Abstract
Background
As in women, regional lymph node status impacts survival in dogs with malignant mammary tumors. However, few studies have evaluated regional lymph node metastases in dogs with malignant mammary gland tumors.
Objectives
To estimate overall survival based on the assessments of the lymph node status and the morphologic and morphometric features in female dogs with malignant mammary gland tumors.
Materials and Methods
In total, 178 lymph nodes from 97 female dogs were assessed and reviewed, and after confirmation by immunohistochemistry (IHC), 161 lymph nodes were selected for analysis of metastases. Animals were considered metastasis‐free (negative lymph nodes) only after IHC analysis for cytokeratin AE1/AE3. The number of positive lymph nodes, the number of metastatic foci, the maximum diameter and the area of metastasis were analyzed, and estimates of overall survival were made.
Results
Dogs with metastasis had lower mean survival than those with metastasis‐free regional lymph nodes, showing a direct relationship between the number of affected lymph nodes and shorter survival. However, histologic analysis of the lymph nodes identified lower survival rates in animals with macrometastases and isolated tumor cells, areas of metastasis >20.11 mm², and metastatic diameters >7.32 mm.
Conclusion
The identification of ≥1 lymph nodes positive for metastasis and morphometric characterization of lymphatic metastases indicate the prognostic relevance of lymph nodes status in dogs with mammary tumors.
Keywords: Canine, Lymph node, Mammary tumors, Metastasis, Survival
Abbreviations
- H&E
hematoxylin‐eosin
- HER‐2
human epidermal growth factor receptor 2
- ICB
Institute of Biological Sciences
- IHC
immunohistochemistry
- ITC
isolated tumor cells
- SD
standard deviation
- SLN
sentinel lymph node
- TMA
total metastatic area
- UFMG
Federal University of Minas Gerais
In human medicine, axillary lymph node status is an important prognostic factor for women with breast cancer.1 Sentinel lymph node (SLN) biopsy is the current standard of care for staging the clinically negative axilla in breast cancer patients, with axillary lymph node dissection reserved for patients with clinical axillary metastases or metastases found on SLN biopsy.2, 3 An SLN is the first node draining the primary tumor in the regional lymphatic basin. The SLNs are identified by the uptake of a tracer (e.g., radioactive tracer, vital dye, or a combination of both) or by the identification of a labeled afferent lymphatic vessel.4 The presence of lymph node metastasis in the absence of recurrence is the single most important prognostic factor in breast cancer, and postoperative adjuvant therapy should be selected according to the number of metastatic lymph nodes, tumor size, histologic grade, and lymphatic vascular invasion, in addition to the patient's age, human epidermal growth factor receptor 2 (HER‐2) status, and hormone receptor status.5, 6
Regional metastases of breast cancer in women are classified as macrometastases when they feature clusters of neoplastic cells >2 mm, whereas those with clusters of neoplastic cells between 0.2 and 2 mm are classified as micrometastases, and those with clusters <0.2 mm are classified as isolated tumor cells (ITC).7 Higher rates of recurrence and death are observed in women with micrometastases compared with women with negative lymph nodes; these assessments, coupled with quantification of metastatic burden, are a clinically relevant predictor of non‐SLN metastasis.8, 9, 10, 11, 12, 13 This type of assessment, however, has been the subject of great debate, because there are no differences in prognosis between women with micrometastases and those with ITC.14, 15, 16, 17, 18, 19, 20, 21
As in women, regional lymph node status has major impact on the survival of dogs with mammary tumors.22, 23, 24, 25, 26, 27 In dogs, the use of tracers for identifying SLNs is rarely used in routine practice, but recent studies have evaluated these techniques.28, 29
Mammary tumors represent 52% of all cancers in female dogs, and 50% are malignant.30, 31 Currently, surgery is the first‐line treatment for mammary tumors in dogs, except for inflammatory carcinoma.32 The extent of surgery depends on patient stage, lymphatic drainage, and size and location of the lesions.33 The lymphatic drainage of neoplastic mammary glands is complex, and the lymph nodes anatomically linked to the mammary glands are found in 2 distinct regions (i.e., axillary and inguinal). The thoracic cranial and caudal glands drain toward the ipsilateral axillary lymph nodes. The abdominal cranial mammary gland often drains to both regions. The caudal and inguinal abdominal glands drain into the ipsilateral superficial inguinal lymph node.28 Furthermore, lymphatic connections between neoplastic and normal adjacent mammary glands are rare but can occur.28 Thus, additional studies related to SLNs in dogs should be developed for further clarification of the lymphatic drainage of neoplastic mammary glands.
In general, the inguinal lymph nodes must be resected en bloc, along the inguinal mammary gland, whenever this gland is removed or, as with axillary lymph nodes, when changes in their shape, volume, or consistency are observed.34
In dogs, the modified TNM classification states that all N1 cases (dogs with histologic or cytologic evidence of metastases) are classified as either stage IV (any T, N1, M0) or stage V (any T, any N, M1).34 This classification, however, does not take into account differences in the location of positive nodes (i.e., ipsilateral or contralateral), the size of the metastatic focus, the clinical relevance of isolated or small clusters of neoplastic cells or the methods used to detect occult micrometastases.35
The identification of locoregional lymph node macrometastases from malignant epithelial mammary tumors is essential for determining postsurgical prognosis.36 However, no differences in disease‐free survival and overall survival times between animals without metastasis and animals with lymph node micrometastases were reported in that study.36
Thus, this study aimed to analyze estimated overall survival based on assessing the classification of lymph node metastasis, the number of metastatic lymph nodes, and the sizes and numbers of metastatic foci present in the inguinal and axillary lymph nodes of female dogs with malignant mammary gland tumors.
