944,487 CYAR (77%), and cats of unknown breeds 2,872 CYAR (<1%). Male cats contributed 635,627 CYAR and female cats 594,073 CYAR. Contributed CYAR per breed is shown in Table 1. Figure 1 shows contributed CYAR in the study population by age category. Mean age in the whole population at risk was 5.5 (±4.1) years, and the mean age differed among breeds. Lowest mean age was found among the Siberian cats with 2.6 (±2.4) years, and the highest in the European Shorthairs with 9.9 (±4.5) years.
Table 1.
Distributions of contributing cat‐years at risk, novel diabetes events, and incidence rates in the Agria database during 2009–2013 per breed category (sorted by descending risk).
| Breed | CYAR | Novel DM Events | IR per 10,000 CYAR (95% CI) |
|---|---|---|---|
| Burmese | 6,558 | 32 | 48.8 (31.9 to 65.7) |
| Russian Blue | 3,174 | 14 | 44.1 (21.0 to 67.2) |
| Norwegian Forest cat | 39,100 | 83 | 21.2 (16.7 to 25.8) |
| European Shorthair | 16,121 | 34 | 21.1 (14.0 to 28.2) |
| Abyssinian | 5,705 | 12 | 21.0 (9.1 to 32.9) |
| Ocicat | 3,036 | 4 | 13.2 (0.3 to 26.1) |
| Devon Rex/Sphynx | 12,931 | 17 | 13.1 (6.9 to 19.4) |
| Domestic cats | 944,487 | 1,142 | 12.1 (11.4 to 12.8) |
| Cornish/German Rex | 10,282 | 12 | 11.7 (5.1 to 18.3) |
| Other purebreds | 2,777 | 3 | 10.8 (−1.4 to 23.0) |
| Somali | 2,487 | 2 | 8.0 (−3.1 to 19.2) |
| Oriental breeds | 14,965 | 12 | 8.0 (3.5 to 12.6) |
| Maine Coon | 23,761 | 15 | 6.3 (3.1 to 9.5) |
| Persian/Exotic | 33,475 | 16 | 4.8 (2.4 to 7.1) |
| British Shorthair | 17,608 | 8 | 4.5 (1.4 to 7.7) |
| Unknown/mix | 2,872 | 1 | 3.5 (−3.3 to 10.3) |
| Birman | 40,900 | 12 | 2.9 (1.3 to 4.6) |
| Ragdoll | 30,874 | 9 | 2.9 (1.0 to 4.8) |
| Siberian | 8,806 | 2 | 2.3 (−0.9 to 5.4) |
| Bengal | 9,782 | 2 | 2.0 (−0.8 to 4.9) |
| Total | 1,229,699 | 1,432 | 11.6 (11.0 to 12.2) |
CYAR, cat‐years at risk; DM, diabetes mellitus; IR, incidence rate; CI, confidence interval.
Figure 1.
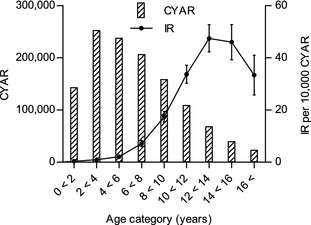
Distributions of contributing cat‐years at risk (CYAR) and incidence rates (IR) of diabetes during 2009–2013 per age category. Error bars represent 95% confidence intervals.
Diagnostic classification
Eighty‐seven percent of the cases were diagnosed as DM, 3% as DM with complication, 4% as DM without complication, and 6% as DM with ketoacidosis. There were no significant differences between the purebred cats and the domestic cats with regard to the diagnostic classification when comparing IRRs between groups.
Incidence rates of diabetes
Overall
The IR of DM in the whole cohort was 11.6 cases per 10,000 CYAR (95% CI, 11.0–12.2). The IRs were similar across study years (Table 2).
Table 2.
