Abstract
Background
Acquired myasthenia gravis (MG) in cats most commonly causes generalized weakness without megaesophagus and is more often associated with a cranial mediastinal mass, compared to dogs.
Hypothesis/Objectives
To extend the clinical findings described in the report of 2000 on MG in cats (J Am Vet Med Assoc 215:55–57).
Animals
Two hundred and thirty‐five cats with MG.
Methods
Retrospective case study to evaluate the long‐term outcome and incidence of spontaneous remission in myasthenic cats. Information including signalment, clinical presentation, presence of and type of cranial mediastinal mass, treatment including surgical versus medical, survival time, and outcome including spontaneous remissions was collected and analyzed in cats diagnosed at the Comparative Neuromuscular Laboratory, University of California San Diego by detection of acetylcholine receptor antibody titers >0.3 nmol/L by immunoprecipitation radioimmunosassay.
Results
Acquired MG in cats is associated with a euthanasia rate of 58%. Abyssinian and Somali cats had an increased incidence of MG compared to mixed breed cats or cats of other breeds. A cranial mediastinal mass, most commonly thymoma, was observed in 52% of the cats, which is higher than in the previous report. Spontaneous remission is not a characteristic of MG in cats.
Conclusions and clinical importance
Myasthenia gravis in cats is a chronic disease associated with a high incidence of a cranial mediastinal mass. Spontaneous remission is not common and clinicians should warn owners of the necessity for long‐term treatment. The clinical outcome with a cranial mediastinal mass did not differ between surgical or medical treatment.
Keywords: Acetylcholine receptor antibody, Feline, Thymoma
Abbreviations
- AChR
acetylcholine receptor
- CI
confidence interval
- MG
myasthenia gravis
Acquired myasthenia gravis (MG) is an antibody‐mediated autoimmune disease in which skeletal muscle weakness occurs as a result of impaired neuromuscular transmission because of loss of nicotinic acetylcholine receptors (AChRs) at the neuromuscular junction and disruption of postsynaptic membrane morphology.1 Acquired MG is a relatively common neuromuscular disease in dogs,2 but is less frequent in cats.3 The most common clinical signs of MG in cats include generalized weakness without megaesophagus and generalized weakness associated with a cranial mediastinal mass.2
Autoantibodies to AChRs are found in most human patients with acquired MG and rarely seen in healthy humans or patients with other diseases.4 A similar specificity of AChR antibodies is also present in dogs and cats with acquired MG.5 The presence of pathogenic AChR antibodies proves an autoimmune response against AChRs, which is not detected with other causes of muscle weakness.
Early reports of acquired MG in cats are limited to case studies6, 7, 8, 9, 10, 11, 12, 13, 14, 15 and retrospective case series.3, 16 Of the 13 cats in these reports, 6 were treated with pyridostigmine alone, 6 were treated with a combination of pyridostigmine and steroids (prednisone or prednisolone), and 1 cat was treated with only prednisolone. Six of these cats had thymomas that were surgically removed.
In the large retrospective study evaluating acquired MG in cats,3 clinical signs and risk factors of 105 cats were described. There is a greater incidence of MG in Abyssinian and Somali cats.3 Generalized weakness was the most common clinical sign seen in 57 cats (54.3%). In addition, 25.7% of cats with MG (27/105) had a mediastinal mass, compared with only 3.4% in dogs2 and approximately 21% in people.17
The aim of this retrospective study was to extend and compare findings from the previous report of 20003 to a larger group of myasthenic cats. We compared signalment, clinical signs, and the presence or absence of a cranial mediastinal mass to the previous retrospective report. In addition, we report the results of medical treatment, including medical versus surgical treatment of a mediastinal mass associated with MG, and evaluated whether cats are similar to dogs in terms of spontaneous remission of acquired MG.