Materials and Methods
All procedures were performed under the guidelines and with the approval of the Ethics Committee in Animal Experimentation at the Federal University of Minas Gerais (UFMG), protocol 174/2011.
Samples
One hundred and seventy‐eight regional lymph nodes (162 inguinal, 14 axillary, and 2 location not available) were assessed from 97 female dogs with malignant mammary tumors. The intact or spayed animals were subjected only to the surgical treatment of their mammary tumors, without adjuvant treatment, at the Hospital of the Veterinary School of the Federal University of Minas Gerais (UFMG) between 2008 and 2013. Before surgery, all animals had complete clinical examinations that included hematology and serum biochemistry. Feed, but not water, was withheld for 36 hours before surgery. Moreover, the dogs underwent thoracic radiography to rule out distant metastasis at the time of diagnosis and abdominal ultrasound examination only when serum biochemical changes or increased abdominal size were observed. The surgical technique (e.g., simple tumor removal, simple mastectomy, regional mastectomy, and radical mastectomy) was chosen according to the number of lesions and sites, considering the lymphatic drainage and established prognostic factors such as lesion size and existence of skin or muscular adherences, as previously described.34 The present study included 3 (3.1%) simple mastectomies, 46 (47.4%) regional mastectomies, and 43 (44.4%) radical mastectomies; the type of surgery was unknown for 5 dogs. All of the tumors were completely excised, and the muscle fascia was not removed in any cases. The inguinal lymph nodes were resected en bloc, along with the inguinal mammary gland, whenever this gland was removed, or in the same manner as the axillary lymph nodes when they were enlarged, adherent, or firm.
The surgical specimens (mammary tumors and lymph nodes) were obtained from archives of the Pathology Department of the Veterinary Clinic and Surgery at the Veterinary School of UFMG and of the Laboratory of Comparative Pathology at the Institute of Biological Sciences (ICB/UFMG) and were analyzed by 3 veterinary pathologists (MRA, EF, and GDC).
Histopathology and Immunohistochemistry
The lymph nodes were sectioned by a single longitudinal slice along the long axis. Larger lymph nodes were sectioned longitudinally and then transversally, with multiple sections obtained. All of the sections were included in different cassettes and were analyzed together, evaluating the long axis of the lymph node. The primary tumors also were sectioned for histopathologic analyses. The tumor samples and regional lymph nodes were fixed in 10% buffered formalin and routinely prepared and stained with hematoxylin‐eosin (H&E). Three veterinary pathologists (MRA, EF, and GDC) histologically examined a minimum of 3 sections (3 μm) of the mammary tumors. When tumors were >5 cm, a minimum of 5 sections were examined. All tumors were classified according to the veterinary histological classification.33, 37, 38 One section (3 μm) of each lymph node was cut and stained with H&E. Three veterinary pathologists (MRA, EF and GDC) reviewed all of the lymph nodes. In addition, the maximum width and length (the 2 greatest dimensions) of all metastatic foci in each positive lymph node were measured, and the larger of the 2 measurements was used as the maximum diameter of the metastatic focus.
To confirm the absence of metastasis, a consecutive section (3 μm) of the same paraffin block obtained for H&E analysis of each lymph node negative for metastasis was cut for IHC analysis. The slides were deparaffinized, rehydrated in graded alcohol, and subjected to heat‐induced antigen retrieval with antigen retrieval solution1 (water bath at 98°C; pH 6.0). The sections were stained with antibodies specific for cytokeratin (CK) AE1/AE31 (1:100, clone AE1AE3), vimentin1 (1:100, clone V9), or P632 (1:80, clone 4A4) and were incubated for 1 hour at ambient temperature. The cytokeratin stain was performed for all H&E lymph node slides that were scored as negative for metastasis. The vimentin and p63 stains were performed only for H&E lymph nodes slides negative for metastasis and with primary diagnoses of carcinosarcoma and malignant adenomyoepithelioma, respectively. The IHC was used only to confirm the absence of metastasis and cases positive for metastasis by IHC were excluded from pathologic analyses. After incubation, the antigen was immunodetected using the Dako Advance (HRP) Visualization Method1 with diaminobenzidine (DAB Substrate System)1 as the chromogen. Sections were counterstained with Mayer's hematoxylin, dehydrated, and mounted in a synthetic medium. Normal mammary glands were used as positive controls. Negative controls were assessed using Ultra V block normal serum3 as the primary antibody.
Neoplastic cells in the lymphoid parenchyma or distributed in the lymphatic sinuses (i.e., subcapsular, medullary, or both) in H&E‐stained sections were considered metastases as previously described.21 When >1 lymph node in the same patient was affected by several metastatic foci, the largest metastatic focus was measured (maximum diameter) for the classification of metastasis as macrometastases, micrometastases, or ITC. Lymph nodes were considered free of metastases only after confirmation by IHC staining.
Images of all metastatic foci were obtained on an Olympus BX41 microscope using a Spot Insight Color Capture System with different objectives depending on the size of the metastatic focus. Metastases were measured using the software Corel Draw 11.
The animals were organized into 5 groups according to a classification of metastasis adapted from human medicine: A (animals without metastasis); B (animals with macrometastases: lymph node metastases >2 mm; Fig 1A); C (animals with micrometastases: foci of neoplastic cells with diameters ranging from 0.2 to 2 mm; Fig 1B); D (animals with ITC: foci of neoplastic cells <0.2 mm; Fig 1C); and E (animals with nonmeasurable metastasis). Group E included animals with lymph nodes that had considerable metastatic involvement but in which it was not possible to measure metastasis (i.e., lymph nodes partially sectioned or lymph nodes with metastatic burden distributed diffusely without defined limits).