Distributions of contributing cat‐years at risk, novel diabetes events, incidence rates, and mean age at first event in the Agria database during 2009–2013 per study year.
| Year | CYAR | Novel DM events | IR per 10,000 CYAR (95% CI) | Mean age (±SD) |
|---|---|---|---|---|
| 2009 | 201,501 | 227 | 11.3 (9.8–12.7) | 10.9 (±3.2) |
| 2010 | 243,399 | 293 | 12.0 (10.7–13.4) | 10.6 (±3.0) |
| 2011 | 252,882 | 276 | 10.9 (9.6–12.2) | 10.5 (±3.3) |
| 2012 | 261,754 | 314 | 12.0 (10.7–13.3) | 10.4 (±3.1) |
| 2013 | 270,164 | 322 | 11.9 (10.6–13.2) | 10.8 (±2.9) |
| Total | 1,229,699 | 1,432 | 11.6 (11.0–12.2) | 10.7 (±3.1) |
CYAR, cat‐years at risk; DM, diabetes mellitus; IR, incidence rate; CI, confidence interval; SD, standard deviation.
Age
Mean age at diagnosis was 10.7 (±3.1) years, and did not differ between male (10.6 ± 3.1 years) and female cats (10.8 ± 3.3 years). The mean age at diagnosis per study year varied from 10.4 (±3.1) in 2012 to 10.9 (±3.2) years in 2009 (Table 2). The IR of DM was close to zero in younger cats, and started to increase at about 6 years of age (Fig 1). The IR peaked at 13 years of age.
Breed
The IR of DM differed across breeds. The Burmese, Russian Blue, Norwegian Forest cat, European Shorthair, and Abyssinian breeds had higher IRs of DM compared to other cats (Table 1). The IRR for DM in Burmese cats compared to all other cats was 4.3 (95% CI, 2.9–6.0; Fig 2), and 5.2 (95% CI, 3.6–7.6) if compared only to other purebred cats. The IRR for Russian Blue cats compared to all other cats and other purebreds, respectively, was 3.8 (95% CI, 2.1–6.4) and 4.5 (95% CI, 2.6–7.7); for Norwegian Forest cats 1.9 (95% CI, 1.5–2.3) and 2.5 (95% CI, 1.9–3.2), and for Abyssinians 1.8 (95% CI, 1.0–3.2) and 2.1 (95% CI, 1.2–3.7). The Bengal, Siberian, Ragdoll, Birman, British Shorthair, Persian, and Maine Coon breeds all had lower IRs compared to all other cats (Table 1), with IRRs indicating a lower risk of DM (Fig 2), and when compared only with other purebreds the lower risk remained for all but the Maine Coon breed. The domestic cats had an IR of 12.1 cases per 10,000 CYAR (95% CI, 11.4–12.8), and the purebred cats had an IR of 10.2 (95% CI, 9.1–11.4). The IRR for domestic cats compared to purebreds was 1.2 (95% CI, 1.0–1.3).
Figure 2.
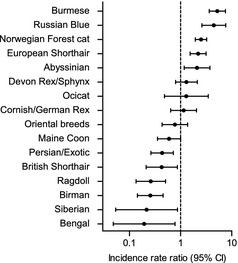
Incidence rate ratios of diabetes risk for different breeds compared to other purebreds. Breeds with at least 3,000 contributed cat‐years at risk are shown. Error bars represent 95% confidence interval (CI).
In the age‐standardized data set, the increased risk for European Shorthairs was no longer apparent. Instead, the Devon Rex/Sphynx breed group was ranked as the third highest IR of DM. The Maine Coon and Siberian breeds also moved from low‐risk breeds in the general data set to an IR of about average in the age‐adjusted data set. The Burmese, Russian Blue, Norwegian Forest cat, and Abyssinian breeds remained at an increased risk of DM also in the age‐adjusted data, and the Bengal, Birman, Persian, Ragdoll, and British Shorthair breeds remained as low‐risk breeds.
Sex
The IR of DM was higher for the male cats (15.4 cases per 10,000 CYAR; 95% CI, 14.4–16.4) than for the female cats (7.6 cases per 10,000 CYAR; 95% CI, 6.9–8.3) in the whole population, with an IRR for male cats compared to the females of 2.0 (95% CI, 1.8–2.3). This increased risk of DM for male cats was seen in most breeds, with a significantly increased risk for male domestic cats, European Shorthairs, Norwegian Forest cats, and Cornish/German Rex cats. An exception was observed for the Burmese breed, where a similar risk for males and females was seen, with an IR for male Burmese cats of 52.1 (95% CI 28.0–76.1), and for females 45.2 (95% CI, 21.5–68.9).
Continuation of insurance after DM claim
Nineteen percent of the cats diagnosed with DM during 2009 retained their insurances for ≥4 years. The median time for continuing insurance after diagnosis in 2009 was 1.1 years, with the lower quartile at 0.6 years and the upper quartile at 3.4 years.