Materials and Methods
Myasthenic cats with a serum AChR antibody titer >0.3 nmol/L diagnosed between 2001 and 2012 were identified for inclusion in this study from the database of the Comparative Neuromuscular Laboratory, University of California, San Diego. The AChR antibody titer was determined by immunoprecipitation radiommunoassay using a feline‐specific antigen as previously described.3 Signalment including sex, neuter status, breed, and year of birth were also obtained from the clinicopathologic database.
For information regarding specific treatment, response to treatment and follow‐up status, referring veterinarians with myasthenic cats included in the study were contacted and asked to submit medical record information. Information requested included presenting clinical signs, date clinical signs were first noted, and vaccination history. Thoracic imaging results indicating the presence or absence of a mediastinal mass and the pathologic diagnosis from biopsy specimens were collected. Information was also collected about surgical versus medical treatment, survival time, and spontaneous remission. Spontaneous remission was defined as the resolution of clinical signs without continued medication and the return of the AChR antibody titer to within the reference range. When myasthenic cats had multiple AChR antibody titers available, an increase or decrease in titer concentration was noted compared to the initial (baseline) titer value.
Kaplan‐Meier survival curves were used to determine if treatment type and the presence or absence of a mediastinal mass had an effect on survival. A Pearson's correlation coefficient was used to evaluate AChR antibody titer and survival. Odds ratios were calculated with a 95% confidence interval (CI) evaluating for risk of age, breed, and sex. Cats tested for acquired MG, but having negative AChR antibody titers, during the same study period were used as control. These control cases were from blood samples submitted to the laboratory with neuromuscular signs, but with negative AChR antibody titers.
Results
From 2001 to 2012, acquired MG was diagnosed in 235 cats at the Comparative Neuromuscular Laboratory, University of California, San Diego. Information was available on breed (218 cases), sex (221 cases), and year of birth (222 cases). Males and females were equal in number and mixed breed cats (80.4%) were more common than purebred cats (19.6%). There was an increased odds ratio for development of MG in Abyssinian (OR = 4.97) and Somali cats (OR = 11.60) compared to other breeds (Table 1). No significant differences in outcomes by sex (P = .7) or breed (P = .24) were found. The age at diagnosis was 8.6 years (mean, range 6 months to 15.4 years). Because medical records with vaccination history were only available for 14 cats, it was not possible to determine a relation in timing of vaccination to onset of clinical signs (range 11–935 days). Five cats were receiving methimazole treatment for hyperthyroidism at the time of diagnosis of MG.
Table 1.
Odds ratios for acquired myasthenia gravis in various breeds of cats
| Breed | Number of Cases | Number of Controls | Odds Ratio | 95% Confidence Interval | P value |
|---|---|---|---|---|---|
| Abyssinian | 24 | 48 | 4.97 | 2.98–8.29 | <.0001 |
| Somali | 5 | 4 | 11.6 | 3.09–43.5 | <.0001 |
| Ocecat | 1 | 4 | 2.27 | 0.25–20.48 | .46 |
| Domestic medium hair | 13 | 88 | 1.33 | 0.73–2.42 | .35 |
| Siamese | 8 | 64 | 1.14 | 0.54–2.41 | .73 |
| Domestic short hair | 140 | 1267 | 1.01 | 0.76–1.34 | .93 |
| Domestic long hair | 22 | 202 | 0.96 | 0.06–1.53 | .88 |
| Burmese | 2 | 23 | 0.79 | 0.18–3.36 | .75 |
| Tonkinese | 1 | 14 | 0.64 | 0.08–4.95 | .68 |
| Himalayan | 2 | 32 | 0.58 | 0.13–2.45 | .46 |
| Maine Coon | 2 | 50 | 0.35 | 0.08–1.48 | .16 |
| Persian | 2 | 63 | 0.28 | 0.06–1.16 | .08 |
| Bengal | 1 | 45 | 0.19 | 0.02–1.44 | .11 |
Presenting clinical signs were well described in the medical records for only 30 cats and included generalized appendicular muscle weakness without regurgitation or dysphagia in 15 cats (50%), focal clinical signs including regurgitation or dysphagia without generalized weakness in 3 cats (10%), and generalized weakness with regurgitation and dysphagia in 12 cats (40%). Results of thoracic radiographs were available in 9 cats with clinical signs of regurgitation. Megaesophagus was found on the radiographs of 5 cats and not noted in 4 cats. In 1 cat with clinical signs of regurgitation and no evidence of megaesophagus, radiographic evidence of aspiration pneumonia was noted. There was no difference in clinical presentation and presence or absence of a mediastinal mass.