Figure 1.
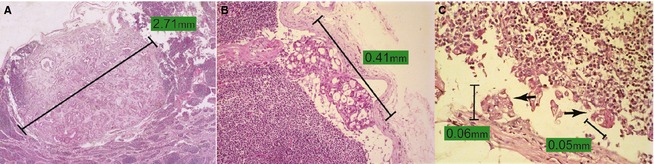
(A) Neoplastic proliferations characterized by numerous structures resembling acini contain significant fibrosis replacing part of the lymphoid parenchyma (macrometastases). 400x. Hematoxylin‐eosin (H&E). (B) Neoplastic proliferation characterized by structures resembling acini localized in the subcapsular sinus (micrometastases). 200x. H&E. (C) Multiple deposits of isolated epithelial neoplastic cells (arrows) in the subcapsular sinus. The size of the largest metastatic deposit, measuring 0.06 mm, was used to classify lymph node metastasis as isolated tumor cells (ITC). 400x. H&E.
In addition, all animals with metastasis were stratified according to the number of lymph nodes involved, the number of metastatic foci, the maximum diameter of metastasis, and the area of metastasis (maximum width × maximum length). Moreover, when >1 metastatic focus was identified in ≥1 lymph nodes in the same patient, individual metastatic areas were summed to calculate the total metastatic area (TMA).8 For all of these analyses, a cut‐off (mean plus 1 standard deviation [SD]) was established for stratification into groups. The number of lymph nodes involved, the maximum diameter of the lymph node metastases and the number of metastatic foci from each animal were used to obtain the averages for each group. All of the metastatic foci were measured to obtain an average metastatic area in animals that had >1 metastatic focus. The TMA from each animal was used to obtain the mean for the group.
Survival Time
The animals with malignant mammary tumors were assessed, and the endpoints were defined according to the type of analyses: histologic tumor types (827 days of follow‐up; 84 animals), presence or absence of lymph node metastasis (1112 days of follow‐up; 84 animals), number of lymph nodes involved (778 days of follow‐up; 84 animals), classification of metastasis (778 days of follow‐up; 84 animals), area of metastasis (285 days of follow‐up; 33 animals), number of metastatic foci (537 days of follow‐up; 33 animals), maximum diameter of metastasis (285 days of follow‐up; 33 animals), and TMA (778 days of follow‐up; 24 animals).
Survival time was defined as the period (days) between surgical tumor removal and date of death from disease. The cause of death was confirmed at the postmortem examination. Animals that were alive and that died of unknown causes or causes unrelated to the tumor were censored. Among the most common causes was visceral (Calazar) leishmaniasis (Leishmania chagasi), which is endemic in the city of Belo Horizonte, Minas Gerais, Brazil, where the research was conducted. Other causes of death were external causes (road accidents), orthopedic surgery, and cardiorespiratory and neurological conditions.
Statistical Analysis
Survival curves were calculated using the Kaplan–Meier estimator, and statistical significance was examined using a log‐rank test, after a normality test, with P < .05 considered significant. Survival data were evaluated according to the histologic tumor types, presence or absence of lymph node metastasis, number of lymph nodes involved, classification of lymph node metastasis, area of metastasis, number of metastatic foci, maximum diameter of metastasis, and TMA.
Results
The ages of the 97 animals at the time of surgery ranged from 4 to 16 years (mean, 10.7 ± 2.7 years). The dogs were predominantly purebred (68/93; 72.34%), and poodles (n = 31) were the most common breed. Mongrel dogs were observed less frequently (25/93; 26.60%), and the breed was not recorded for 4 dogs. The dogs were intact (52/76; 68.42%) and spayed (24/76; 31.58%); reproductive status was not available for 21 dogs. The inguinal and caudal abdominal glands were the predominant sites of the lesions (31/93; 33.33% each) in relation to the multi‐centric localization (15/93; 16.13%), cranial abdominal glands (12/93; 12.90%), and thoracic glands (4/93; 4.31%). In 4 cases, the site of the lesions was not confirmed. None of the animals had pulmonary changes on radiographic examination, changes in abdominal ultrasound findings, or both at the time of surgery.
Initially, 178 lymph nodes (162 inguinal, 14 axillary and 2 location not confirmed) of 97 dogs were accessed. However, after IHC analysis, 161 lymph nodes of 90 dogs were considered for study. Among 161 lymph nodes, 49.7% (80) were diagnosed as metastatic. One axillary lymph node and multiple ipsilateral axillar lymph nodes were removed from 5.7% (5/88) and 3.4% (3/88) of dogs, respectively. One ipsilateral inguinal lymph node and multiple ipsilateral inguinal lymph nodes were identified in 39.8% (35/88) and 47.7% (42/88) of dogs, respectively. Both axillary and inguinal lymph nodes were excised from 3.4% (3/88) of dogs. In 2 dogs, the lymph node site was not recorded. Dogs with lymph node metastasis were predominantly observed (57/90; 63.3%) relative to dogs without lymph node metastasis (33/90; 36.7%, Table 1). The mean number of evaluated nodes per animal was 1.78 (SD, 1.01), with a maximum of 6 and a minimum of 1 lymph node. The mean number of metastatic lymph nodes per animal was 1.4 (SD, 0.75), with a maximum of 5 and a minimum of 1 metastatic lymph node. The inguinal lymphatic region was the predominant site of metastasis (45/55; 81.8%) relative to the axillary lymphatic region (8/55; 14.5%) and simultaneous occurrence (inguinal and axillary; 2/55; 3.6%). The site of metastasis was not confirmed in 2 dogs.