Discussion
In this large insurance register‐based study, we were able to estimate the incidence of DM in cats and to stratify results by age, breed, and sex. We confirmed previous studies on the predisposition to DM in the Burmese breed, and also extend these findings to describe an increased risk, as well as a decreased risk, in several different breeds. Furthermore, we found that the commonly reported male overrepresentation was not seen in Burmese cats.
Several cat breeds with an increased risk of feline DM were identified. The Burmese breed exhibited a 5‐fold increased risk of DM as compared to other purebred cats. The Burmese predisposition in our study verifies previous results from European8 and Australian9, 10 studies. A Burmese breed predisposition has not been found in the United States.1, 6 A reason for this difference may be that the European and American Burmese breed lines have been kept separate for decades, and thus represent two different populations.19 Another possible explanation is that too few purebred cats were included in the previous studies1, 6 to be able to detect breed predispositions. The reason for the Burmese cats having an increased risk of developing DM is still unknown, but a dyslipidemia reflecting an inborn error of lipid metabolism has been described in the breed.20 Studies have shown a proportion of Australian Burmese cats to have fasting hypertriglyceridemia and an exaggerated postprandial triglyceride response after an oral fat tolerance test.21 Moreover, apparently healthy Burmese cats have been shown to have higher fasting glucose concentrations and lower glucose tolerance than non‐Burmese cats matched by age and body condition score.22 Furthermore, lean Burmese cats have been shown to have decreased adiponectin concentrations, similar to the concentrations observed in obese cats, suggesting they may be in a prodromal obese metabolic state.23 In the same study, lean Burmese cats also had aberrations in their cholesterol lipoprotein fraction profiles, similar to those of obese cats.23 In humans, certain ethnic groups with a high propensity for obesity and type 2 diabetes have lower adiponectin concentrations compared to other groups, and this difference was not explained by differences in percent body fat among groups. Instead, the degree of hypoadiponectinemia seemed to be more related to the degree of insulin resistance and hyperinsulinemia than to the degree of adiposity.24 The dyslipidemia and insulin resistance observed in Burmese cats, thus may be related to the increased risk of DM in the breed. Additional investigations are needed to unravel the underlying genetic risk factors to the dyslipidemia and predisposition for DM in this breed.
Three previously unreported high‐risk breeds for DM (the Russian Blue, Norwegian Forest cat, and Abyssinian) were identified in our study. A previous study using insurance data to report the prevalence of DM8 did not detect significant differences among individual breeds, aside from a higher risk for the Burmese cats. The larger population included in our study may have contributed to the fact that breed variations were detected. The high‐risk breeds identified in this study, including the Burmese, do not seem to be closely related or share a common ancestry, and the level of inbreeding also varies among breeds.19 All 4 breeds were at increased risk in both the general and the age‐adjusted data sets. Moreover, domestic cats had a higher risk of DM as compared to purebred cats, but the difference was small. Furthermore, our study identified several breeds with a lower risk of DM. The Bengal, Birman, Persian, Ragdoll, and British Shorthair cat breeds all were less prone to develop DM in our study, in both the general and the age‐adjusted data, as compared with all other cats as well as compared with other purebred cats. Low‐risk cat breeds for DM to our knowledge have not been reported previously.
Diabetes mellitus is a disease seen most frequently in older cats, with a mean age of diagnosis at approximately 11 years in this case material, which is consistent with the results of the previous studies.1, 6, 10 Our results demonstrate that the risk of developing DM continues to increase with age, with a peak risk at 13 years of age. Furthermore, our data indicate that the incidence seems to decrease at older ages. At the same time, the population at risk also decreases, making statistical evaluation less reliable in this geriatric group. Possibly, owners may choose elective euthanasia rather than treatment, and therefore no claim for the veterinary care insurance will be registered and consequently cases may be missed. Possibly also, the occurrence of DM may actually decrease because the presence of overweight and obesity decreases in geriatric cats.25
Because age is a risk factor for DM, we also standardized our data to adjust for age differences among breeds. With the standardized age rates, the European Shorthair breed was no longer at increased risk for DM. Mean age in this breed group was higher compared to other breeds, explaining the high IR in the general data set. On the other hand, the Devon Rex/Sphynx breed group showed an increased risk of DM in the age‐adjusted data set, whereas the Maine Coon and Siberian breeds changed from low‐risk to average risk breeds. In the age‐adjusted data, the Burmese, Russian Blue, Norwegian Forest cat, and Abyssinian breeds still remained at an increased risk of developing DM. The Bengal, Birman, Persian, Ragdoll, and British Shorthair breeds remained at low‐risk in the age‐adjusted data set.
