Abbreviations
- EGGD
equine glandular gastric disease
- EGUS
equine gastric ulcer syndrome
- ESGD
equine squamous gastric disease
- GERD
gastroesophageal reflux disease
- PUD
peptic ulcer disease
Terminology
The term Equine Gastric Ulcer Syndrome (EGUS) was first used in 1999 to describe gastric ulceration in the horse.1 However, as discussed by Merritt,2 the terminology is commonly misused. The committee reinforces the importance of distinguishing between diseases of the squamous and glandular mucosa because, as discussed in this statement, important differences exist between the two. In human medicine, the term peptic ulcer disease (PUD) is used as an umbrella term to describe erosive and ulcerative diseases of the stomach and it is recognized that a large number of individual diseases are present under the term.3 Furthermore, while some different diseases might share similarities in pathophysiology and treatment regimens, it is recognized in human medicine that the direct extrapolation of either from one specific disease (such as NSAID‐associated ulceration) to another (such as Helicobacter pylori associated ulceration) is inappropriate.3
The committee recognizes that the terminology for EGUS requires clarification and proposes that the nomenclature be: Equine Gastric Ulcer Syndrome (EGUS) as a general all encompassing term to describe erosive and ulcerative diseases of the stomach consistent with the use of the term PUD in man; Equine Squamous Gastric Disease (ESGD) and Equine Glandular Gastric Disease (EGGD) as terms that more specifically describe the affected region anatomically. Within ESGD, both primary and secondary disease is recognized. Primary ESGD, the more common of the 2 forms, occurs in animals with an otherwise normal gastrointestinal tract. In contrast, secondary ESGD occurs in animals with delayed gastric outflow secondary to an underlying abnormality such as pyloric stenosis.4 The pathophysiology of EGGD remains to be elucidated and as such further subclassification of lesion type is not possible at this time. Instead, the committee recommends the use of descriptive terminology with a clear distinction of the anatomical region affected (cardia, fundus, antrum, or pylorus as shown in Figure 2) and the gross appearance of the lesion. The committee emphasizes that the affected region of the stomach should be clearly identified when communicating research and clinical findings. A summary of the proposed terminology is depicted in Figure 1.
Figure 2.
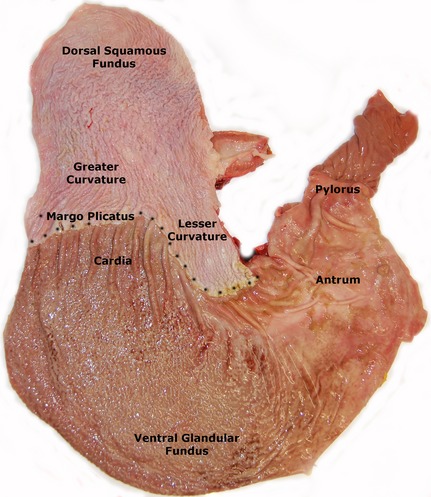
A postmortem specimen of the equine stomach depicting the anatomical regions of the stomach.
Figure 1.
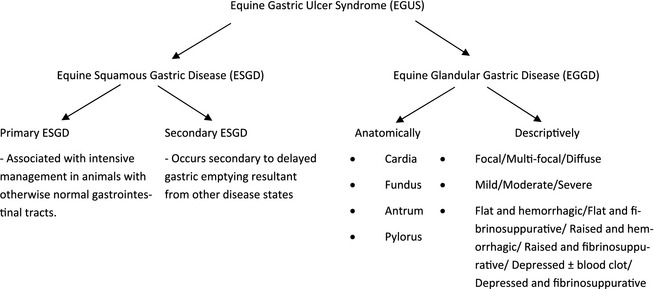
A summary of the proposed terminology for describing erosive and ulcerative diseases of the horse's stomach.
Recommendation: Expansion of the existing EGUS terminology to specifically identify squamous and glandular disease as ESGD and EGGD, respectively, as shown in Figure 1.