Table 1.
Distribution of histologic tumor types and lymph node features according to the adapted metastasis classification used in humans
| Absence of Metastasis Group A (n [%]) (n = 33) | Macrometastases Group B (n [%]) (n = 13) | Micrometastases Group C (n [%]) (n = 15) | Isolated Tumor Cells Group D (n [%]) (n = 9) | No Measurable Metastasis Group E (n [%]) (n = 20) | |
|---|---|---|---|---|---|
| Histologic types (n = 90) | |||||
| MTC | 24 [72.7] | 4 [30.8] | 3 [20.0] | 3 [33.3] | 1 [5.0] |
| SC | 3 [9.1] | 1 [7.7] | 2 [13.3] | 1 [11.1] | 9 [45.0] |
| RTMT* | 2 [6.1] | 6 [46.2] | 2 [13.3] | 0 [0] | 8 [40.0] |
| PTC** | 2 [6.1] | 0 [0] | 7 [46.7] | 3 [33.3] | 1 [5.0] |
| CSS | 2 [6.1] | 2 [15.4] | 1 [6.7] | 2 [22.2] | 1 [5.0] |
| Lymph node metastasis area (n = 33) | |||||
| <20.11 mm² | 0 [0] | 6 [54.5] | 14 [100] | 8 [100] | 0 [0] |
| >20.11 mm² | 0 [0] | 5 [45.5] | 0 [0] | 0 [0] | 0 [0] |
| Lymph node metastatic foci (n = 33) | |||||
| <28 foci | 0 [0] | 11 [100] | 13 [92.9] | 6 [75] | 0 [0] |
| >28 foci | 0 [0] | 0 [0] | 1 [7.1] | 2 [25] | 0 [0] |
| Lymph node maximum diameter (n = 33) | |||||
| <7.32 mm | 0 [0] | 5 [45.5] | 14 [100] | 8 [100] | 0 [0] |
| >7.32 mm | 0 [0] | 6 [54.5] | 0 [0] | 0 [0] | 0 [0] |
| Lymph node total metastatic area (n = 24) | |||||
| <88.92 mm² | 0 [0] | 3 [50] | 13 [100] | 5 [100] | 0 [0] |
| <88.92 mm² | 0 [0] | 3 [50] | 0 [0] | 0 [0] | 0 [0] |
MTC, carcinoma in mixed tumor; SC, solid carcinoma; RTMT*, rare type malignant mammary tumors (including micropapillary carcinoma, n = 14; lipid‐rich carcinoma, n = 1; pleomorphic lobular carcinoma, n = 1; malignant adenomyoepithelioma, n = 1; and malignant phyllodes tumor, n = 1); PTC**, (including papillary carcinoma, n = 10; and tubular carcinoma, n = 3); CSS, carcinosarcoma.
Carcinomas in mixed tumors were the most common cancer, and the rarest tumors were lipid‐rich carcinoma, malignant adenomyoepithelioma, pleomorphic lobular carcinoma, malignant phyllodes tumor, and invasive micropapillary carcinoma, all of which were included in the group of rare types of malignant mammary tumors (Table 1). The animals with carcinoma in mixed tumors, rare types of malignant tumors, solid carcinoma, papillary and tubular carcinoma and carcinosarcoma had mean respective survival times of 485.5 ± 259.4 days (not reaching median survival), 227.1 ± 235.2 days (median, 180 days), 228.2 ± 253.4 days (median, 195 days), 401.7 ± 196.8 days (not reaching median survival), and 163.3 ± 115.7 days (median, 185 days). Animals with solid carcinoma, carcinosarcoma, and rare types of malignant mammary tumors had lower survival rates than animals with carcinoma in mixed tumors and papillary and tubular carcinoma (P < .0001; Fig 2A).
Figure 2.
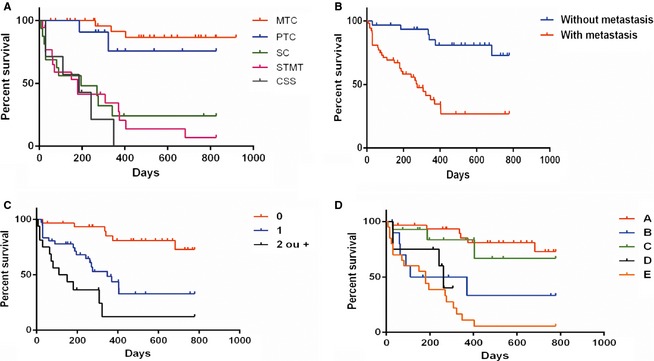
(A) Kaplan–Meier survival curve for the animals according to the histologic types: MTC (carcinoma in mixed tumor; n = 32); PTC (papillary and tubular carcinoma; n = 11); SC (solid carcinoma; n = 16; median, 195 days); RTMT (rare type malignant mammary tumors; n = 18; median, 180 days); and CSS (carcinosarcoma; n = 7; median, 185 days). Animals with MTC and PTC not did reach median survival (P < .0001). (B) Kaplan–Meier survival curve for the animals with metastasis (n = 53, median, 275 days) and without metastasis (n = 31, not reaching median survival) (P < .0001).(C) Kaplan–Meier survival curve for the animals according to the number of lymph nodes involved: 0 (no metastatic lymph nodes; n = 31, not reaching median survival), 1 (1 metastatic lymph node; n = 37; median, 348 days), and ≥2 (≥2 metastatic lymph nodes; n = 16; median, 130 days) (P < .0001). (D) Kaplan–Meier survival curve for the animals according to the classification of metastasis: A (absence of metastasis; n = 31), B (macrometastases; n = 11; median, 240 days), C (micrometastases; n = 14), D (isolated tumor cells; n = 8; median, 262 days), and E (no measurable metastasis; n = 20; median, 180 days) (P < .01). The animals in groups A and C did not reach median survival.