Our findings support previously published data on sex predilection, with an increased risk of DM in the male compared to the female cats.1, 6, 8, 10 The male predisposition to DM also was significant in several individual breeds (e.g., European Shorthair, German/Cornish Rex, and Norwegian Forest cats), as well as in the domestic cats. The increased risk of DM in male cats may be related to 2 factors. First, male cats are predisposed to obesity,25 a major risk factor for DM, and secondly, male cats tend to have lower insulin sensitivity than female cats.26 The male predilection for DM interestingly was not evident in the Burmese breed, in concordance with other studies.8, 9 The equal risk for developing DM in male and female Burmese cats may reflect different genetic risk factors for the increased risk of developing DM in this breed compared to other breeds.
A shortcoming of our study is the lack of information on neutering status. Neutering has been recognized as a risk factor for DM in both male and female cats.1, 6, 8 Neutering alone does not seem to decrease insulin sensitivity, but rather increases the risk of obesity. Neutered cats gain weight more easily compared to intact cats,27, 28, 29 and subsequent weight gain leads to decreased insulin sensitivity.26, 30 Therefore, according to these studies, it is the increased risk of obesity after neutering that appears to be the contributing factor to the increased occurrence of DM in neutered cats.8, 31 However, other studies show conflicting results and it remains unclear whether male or female cats are more prone to weight gain after neutering.27, 29, 32 In Sweden, more than 80% of all cats are neutered, with somewhat more male cats (88%) neutered compared to female cats (73%).16
Because obesity is one of the major risk factors for DM in cats,4 and the incidence of obesity is a growing problem in the cat population,33 an increase in the incidence of DM in cats could be anticipated. However, no increase in the incidence of DM was evident in our data during the period of 2009–2013. A possible explanation for the lack of increase in incidence in our study is the relatively short study time period of 5 years, which can be compared to a previous study showing an increased prevalence of DM based on data from several decades.1 Previous studies reporting occurrence of DM have been based on insurance data8 or hospital records,1, 6 and may not accurately reflect the incidence of the disease, as the measurement of prevalence is affected by case survival. Moreover, awareness and knowledge about the disease, better prognosis, as well as willingness of both owners and veterinarians to diagnose and treat diabetic cats, together with an increasingly older cat population, are likely to be at least partly responsible for the increase in prevalence reported in previous studies.
The diagnostic codes used in this study were assigned by the attending veterinarian, and agreement between veterinary records and the database has been evaluated previously and found to be fair.15 A DM event in our insurance database, thus likely represents a true case. However, some cats presenting with stress hyperglycemia might have been falsely misdiagnosed, and cases also may have gone undetected. In order to be visible in the database, the cost of the veterinary care event must be high enough to reach the deductible. For example, cats euthanized at the time of diagnosis may not always be visible as cases in the insurance database. As a consequence, not all cases will be registered, leading to an underestimation of the true incidence.
Another possible disadvantage of our study is the risk of cats diagnosed with DM before the study period appearing as incident cases in our material. Because we had access to the data from 2008, we decided to exclude cats with a diagnosis of DM during that year to decrease the risk of including prevalent cases. The potential bias of including prevalent cases may impact the estimated mean age at first event, as well as the age‐specific IRs, and also the incidence per year. The problem is anticipated to be most important during the first study years. The mean age was slightly higher during 2009 compared with subsequent years, which would indicate some cases falsely appearing as first events during the early study period.
Another possible limitation of this study is the fact that a smaller proportion of the domestic cats compared to the pedigree cats are insured. In our data, the purebred cats contributed to 23% of CYAR, which is higher than the proportion of pedigree cats of nearly 10% in the whole Swedish population.16 It is also unknown to what extent insured cats are typical of all Swedish cats, or of cats in general. Insured cats may be more highly cared for and have increased access to veterinary care, and insured cats may thus may represent a healthier population compared to uninsured cats.