Medical records of 133 cats included reports of thoracic radiographs and the presence or absence of a cranial mediastinal mass. A cranial mediastinal mass was observed in 69 (52%) cats. Histopathologic diagnosis of the mass was available for 36 cases via surgical biopsy or fine‐needle aspiration. Thymoma was diagnosed in 35 (97.7%) cats and lymphoma in 1 cat (2.3%).
Cats with MG were treated with medical management only (82 cats), surgery only (5 cats), or both medical and surgical management (35 cats). Medical treatment in 117 cats included steroids (prednisone, prednisolone, or triamcinolone) alone in 20 cats, pyridostigmine alone in 17 cats, a combination of steroids and pyridostigmine treatment in 65 cats, or combination of one or both of these with other immunomodulatory medications (cyclosporine, chlorambucil, or both) in 15 cats.
Serial AChR antibody titers were performed in 54 cats, of which 29 cats had thymoma, 19 cats without thymoma, and unknown in 6 cats (Fig. 1). Of the 29 cats with thymoma, 17 cats (57%) had a decrease in AChR antibody titers. Of these 17 cats, 12 (70%) had surgery and medications, 3 (18%) had only medications, and 2 had unknown treatment (12%). The titers increased in 7/29 (24%) of the thymoma cases with 6 (86%) cats having surgery and 1 cat having an unknown treatment. Of these 6 cats with surgical mass removal, 5 also had concurrent medical treatment for MG; the concurrent treatment was unknown for 1 cat. Antibody titers increased and then decreased from baseline in 3/29 (10%) and decreased and then increased in 2/29 (7%) of the thymoma‐associated MG cases. These latter 2 groups of 5 cats had both surgery and medical treatment. Cats in the thymoma group did not go into immune remission as continued medication was required and the AChR antibody titer remained elevated. Of the 19 cats without thymoma, the AChR antibody titer did not initially increase on follow‐up testing in any cat, 14 of the cats (73%) had a decrease in antibody titer, 3 cats (15%) had an initial increase in titer and then a decrease, and 2 cats (10%) had an initial decrease in titer and then an increase.
Figure 1.
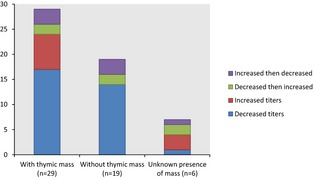
Serial measurements of acetylcholine receptor antibody titer after treatment (n = 54).
Comorbidities were reported in the medical records of 56 cats with acquired MG and many of these cats had multiple disease processes in addition to MG. Fourteen (25%) of these cats had a heart murmur or cardiomyopathy with 2/14 having a history of congestive heart failure. Ten cats (18%) had hyperthyroidism, 6 cats (11%) had diabetes mellitus, and 5 cats (9%) had a history of pneumonia (presumed to be secondary to megasophagus in 4/5 cats). Corneal ulceration, dermatitis, and pleural effusion were described in 4 cats each. Three cats were identified as having seizures and 3 cats were reported to have dental disease. The following diseases were described in 2 cats for each: herpes, urinary obstruction, diffuse bronchial disease, asthma, feline lower urinary tract disease, cholangiohepatitis, upper respiratory infection, and erythema multiforme. The following diseases were identified in at least 1 cat: keratoconjunctivitis sicca, otic polyp, retropharyngeal abscess, hypertension, right femoral fracture, gastrointestinal illness, possible vaccine reaction, hydronephrosis, hepatic lipidosis, pancreatitis, pemphigus foliaceous, chorioretinitis, Bartonella infection, obesity, renal insufficiency, renal failure, pancreatic cancer, neoplastic skin disease, and renal carcinoma. The cat with renal carcinoma and the cat with neoplastic skin disease had a concurrent thymoma; the other 2 cats with pancreatic neoplasia did not have evidence of a thymic mass on radiography.