Regarding the presence or absence of lymph node metastasis, the animals with metastasis had lower survival times (mean, 253.9 ± 244.2 days and median, 275 days) compared with animals without metastasis (mean 530.6 ± 286.8 days and not reaching median survival; P < .0001; Fig 2B).
When the number of lymph nodes involved was evaluated, the presence of ≥2 metastatic ipsilateral lymph nodes (equal or different lymphatic basins) was associated with lower survival (P < .0001; Fig 2C). The mean survival times of animals with lymph nodes without metastasis, with 1 metastatic lymph node, and with ≥2 metastatic lymph nodes were 497.6 ± 238.4 days (not reaching median survival), 275.3 ± 223.2 days (median, 348 days), and 174.2 ± 192.8 days (median, 130 days), respectively.
Animals with isolated tumor cells (group D; mean, 200.1 ± 108.0 days and not reaching median survival), nonmeasurable metastases (group E; mean, 191.8 ± 186.9 days and median, 180 days), and macrometastases (group B; mean, 248.8 ± 277.8 days and median, 240 days) had lower survival times than those without metastasis (group A; mean, 497.6 ± 238.37 days and not reaching median survival) and those with micrometastases (group C; mean, 342.9 ± 238.9 days and not reaching median survival). Lower survival with statistical significance was observed in animals from groups B×A (P = .002), D×A (P = .001) and E×A (P < .0001) and in animals from group E relative to group C (P < .001; Fig 2D).
Stratification of animals with measurable metastasis (i.e., macrometastases, micrometastases, and ITC) distributed in groups according to the area of metastasis, number of metastatic foci, maximum diameter, and TMA is presented in Table 1.
The mean area of metastasis was 2.95 mm² (range, 0.0001–169.88 mm²; SD, 17.16 mm²; n = 33). When stratified by area, animals with areas <20.11 mm² (mean, 207.1 ± 93.4 days and not reaching median survival) had a greater survival than those with areas >20.11 mm² (mean, 103.0 ± 107.1 days and median, 86.5 days; P = .0457; Fig 3A).
Figure 3.
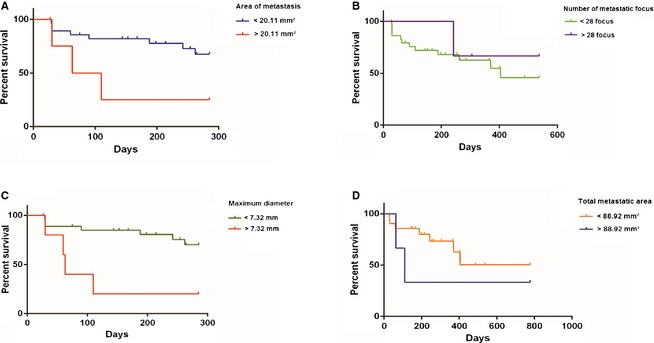
(A) Kaplan–Meier survival curve for the animals according to the area of metastasis: <20.11 mm² (n = 28; not reaching median survival) and >20.11 mm² (n = 5; median, 86.5 days) (P = .0457). (B) Kaplan–Meier survival curve for the animals according to the number of metastatic foci: <28 foci (n = 30; median, 404 days) and >28 foci (n = 3, not reaching median survival) (P = .629). (C) Kaplan–Meier survival curve for the animals according to the maximum diameter of metastasis: <7.32 mm (n = 27; not reaching median survival) and >7.32 mm (n = 6; median, 63 days) (P = .0068). (D) Kaplan–Meier survival curve for the animals according to the total metastatic area (TMA): <88.92 mm² (n = 21; not reaching median survival) and >88.92 mm² (n = 3; median, 110 days) (P = .3329).
The mean number of metastatic foci was 10 (range, 1–97 foci; SD, 18 foci; n = 33). There were no statistically significant differences in the survival of animals with <28 foci (mean, 237.1 ± 174.5 days and median, 404 days) and >28 foci (mean, 361.3 ± 155.3 days and not reaching median survival; Fig 3B).
The mean maximum diameter of the metastases was 2.86 mm (range, 0.06–16.92 mm; SD, 4.46 mm; n = 33). When stratified by maximum diameter, the survival of animals with diameters >7.32 mm (mean, 95.8 ± 97.4 days and not reaching median survival) was lower than animals with diameters <7.32 mm (mean, 212.6 ± 90.6 days and median, 63 days; P = .0068; Fig 3C).
Finally, the mean TMA obtained for animals with >1 metastatic focus was 26.04 mm² (range, 0.0196–239.41 mm²; SD, 62.88 mm²; n = 24). When stratified by TMA, no significant difference was found in survival of the animals with TMAs <88.92 mm² (mean, 303.3 ± 210.1 days and not reaching median survival) and with TMAs >88.92 mm² (mean, 317.0 ± 399.9 days and median, 110 days; Fig 3D).