Other limitations of this study include lack of information about environmental risk factors such as diet, activity, body condition score, and other factors that are unavailable in the database, but could influence the risk of developing DM. Moreover, the continuation of insurance as a proxy for survival is not optimal because information about the exact time of death or why owners choose to cancel their insurance is not always available or validated in the database. However, 19% of the cats remained insured for at least 4 years after diagnosis.
In conclusion, our study identified several previously unreported breeds associated with either increased or decreased risk of developing DM. We also were able to confirm the previously known Burmese breed predisposition for DM. The lack of male overrepresentation in the Burmese breed, clearly seen in other breeds, indicates that different genetic risk factors are responsible for the increased risk of developing DM in this breed, which warrants further investigation. No evidence of an increasing incidence of DM in cats during the years 2009–2013 was detected.
Acknowledgments
The authors thank Agria Pet Insurance for allowing access to the database.
Funding: The research project was funded by the Future Animal Health and Welfare research platform, Swedish University of Agricultural Sciences.
Conflict of Interest Declaration: Tove Fall has received honorarium for lecturing from MSD Animal Health. Brenda Bonnett is presently an independent, part‐time consultant to the Agria Insurance Company.
Presentation at meetings: The study was presented at the 40th World Small Animal Veterinary Association Congress (Bangkok, Thailand, 2015), and at the Animal Obesity Congress (Uppsala, Sweden, 2015).
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Footnotes
Agria Pet Insurance, Stockholm, Sweden.
SAS Institute Inc., Cary, NC.
References
- 1. Prahl A, Guptill L, Glickman NW, et al. Time trends and risk factors for diabetes mellitus in cats presented to veterinary teaching hospitals. J Feline Med Surg 2007;9:351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus‐present and future perspectives. Nat Rev Endocrinol 2012;8:228–236. [DOI] [PubMed] [Google Scholar]
- 3. Slingerland LI, Fazilova VV, Plantinga EA, et al. Indoor confinement and physical inactivity rather than the proportion of dry food are risk factors in the development of feline type 2 diabetes mellitus. Vet J 2009;179:247–253. [DOI] [PubMed] [Google Scholar]
- 4. Scarlett JM, Donoghue S. Associations between body condition and disease in cats. J Am Vet Med Assoc 1998;212:1725–1731. [PubMed] [Google Scholar]
- 5. Rand JS, Fleeman LM, Farrow HA, et al. Canine and feline diabetes mellitus: nature or nurture? J Nutr 2004;134:2072S–2080S. [DOI] [PubMed] [Google Scholar]
- 6. Panciera DL, Thomas CB, Eicker SW, et al. Epizootiologic patterns of diabetes mellitus in cats: 333 cases (1980‐1986). J Am Vet Med Assoc 1990;197:1504–1508. [PubMed] [Google Scholar]
- 7. Westermark P, Wernstedt C, O'Brien T, et al. Islet amyloid in type 2 human diabetes mellitus and adult diabetic cats contains a novel putative polypeptide hormone. Am J Pathol 1987;127:414. [PMC free article] [PubMed] [Google Scholar]
- 8. McCann TM, Simpson KE, Shaw DJ, et al. Feline diabetes mellitus in the UK: the prevalence within an insured cat population and a questionnaire‐based putative risk factor analysis. J Feline Med Surg 2007;9:289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rand JS, Bobbermien LM, Hendrikz JK, et al. Over representation of Burmese cats with diabetes mellitus. Aust Vet J 1997;75:402–405. [DOI] [PubMed] [Google Scholar]
- 10. Lederer R, Rand JS, Jonsson NN, et al. Frequency of feline diabetes mellitus and breed predisposition in domestic cats in Australia. Vet J 2009;179:254–258. [DOI] [PubMed] [Google Scholar]