Clinical outcomes were available in 92 cats. Only 35 cats (38%) were alive at the time of this study (range 11 days to 115 months from diagnosis). Four cats (4%) died and 53 (58%) were euthanized (range 1 day to 97 months from diagnosis). After treatment, only 5 of the surviving cats went into spontaneous remission and showed normalization of the AChR antibody titers. An additional cat went into clinical disease remission for 1 year and then came out of remission. This cat did not have a thymoma and was euthanized 507 days after diagnosis.
Clinical outcome was not associated with the presence or absence of a cranial mediastinal mass (P = .49). In the population of cats with a cranial mediastinal mass, there was no significant difference in outcome by medical treatment (P = .67) or surgery (P = .09). There was a significant negative correlation (r = −0.3, P = .001) between the value of the antibody titer and survival time (Fig. 2).
Figure 2.
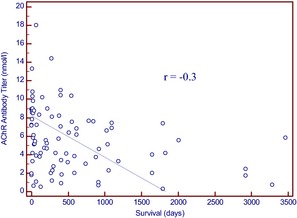
Negative correlation (r = −0.3) between acetylcholine receptor antibody titer and survival (in days).
Discussion
Similar to our previous study on acquired MG in 105 cats,3 this study in 235 myasthenic cats identified an increased relative risk in Abyssinian and Somali cats. Consistent with the previous report, there was no sex predisposition. In dogs, there appears to be an increased risk for developing acquired MG in the Akita, Scottish Terriers, German Shorthaired pointers, and Chihuahuas.2 In people, there is an increased risk of various types of MG based on race, sex, and age; muscle‐specific kinase antibody‐positive MG appears to be more common in African Americans with a younger age of onset and AChR antibody‐positive MG is more commonly seen in males at an older age without a race predilection and is associated with thymoma.18
Only 14 cats had information regarding vaccine history in the medical record. In a previous report, there was an exacerbation of clinical signs in a dog with acquired MG approximately 6 weeks after routine vaccination.19 Because of the lack of medical record data and many unvaccinated indoor cats, we are unable to detect an association with vaccination to trigger the disease or vaccination‐induced exacerbation of MG in cats.
Acquired MG occurs in cats after methimazole treatment for hyperthyroidism because of the immunomodulatory properties of the medication.13, 20 In this cohort of cats, 5 cats were receiving methimazole treatment at the time of diagnosis. One of the cats was still alive 3 years after diagnosis and the other was alive about 2.3 years after diagnosis. Both cases were then lost to follow‐up. There is no information about the treatment or outcome of the other 3 cases. Two additional cats had previously been treated with methimazole, but then had I131 treatment, and were not on methimazole treatment at the time of diagnosis. Another cat was started on methimazole treatment after diagnosis of MG with no report of worsening of clinical signs.
Generalized weakness without megaesophagus or dysphagia was present in 50% of the cats in this study; this is lower than in our previous study (64%). Because of the retrospective nature and reliance on medical records data, clinical signs were only accurately noted in 30 cats out of the cohort of 235 cats, which is a weakness of this study. In another study from a different group, about 85% (17/20) of cats with MG showed generalized weakness.16 Generalized weakness with megaesophagus or dysphagia occurred in 40% of the cats in this study; this is higher than in the previous study (15%). Unlike in dogs where focal MG is relatively common, clinical signs localized to the esophagus or pharynx only (focal MG) composed about 10% of the cases; this is down from 21% in our previous study. Megaesophagus is not as common in cats compared to dogs, because of the increased proportion of smooth muscle in the esophagus compared to the canine esophagus, which contains mostly skeletal muscle.