Discussion
As in women, regional lymph node status has major impact on survival in dogs with mammary tumors.22, 23, 24, 25, 26, 27 In the present study, animals with metastasis had lower survival times than did animals without metastasis. These findings are similar to those reported in the literature.22, 23, 24, 25, 26
To our knowledge, this study is the first to describe the prognostic significance of identifying a higher number of lymph nodes positive for metastasis in dogs, as has been reported in humans.1, 39 Another novel aspect is the use of a more accurate technique for measuring metastases, which yields a more detailed representation of the metastatic load of the lymph nodes in these animals. In this study, dogs with lymph node metastases with diameters >7.32 mm had lower overall survival. Another interesting finding was that the presence of ITC in the lymph nodes of dogs with malignant mammary tumors was associated with lower overall survival in comparison to dogs with no metastatic malignant mammary tumors.
Histological type is one of the most important prognostic factors in dogs with mammary tumors and, our results are similar to those described in the literature.23, 24 We found that solid carcinomas are extremely aggressive histologically and have a worse prognosis compared with histologically well‐differentiated tumors, such as carcinomas in mixed tumors, papillary carcinoma, and tubular carcinoma. Tumors subclassified as micropapillary carcinomas were particularly noteworthy in this study. These tumors were associated with lower overall survival compared with other tumors, as described in previous studies.40, 41, 42, 43
In this study, metastatic involvement of >1 lymph node showed a direct correlation with worse prognosis. This parameter may become an important prognostic factor in veterinary clinical practice and has not previously been described in dogs. In women, a higher number of affected axillary lymph nodes is associated with shorter survival times, which is a well‐recognized prognostic factor in breast cancer.1, 39
The presence of lymph node macrometastases is also a clinically relevant prognostic factor in dogs with mammary tumors.36 However, a novel finding of similar prognostic relevance was the identification of isolated cells in these animals. In this study, 33.33% of the animals diagnosed with ITC had more aggressive tumors, such as carcinosarcoma and solid carcinoma. Such behavior may be similar to that observed in women with breast cancer, in whom the clinical relevance of identifying cell clusters <0.2 mm has shown conflicting results and may be directly related to the histologic type studied.13, 16, 18, 21
The differences in animal survival demonstrate that metastatic area measurements can be used to characterize regional lymph node metastases in female dogs with malignant mammary tumors. However, this characterization may not be practical in routine laboratory pathology because it would be necessary to measure all metastatic foci to obtain the mean metastatic area.
The maximum diameter of metastases currently is used to classify metastases as macrometastases, micrometastases, or ITC in humans.7 In the present study, the classification of metastasis used in humans was made, along with stratification to the maximum diameter (cut‐off: mean plus SD = 7.32 mm). Lower survival was observed in animals with a maximum diameter of lymph node metastasis >7.32 mm relative to animals with a maximum diameter of lymph node metastasis <7.32 mm. Thus, the behavior of macrometastases and staging in dogs can vary, and the current classification system based on humans (macrometastases: metastasis >2 mm) may not be the most appropriate. Other authors have discussed the use of classification systems other than the classification systems used in humans that have been adapted to veterinary medicine.35 A subclassification system for macrometastases should be considered because the lymph node metastasis can reach considerable size in female dogs, similar to findings in the present study.
The TMA measurements in SLNs can predict non‐SLN metastasis in women with breast cancer.8 The present study found no differences in animal survival between a TMA (cut‐off: mean plus SD) >88.92 mm² and a TMA <88.92 mm². Thus, to obtain prognostic information, we consider that the maximum diameter measurement could be one of the most suitable methods when analyzing single sections of lymph nodes in female dogs with malignant mammary tumors.
In the clinical studies of humans, substantial advances are described in relation to the standardization of gross sectioning and the number of histologic sections to be analyzed in SLNs of women with breast cancer.44 In veterinary medicine, evaluating lymph node metastases, determining the prognostic value of metastatic size, and even establishing the number of histologic sections to be analyzed should be investigated in additional studies, especially when using SLN mapping in mammary tumors of dogs. The findings in this study may help in future proposals for the standardization of these methods of analysis, showing the real prognostic relevance of the occurrence of lymph node metastasis in malignant mammary tumors of dogs.
Corroborating previous findings,22, 23, 24, 25, 26 our study showed that the presence of lymph node metastasis is a negative prognostic factor. However, the analysis of these data with tumor size, histologic type, mitotic index, histologic grading, status of hormonal receptors, and cell proliferation markers may provide more consistent information in a multivariate analysis of the possible independent prognostic factors in dogs with malignant mammary tumors and may provide actual practical applications for the data presented in this study for clinical practice. However, the independent prognostic relevance of lymph node status in dogs with mammary tumors is still poorly established in multivariate analysis.23, 45, 46
Conclusions
The identification of ≥1 metastatic lymph nodes is considered a parameter for prognostic assessment in affected animals. Moreover, macrometastases and ITC in regional lymph nodes are associated with a worse prognosis in dogs with malignant mammary tumors subjected only to surgical treatment. A more detailed classification system and staging that takes into account differences in the sizes of macrometastases identified in female dogs should be considered, and additional studies should be conducted to confirm these findings.
Acknowledgments
Grant support: This work was financially supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Fundação de Amparo a Pesquisa de Minas Gerais (FAPEMIG), Brazil.
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Footnotes
Dako, Carpinteria, CA.
NeoMarkers Inc., Fremont, CA.
Thermo Fisher Scientific, LabVision Corporation, Fremont, CA.