- 11. Cowie CC, Rust KF, Byrd‐Holt DD, et al. Prevalence of diabetes and high risk for diabetes using A1C criteria in the U.S. population in 1988–2006. Diabetes Care 2010;33:562–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sallander M, Eliasson J, Hedhammar A. Prevalence and risk factors for the development of diabetes mellitus in Swedish cats. Acta Vet Scand 2012;54:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Egenvall A, Bonnett BN, Haggstrom J, et al. Morbidity of insured Swedish cats during 1999‐2006 by age, breed, sex, and diagnosis. J Feline Med Surg 2010;12:948–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Egenvall A, Nodtvedt A, Haggstrom J, et al. Mortality of life‐insured Swedish cats during 1999–2006: age, breed, sex, and diagnosis. J Vet Intern Med 2009;23:1175–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Egenvall A, Bonnett BN, Olson P, et al. Validation of computerized Swedish dog and cat insurance data against veterinary practice records. Prev Vet Med 1998;36:51–65. [DOI] [PubMed] [Google Scholar]
- 16. Hundar, katter och andra sällskapsdjur 2012 In: Statistik från en undersökning på uppdrag av Agria Djurförsäkring, Jordbruksverket, Royal Canin och Svenska Kennelklubben. Örebro: Statistiska Centralbyrån; 2012: 4–14. [Google Scholar]
- 17. Olson P Kängström LE. Svenska djursjukhusföreningens diagnosregister för häst, hund och katt. Taberg: Svenska djursjukhusföreningen; 1993. [Google Scholar]
- 18. Rothman KJ. Epidemiology: an introduction, 2nd edn New York: Oxford University Press; 2012. [Google Scholar]
- 19. Alhaddad H, Khan R, Grahn RA, et al. Extent of linkage disequilibrium in the domestic cat, Felis silvestris catus, and its breeds. PLoS ONE 2013;8:e53537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Crispin S. Ocular lipid deposition and hyperlipoproteinaemia. Prog Retin Eye Res 2002;21:169–224. [DOI] [PubMed] [Google Scholar]
- 21. Kluger EK, Hardman C, Govendir M, et al. Triglyceride response following an oral fat tolerance test in Burmese cats, other pedigree cats and domestic crossbred cats. J Feline Med Surg 2009;11:82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lederer R, Rand J, Morton JM. Fasting glucose concentrations are higher and glucose tolerance is lower in Burmese cats compared to matched non‐Burmese cats. J Vet Intern Med 2005;19:462–463. [Google Scholar]
- 23. Lee P, Mori A, Coradini M, et al. Potential predictive biomarkers of obesity in Burmese cats. Vet J 2013;195:221–227. [DOI] [PubMed] [Google Scholar]
- 24. Weyer C, Funahashi T, Tanaka S, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab 2001;86:1930–1935. [DOI] [PubMed] [Google Scholar]
- 25. Lund EM, Armstrong PJ, Kirk CA, et al. Prevalence and risk factors for obesity in adult cats from private US veterinary practices. Int J Appl Res Vet Med 2005;3:88–96. [Google Scholar]
- 26. Appleton DJ, Rand JS, Sunvold GD. Insulin sensitivity decreases with obesity, and lean cats with low insulin sensitivity are at greatest risk of glucose intolerance with weight gain. J Feline Med Surg 2001;3:211–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nguyen PG, Dumon HJ, Siliart BS, et al. Effects of dietary fat and energy on body weight and composition after gonadectomy in cats. Am J Vet Res 2004;65:1708–1713. [DOI] [PubMed] [Google Scholar]
- 28. Fettman M, Stanton C, Banks L, et al. Effects of neutering on bodyweight, metabolic rate and glucose tolerance of domestic cats. Res Vet Sci 1997;62:131–136. [DOI] [PubMed] [Google Scholar]
- 29. Martin L, Siliart B, Dumon H, et al. Leptin, body fat content and energy expenditure in intact and gonadectomized adult cats: a preliminary study. J Anim Physiol Anim Nutr 2001;85:195–199. [DOI] [PubMed] [Google Scholar]
- 30. Biourge V, Nelson RW, Feldman EC, et al. Effect of weight gain and subsequent weight loss on glucose tolerance and insulin response in healthy cats. J Vet Intern Med 1997;11:86–91. [DOI] [PubMed] [Google Scholar]
- 31. Hoenig M, Ferguson DC. Effects of neutering on hormonal concentrations and energy requirements in male and female cats. Am J Vet Res 2002;63:634–639. [DOI] [PubMed] [Google Scholar]
- 32. Fettman MJ, Stanton CA, Banks LL, et al. Effects of weight gain and loss on metabolic rate, glucose tolerance, and serum lipids in domestic cats. Res Vet Sci 1998;64:11–16. [DOI] [PubMed] [Google Scholar]
- 33. German AJ. The growing problem of obesity in dogs and cats. J Nutr 2006;136:1940S–1946S. [DOI] [PubMed] [Google Scholar]