An important finding in our study is that independently of the clinical presentation, there was a higher incidence of a cranial mediastinal mass (52%) than reported in our previous study (26%).3 Our earlier report on MG in cats documented a greater association of a cranial mediastinal mass in MG in cats compared with MG in dogs.3 The incidence of acquired MG in cats evaluated primarily for thymoma is between 4.7 and 6% of cases.21, 22 On the basis of the relatively common association of MG and thymoma as shown in our study, we recommend AChR antibody testing on all cats with confirmed or suspected thymoma. This recommendation is standard in human medicine.23, 24
In this study, 40 myasthenic cats with a cranial mediastinal mass underwent surgical removal. Thirty‐five of these cats survived the immediate postoperative period and all but one of the surviving cats (34 cats) required medical management to control clinical signs of MG after thymectomy. This finding correlates with the veterinary literature where almost all cases of cats with MG‐associated thymoma required postsurgical medical management to control signs of MG.7, 11, 12 There is only 1 case of a cat reported with a cystic thymoma and associated MG that recovered postthymectomy without a relapse of clinical signs of MG.9
In this study, no information was available regarding completeness of tumor resection. Based on the results of our study, it may be rare to have resolution of thymoma‐associated MG after removal of the tumor. Therefore, owners should be alerted to the likelihood of continued medical management of MG after surgical removal of a thymoma. Because of the retrospective nature of this study, the variability in treatments and limited information available in the medical records, we were unable to reach any conclusions regarding best management of thymoma‐associated MG. Further long‐term prospective investigations of postoperative surgical cases are warranted to determine resolution of weakness and if normalization of serial AChR antibody titers would occur over time.
In both the human and veterinary literature, there are conflicting reports of continued clinical signs of MG versus remission of clinical signs after surgical removal of thymoma.7, 11, 12, 25, 26, 27, 28, 29, 30, 31, 32, 33 Studies in myasthenic people include both cases of thymic hyperplasia and thymoma. The outcome of thymic hyperplasia cannot be directly compared to that of myasthenic cats with thymoma. One study, which followed myasthenic people after a thymectomy for various thymic masses, reported long‐term improvement in the majority of patients with 34% showing complete remission and 33% showing substantial decrease in the requirement for medication.32 In another study, the mean survival time in the 33% of patients showing no response postthymoma removal was decreased compared with survival time in patients showing a partial response (16.7%) and those with a complete remission (50%)34 of MG clinical signs. This suggests that there is a decreased morbidity and mortality in patients who continue to show clinical signs of MG after thymic mass removal.
In this study, the majority of medically treated cats (56%) were treated with a combination of steroids and pyridostigmine (65/117) and the other cats were treated with steroids only (17%), only pyridostigmine treatment (14%), or with a combination of steroids +/‐ pyridostigmine with other immunomodulatory medications (13%). In this cohort, 15 cases where treated with adjunctive immunomodulatory medications that had not been previously reported in the literature as being used in cats with acquired MG. This included 13 cats treated with cyclosporine (with steroids ± pyridostigmine), 1 cat treated with chlorambucil (with steroids and pyridostigmine), and 1 cat treated with a combination of cyclosporine, chlorambucil, steroids, and pyridostigmine. Because of the retrospective nature of this study limiting the data available on outcomes in addition to the variety of treatments and dosages used, there is not enough information to correlate medical treatment with outcome. Future prospective studies evaluating medical treatment protocols would be needed to determine which treatment option may prove be the most beneficial in this disease.
To determine the course of the disease and possible immune remission, subsequent AChR antibody titers were performed in 54 cats with and without thymoma. There was no significant change in AChR antibody titers in cats with thymoma immediately after surgical intervention compared to pretreatment titers, which corresponds with a previous feline case report10 and cases in the human literature.35 Five of 54 (9.2%) MG cats went into spontaneous remission after treatment, requiring no further medication and with AChR antibody titers <0.3 nmol/L (4 cases not associated with thymoma and 1 with thymoma). Only 1 of the cats in remission had myasthenia associated thymoma, which was treated with surgical removal and a combination of steroids and pyridostigmine. Treatment for 2 of the other cats what went into remission consisted of steroids and pyridostigmine and 1 cat was treated with a combination of steroids, pyridostigmine, and chlorambucil. Information was lacking about the specific treatment used in 1 of the 5 cats that showed normalization of AChR antibody titer. Spontaneous immune remission is reported to occur in about 88.7% of canine acquired nonthymoma MG cases and clinical remission in 19.2–77.6% of human acquired MG cases.36, 37 Based on the results of this study and previous reports, the overall rate of spontaneous remission appears to be less in cats compared to dogs and humans.