References
- 1. Carter CL, Allen C, Henson DE. Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer 1989;63:181–187. [DOI] [PubMed] [Google Scholar]
- 2. Veronesi U, Paganelli G, Viale G, et al. A randomized comparison of sentinel‐node biopsy with routine axillary dissection in breast cancer. N Engl J Med 2003;349:546–553. [DOI] [PubMed] [Google Scholar]
- 3. Lyman GH, Temin S, Edge SB, et al. Sentinel lymph node biopsy for patients with early‐stage breast cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2014;32:1365–1383. [DOI] [PubMed] [Google Scholar]
- 4. Kuehn T, Bembenek A, Decker T, et al. A concept for the clinical implementation of sentinel lymph node biopsy in patients with breast carcinoma with special regard to quality assurance. Cancer 2005;103:451–461. [DOI] [PubMed] [Google Scholar]
- 5. Fitzgibbons PL, Page DL, Weaver D, et al. Prognostic factors in breast cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med 2000;124:966–978. [DOI] [PubMed] [Google Scholar]
- 6. Goldhirsch A, Ingle JN, Gelber RD, et al. Thresholds for therapies: Highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer. Ann Oncol 2009;20:1319–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Greene FL, Page DL, Fleming ID, et al. Breast In: Greene FL, Page DL, Fleming ID, Fritz A, Balch CM, Haller DG, Morrow M, eds. AJCC Cancer Staging Manual, 6th ed New York: Springer; 2002:223–240. [Google Scholar]
- 8. Chae AW, Vandewalker KM, Li YJ, et al. Quantitation of sentinel node metastatic burden and Her‐2/neu over‐expression accurately predicts residual axillary nodal involvement and extranodal disease in breast cancer. Eur J Surg Oncol 2013;39:627–633. [DOI] [PubMed] [Google Scholar]
- 9. Fisher ER, Palekar A, Rockette H, et al. Pathologic findings from the National Surgical Adjuvant Breast Project (Protocol No. 4). V. Significance of axillary nodal micro and macrometastases. Cancer 1978;42:2032–2038. [DOI] [PubMed] [Google Scholar]
- 10. Fisher B, Gebhardt MC. The evolution of breast cancer surgery: Past, present, and future. Semin Oncol 1978;5:385–394. [PubMed] [Google Scholar]
- 11. Cote R, Peterson H, Chaiwun B, et al. Role of immunohistochemical detection of lymph‐node metastases in management of breast cancer. Lancet 1999;354:896–900. [DOI] [PubMed] [Google Scholar]
- 12. Viale G, Maiorano E, Pruneri G, et al. Predicting the risk for additional axillary metastases in patients with breast carcinoma and positive sentinel lymph node biopsy. Ann Surg 2005;241:319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cserni G, Bianchi S, Vezzosi V, et al. Variations in sentinel node isolated tumour cells/micrometastases and non‐sentinel node involvement rates according to different interpretations of the TNM definitions. Eur J Cancer 2008;44:2185–2191. [DOI] [PubMed] [Google Scholar]
- 14. Nagashima T, Sakakibara M, Nakano S, et al. Sentinel node micrometastases and distant failure in breast cancer patients. Breast Cancer 2006;13:186–191. [DOI] [PubMed] [Google Scholar]
- 15. Langer I, Marti WR, Guller U, et al. Axillary recurrence rate in breast cancer patients with negative sentinel lymph node (SLN) or SLN micrometastases. Ann Surg 2005;241:152–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Imoto S, Ochiai A, Okumura C, et al. Impact of isolated tumor cells in sentinel lymph nodes detected by immunohistochemical staining. Eur J Surg Oncol 2006;32:1175–1179. [DOI] [PubMed] [Google Scholar]
- 17. Cserni G, Amendoeira I, Apostolikas N, et al. Pathological work‐up of sentinel lymph nodes in breast cancer. Review of current data to be considered for the formulation of guidelines. Eur J Cancer 2003;39:1654–1667. [DOI] [PubMed] [Google Scholar]
- 18. De Boer M, Van Deurzen CH, Van Dijck JA, et al. Micrometastases or isolated tumor cells and the outcome of breast cancer. N Engl J Med 2009;361:653–663. [DOI] [PubMed] [Google Scholar]
- 19. Patani N, Mokbel K. The clinical significance of sentinel lymph node micrometastases in breast cancer. Breast Cancer Res Treat 2009;114:393–402. [DOI] [PubMed] [Google Scholar]
- 20. Patani N, Mokbel K. Clinical significance of sentinel lymph node isolated tumour cells in breast cancer. Breast Cancer Res Treat 2011;127:325–334. [DOI] [PubMed] [Google Scholar]
- 21. Ahmed SS, Thike AA, Iqbal J, et al. Sentinel lymph nodes with isolated tumour cells and micrometastases in breast cancer: Clinical relevance and prognostic significance. J Clin Pathol 2014;67:243–250. [DOI] [PubMed] [Google Scholar]
- 22. Kurzman ID, Gilbertson SR. Prognostic factors in canine mammary tumors. Semin Vet Med Surg (Small Animal) 1986;1:25–32. [PubMed] [Google Scholar]
- 23. Hellmén E, Bergström R, Holmberg L, et al. Prognostic factors in canine mammary tumors: A multivariate study of 202 consecutive cases. Vet Pathol 1993;30:20–27. [DOI] [PubMed] [Google Scholar]