In all MG cases in cats (both with and without a cranial mediastinal mass), there was a significant correlation between higher AChR antibody titers and decreased survival time (Fig. 2). As a normal distribution was not found when evaluating AChR antibody titer and survival times, no cutoff value could be determined that would help in assessing cats above or below a threshold value clinically. In human MG, there is also a more favorable outcome in patients with low or negative antibody levels.38, 39, 40
Radiation therapy is another potential treatment option for myasthenic cats with thymoma. Reports of outcomes are limited.34, 41 One study suggests a median survival time of 720 days in cats undergoing radiation therapy41 versus 3.71 years in cats undergoing surgery for thymoma.22 There is no information about the effect on clinical signs of MG in cats treated for thymoma with radiation treatment or surgery. Radiation could be considered as a potential treatment and may decrease the morbidity of surgery in the cat population, but more data are necessary. None of the cats in our population were treated with radiation therapy.
A surprising finding in our study was the large percentage of cats (58%) that were euthanized for this condition. A reason for euthanasia was not evident from the medical records. Possible reasons for euthanasia include inability to pursue surgical removal of a thoracic mass, difficulty administering medication to cats, limited information about outcome and prognosis, and client's inability to care for a cat with a neuromuscular disease. Further study into why clients decide to euthanize myasthenic cats is needed to determine ways to decrease morbidity and mortality.
In conclusion, MG in cats is associated with a high rate of euthanasia. A thymoma‐associated cranial mediastinal mass occurs more frequently in cats than previously reported. There was a significant negative correlation (r = −0.3, P = .001) between the value of the antibody titer and survival time. Because of the nature of the study and missing data in medical records, the absolute incidence of spontaneous remission could not be determined with certainty, but as far as could be determined, immune remissions are not a characteristic of MG in cats compared to MG in dogs.
Acknowledgments
The authors thank all the veterinarians who provided serum samples on myasthenic cats and follow‐up data for this study.
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: The authors declare no off‐label use of antimicrobials.
This work was performed in and supported by the Comparative Neuromuscular Laboratory, University of California, San Diego.
This study was presented as an abstract at the 2013 European College of Veterinary Neurology (ECVN) Symposium, Paris, France.
References
- 1. Lindstrom JM. Acetylcholine receptors and myasthenia. Muscle Nerve 2000;23:453–477. [DOI] [PubMed] [Google Scholar]
- 2. Shelton GD, Schule A, Kass PH. Risk factors for acquired myasthenia gravis in dogs: 1154 cases (1991–1995). J Am Vet Med Assoc 1997;211:1428–1431. [PubMed] [Google Scholar]
- 3. Shelton GD, Ho M, Kass PH. Risk factors for acquired myasthenia gravis in cats: 105 cases (1986–1998). J Am Vet Med Assoc 2000;216:55–57. [DOI] [PubMed] [Google Scholar]
- 4. Lindstrom JM, Seybold ME, Lennon VA, et al. Antibody to acetylcholine receptor in myasthenia gravis. Neurology 1998;51:933. [DOI] [PubMed] [Google Scholar]
- 5. Shelton GD. Routine and specialized laboratory testing for the diagnosis of neuromuscular diseases in dogs and cat. Vet Clin Pathol 2010;39:278–295. [DOI] [PubMed] [Google Scholar]
- 6. Dawson JRB. Myasthenia gravis in a cat. Vet Rec 1970;85:562–563. [DOI] [PubMed] [Google Scholar]
- 7. Scott‐Moncrieff JC, Cook JR Jr, Lantz GC. Acquired myasthenia gravis in a cat with thymoma. J Am Vet Med Assoc 1990;196:1291–1293. [PubMed] [Google Scholar]
- 8. Cuddon PA. Acquired immune‐mediated myasthenia gravis in a cat. J Small Anim Pract 1989;30:511–516. [Google Scholar]
- 9. O'Dair HA, Holt PE, Pearson GR, et al. Acquired immune‐mediated myasthenia gravis in a cat associated with a cystic thymus. J Small Anim Pract 1991;32:198–202. [Google Scholar]
- 10. Gores BR, Berg J, Carpenter JL, et al. Surgical treatment of thymoma in cats: 12 cases (1987–1992). J Am Vet Med Assoc 1994;204:1782–1785. [PubMed] [Google Scholar]
- 11. Zitz JC, Birchard SJ, Couto GC, et al. Results of excision of thymoma in cats and dogs: 20 cases (1984‐2005). J Am Vet Med Assoc 2008;232:1138–1192. [DOI] [PubMed] [Google Scholar]
- 12. Shilo Y, Pypendop BH, Barter LS, et al. Thymoma removal in a cat with acquired myasthenia gravis: A case report and literature review of anesthetic techniques. Vet Anaesth Analg 2011;38:603–613. [DOI] [PubMed] [Google Scholar]
- 13. Bell ET, Mansfield CS, James FE. Immune‐mediated myasthenia gravis in a methimazole‐treated cat. J Small Anim Pract 2012;53:661–663. [DOI] [PubMed] [Google Scholar]
- 14. Mason KV. A case of myasthenia gravis in a cat. J Small Anim Pract 1976;17:467–472. [DOI] [PubMed] [Google Scholar]
- 15. Joseph RJ, Carrillo JM, Lennon VA. Myasthenia gravis in the cat. J Vet Intern Med 1988;2:75–79. [DOI] [PubMed] [Google Scholar]
- 16. Ducote JM, Dewey CW, Coates JR. Clinical forms of acquired myasthenia gravis in cats. Compend Contin Educ Pract Vet 1999;21:440–488. [Google Scholar]
- 17. Mao ZF, Mo X, Qui C, et al. Incidence of thymoma in myasthenia gravis: A systematic review. J Clin Neurol 2012;8:161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Oh SJ, Morgan MG, Liang Lu, et al. Different characteristic phenotypes according to antibody in myasthenia gravis. J Clin Neuromuscul Dis 2012;14:57–65. [DOI] [PubMed] [Google Scholar]
- 19. Garlepp MJ, Kay PH, Farrow BR, et al. Autoimmunity in spontaneous myasthenia gravis in dogs. Clin Immunol Immunopathol 1984;31:301–306. [DOI] [PubMed] [Google Scholar]
- 20. Trepanier LA. Medical management of hyperthyroidism. Clin Tech Small Anim Pract 2006;21:22–28. [DOI] [PubMed] [Google Scholar]
- 21. Aronsohn MG, Schunk KL, Carpenter JL, et al. Clinical and pathological features of thymoma in 15 dogs. J Am Vet Med Assoc 1984;184:1355–1362. [PubMed] [Google Scholar]
- 22. Garneau MS, Price LL, Withrow SJ, et al. Perioperative mortality and long‐term survival in 80 dogs and 32 cats undergoing excision of thymic epithelial tumors. Vet Surg 2014;44:557–564. doi:10.1111/j.1532‐950X.2014.12304.x. [DOI] [PubMed] [Google Scholar]
- 23. Fuji Y. The thymus, thymoma, and myasthenia gravis. Surg Today 2013;43:461–466. [DOI] [PubMed] [Google Scholar]