- 24. Yamagami T, Kobayashi T, Takahashi K, Sugiyama M. Prognosis for canine malignant mammary tumors based on TNM and histologic classification. J Vet Med Sci 1996;58:1079–1083. [DOI] [PubMed] [Google Scholar]
- 25. Pérez Alenza MD, Peña L, Nieto AI, Castaño M. Clinical and pathological prognostic factors in canine mammary tumors. Ann Ist Super Sanita 1997;33:581–585. [PubMed] [Google Scholar]
- 26. Nieto A, Peña L, Pérez‐Alenza MD, et al. Immunohistologic detection of estrogen receptor alpha in canine mammary tumors: Clinical and pathologic associations and prognostic significance. Vet Pathol 2000;37:239–247. [DOI] [PubMed] [Google Scholar]
- 27. Chang SC, Chang CC, Chang TJ, Wong ML. Prognostic factors associated with survival two years after surgery in dogs with malignant mammary tumors: 79 cases (1998–2002). J Am Vet Med Assoc 2005;227:1625–1629. [DOI] [PubMed] [Google Scholar]
- 28. Patsikas MN, Karayannopoulou M, Kaldrymidoy E, et al. The lymph drainage of the neoplastic mammary glands in the bitch: A lymphographic study. Anat Histol Embryol 2006;35:228–234. [DOI] [PubMed] [Google Scholar]
- 29. Beserra HEO, Grandi F, Ibañez JF, et al. Sentinel lymph node identification: The importance of new methodologies and preclinical studies in dogs. Braz J Vet Pathol 2013;6:5. [Google Scholar]
- 30. Daleck CR, Franceschini PH, Alessi AC, et al. Canine mammary neoplasia: Clinical and surgical evolution. Cienc Rural 1998;28:95–100. [Google Scholar]
- 31. Morrison WB. Canine and feline mammary tumors In: Morrison WB, ed. Cancer in Dogs and Cats: Medical and Surgical Management, 1st ed Philadelphia, PA: Linppincott Williams & Wilkins; 1998:591–598. [Google Scholar]
- 32. Novosad CA. Principles of treatment for mammary gland tumors. Clin Tech Small Anim Pract 2003;18:107–109. [DOI] [PubMed] [Google Scholar]
- 33. Cassali GD, Lavalle GE, Ferreira E, et al. Consensus for the diagnosis, prognosis and treatment of canine mammary tumors – 2013. Braz J Vet Pathol 2014;7:38–69. [Google Scholar]
- 34. Sorenmo KU, Worley DR, Goldschmidt MH. Tumors of the mammary gland In: Withrow SJ, Vail DM, Page RL, eds. Withrow & MacEwen's Small Animal Clinical Oncology, 5th ed Philadelphia, PA: Saunders Elsevier; 2013:538–556. [Google Scholar]
- 35. Matos AJ, Faustino AM, Lopes C, et al. Detection of lymph node micrometastases in malignant mammary tumours in dogs by cytokeratin immunostaining. Vet Rec 2006;158:626–630. [DOI] [PubMed] [Google Scholar]
- 36. Szczubial M, Lopuszynski W. Prognostic value of regional lymph node status in canine mammary carcinomas. Vet Comp Oncol 2011;9:296–303. [DOI] [PubMed] [Google Scholar]
- 37. Misdorp W, Else RW, Hellmen E. Histological Classification of Mammary Tumors of the Dog and the Cat. Geneva, Switzerland: World Health Organization; 1999. [Google Scholar]
- 38. Araújo MR, Damasceno KA, Gamba CO, et al. Morphological and immuno‐histochemical characterization of five phyllodes mammary gland tumors in dogs. Onl J Vet Res 2014;18:688–695. [Google Scholar]
- 39. Silverstein MJ, Gierson ED, Waisman JR, et al. Axillary lymph node dissection for T1a breast carcinoma. Is it indicated?. Cancer 1994;73:664–667. [DOI] [PubMed] [Google Scholar]
- 40. Cassali GD, Gärtner F, Vieira da Silva MJ, Schmitt FC. Cytological diagnosis of a metastatic canine mammary tumor in pleural effusion. Arq Bras Med Vet Zootec 1999;51:307–310. [Google Scholar]
- 41. Cassali GD, Serakides R, Gartner F, Schmitt FC. Invasive micropapillary carcinoma of the dog mammary gland. A case report. Arq Bras Med Vet Zootec 2002;54:366–369. [Google Scholar]
- 42. Gama A, Alves A, Schmitt FC. Clinicopathologic features of mammary invasive micropapillary carcinoma (IMC) in dogs. Vet Pathol 2008;45:600–601. [DOI] [PubMed] [Google Scholar]
- 43. Gamba CO, Dias EJ, Ribeiro LG, et al. Histopathological and immunohistochemical assessment of invasive micropapillary mammary carcinoma in dogs: A retrospective study. Vet J 2013;196:241–246. [DOI] [PubMed] [Google Scholar]
- 44. Weaver DL. Pathology evaluation of sentinel lymph nodes in breast cancer: Protocol recommendations and rationale. Mod Pathol 2010;23(Suppl 2):S26–S32. [DOI] [PubMed] [Google Scholar]
- 45. De Las Mulas JM, Millán Y, Dios R. A prospective analysis of immunohistochemically determined estrogen receptor alpha and progesterone receptor expression and host and tumor factors as predictors of disease‐free period in mammary tumors of the dog. Vet Pathol 2005;42:200–212. [DOI] [PubMed] [Google Scholar]
- 46. Santos AA, Lopes CC, Ribeiro JR, et al. Identification of prognostic factors in canine mammary malignant tumours: A multivariable survival study. BMC Vet Res 2013;9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]