- 24. Attaran S, Acharya M, Anderson JR, Punjabi PP. Does surgical debulking for advanced stages of thymoma improve survival? Interact Cardiovasc Thorac Surg 2012;15:494–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shelton GD. Myasthenia gravis and disorders of neuromuscular transmission. Vet Clin North Am Small Anim Pract 2002;32:189–206. [DOI] [PubMed] [Google Scholar]
- 26. Gilhus NE, Owe JF, Hoff JM, et al. Myasthenia gravis: A review of available treatment approaches. Autoimmune Dis 2011;2011:847393. Epub 2011 Oct 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Silvestri NG, Wolfe GI. Myasthensia gravis. Semin Neurol 2012;32:215–226. [DOI] [PubMed] [Google Scholar]
- 28. Atwater SW, Powers BE, Park RD, et al. Canine thymoma: 23 cases (1980–1991). J Am Vet Med Assoc 1994;205:1007–1013. [PubMed] [Google Scholar]
- 29. Uchida K, Awamura Y, Nakamura T. Thymoma and multiple thymic cysts in a dog with acquired myasthenia gravis. J Vet Med Sci 2002;64:637–640. [DOI] [PubMed] [Google Scholar]
- 30. Lainesse MF, Taylor SM, Myers SL, et al. Focal myasthenia gravis as a paraneoplastic syndrome of canine thymoma: Improvement following thymectomy. J Am Anim Hosp Assoc 1996;32:111–117. [DOI] [PubMed] [Google Scholar]
- 31. Klebanow ER. Thymoma and acquired myasthenia gravis in the dog: A case report and review of 13 additional cases. J Am Anim Hosp Assoc 1992;28:63–69. [Google Scholar]
- 32. Spillane J, Hayward M, Hirsch NP, et al. Thymectomy: Role in the treatment of myasthenia gravis. J Neurol 2013;260:1798–1801. [DOI] [PubMed] [Google Scholar]
- 33. Vachlas K, Zisis C, Rontogianni D, et al. Thymoma and myasthenia gravis: Clinical aspects and prognosis. Asian Cardiovasc Thorac Ann 2012;20:48–52. [DOI] [PubMed] [Google Scholar]
- 34. Kaser‐Holt B, Rohrer CR, Fidel JL, et al. Radiotherapy in three suspect cases of feline thymoma. J Am Anim Hosp Assoc 2001;37:483–488. [DOI] [PubMed] [Google Scholar]
- 35. Nakajima J, Murakawa T, et al. Postthymectomy myasthenia gravis: Relationship with thymoma and antiacetylcholine receptor antibody. Ann Thor Surg 2008;86:941–945. [DOI] [PubMed] [Google Scholar]
- 36. Shelton GD, Lindstrom JM. Spontaneous remission in canine myasthensia gravis: Implications for assessing human MG therapies. Neurology 2001;57:2139–2141. [DOI] [PubMed] [Google Scholar]
- 37. Mao ZF, Mo XA, Qin C, et al. Course and prognosis of myasthenia gravis: A systemic review. Eur J Neurol 2010;7:913–921. [DOI] [PubMed] [Google Scholar]
- 38. Sun X, Wang Y, Liu Y, et al. Myasthenia gravis appearing after thymectomy. J Clin Neurosci 2011;18:57–60. [DOI] [PubMed] [Google Scholar]
- 39. Deymeer F, Gungor‐Tuncer O, Yilmaz V. Clinical comparison of anti‐MuSK‐ vs anti‐AChR‐positive and seronegative myasthenia gravis. Neurology 2007;68:609–611. [DOI] [PubMed] [Google Scholar]
- 40. Nikolic A, Djukic P, Basta I, et al. The predictive value of the presence of different antibodies and thymus pathology to the clinical outcome in patients with generalized myasthenia gravis. Clin Neurol Neurosurg 2013;115:432–437. [DOI] [PubMed] [Google Scholar]
- 41. Smith AN, Wright JC, Brawner WR, et al. Radiation therapy in the treatment of canine and feline thymomas: A retrospective study (1985–1999). J Am Anim Hosp Assoc 2001;37:489–496. [DOI] [PubMed] [Google Scholar]