Prevalence
The prevalence of gastric ulceration varies with breed, use, level of training, as well as between ESGD and EGGD. The highest prevalence of ESGD occurs in Thoroughbred racehorses with 37% of untrained horses affected, increasing to 80–100% within 2–3 months of race training.5, 6, 7 Standardbred racehorses have a similar overall ESGD prevalence of 44% that rises up to 87% in training,8, 9, 10 while 17–58% show/sport horses and 37–59% of pleasure horses are affected.11, 12, 13, 14, 15 Endurance horses have an ESGD prevalence of 48% during the out of competition period that rises to 66–93% during the competitive period, with lesions most prevalent in elite horses.16, 17 Horses that are rarely competed and predominantly used in their home environment have the lowest ESGD prevalence of 11%.18
The prevalence of EGGD is less well understood. Australian Thoroughbred racehorses have reported prevalences of between 47%6 and 65%.19 In endurance horses, the prevalence is 16% outside of the competition period and 27–33% while competing.16, 17 A retrospective study in the United Kingdom found EGGD in 54% of 191 leisure horses and in 64% of 493 sport horses.20 Comparably, 57% of horses used for a variety of purposes were reported to have EGGD in 2 separate studies.14, 21 The majority of EGGD lesions in all of the above studies were found within the pyloric antrum.
Epidemiology
A postmortem study of 3,715 horses over 72 years found significant associations between the presence of ulceration and breed (Thoroughbred and Standardbred breeds were more likely to have ulcers than cold‐blooded horses) and sex (a higher prevalence was reported in stallions than mares and geldings).22 In contrast, 2 large cross‐sectional studies of Thoroughbred racehorses documented no significant effect of age or sex on the likelihood of having ESGD.7, 23 Similarly, a large study in Standardbreds found no association between the presence of ESGD and age; however, there was an association between increasing age and worsening ulcer severity, the relative risk for which was greatest in geldings.8 In a retrospective study of 684 sport and leisure horses in the United Kingdom, no effect of age, sex or month of presentation was found on prevalence of ESGD or EGGD. However, a significant association was found between the Thoroughbred breed and the presence of ESGD in horses of any age.20 Together, these findings suggest that other factors such as intensity or duration of exercise outweigh any potential age or sex effect, but that a breed effect might be present with Thoroughbreds predisposed to ESGD.
There are few large scale epidemiologic studies that investigated other risk factors for EGUS. Of those available, significant associations have been shown between ESGD and individual trainers, a metropolitan yard location (horses trained in urban areas were 3.9 × more likely to have gastric ulcers), a lack of direct contact with other horses, solid barriers instead of rails, and talk rather than music radio in the barn.23 Straw feeding and a lack of access to water in the paddock have been associated with an increased risk of EGUS in general.24 Further large scale work is required to better understand the epidemiologic factors which influence disease development, particularly EGGD which is largely unstudied at this point in time.
Nutritional Risk Factors
Pasture turnout is considered to reduce the risk of EGUS although evidence supporting this belief if cnflicting. Horses with access to some turnout were less likely to have ESGD, and this risk was even lower if they were turned out with other horses in one study of Thoroughbred racehorses in training.23 Conversely no effect of quality of pasture, or time at pasture (stabled, stable and pasture, pastured) was shown on ESGD prevalence in another study of Thoroughbred racehorses.25 In addition, there were no differences observed on intragastric pH in horses fed ad libitum grass hay and grain twice a day (1 kg/100 kg/d) when they were housed in a grass paddock, in a stall on their own or in a stall with an adjacent companion, suggesting that pasture turnout on its own might not affect gastric pH per se.26
Similarly, free access to fibrous feed or frequent forage feeding is widely considered to reduce the risk of gastric ulceration although strong evidence supporting this belief if also lacking. Feeding alfalfa hay and grain results in higher gastric pH and less peptic injury to the gastric squamous mucosa than feeding brome grass hay or coastal Bermuda hay with no grain.27, 28 Furthermore, in a study evaluating the influence of a high fiber diet versus an iso‐energetic low fiber diet both the number and severity of ESGD lesions was greater in the high fiber diet group.29 Together, these findings suggest that that the impact of forage feeding in the absence of other risk factor reduction might not be as great as previously believed. There is an increased likelihood of ESGD (severity score ≥2/5) when straw is the only forage provided24 suggesting that forage type might also be important. An increased time between forage meals (>6 hours between meals), compared with more frequent forage feeding (<6 hours between meals) increases the likelihood of ESGD.24
A more consistent effect is observed with increased starch/grain intake and this been associated with an increased risk of ESGD in animals working at various levels of intensity in a number of studies. There is a marked increase in ulceration when nonexercising animals are stabled and fed grain at 1% of BW, 1 hour before hay is fed.30 Similarly, exceeding 2 g/kg BW of starch intake per day is associated with an approximately 2‐fold increase in the likelihood of ESGD grade ≥2/5.24 ESGD developed in all horses within 14 days of their removal from pasture, stabling (fed 6 kg concentrate feed/day) and entering a simulated training regimen.31
Intermittent access to water increases the risk of EGUS as it has been shown that horses without access to water in their paddock are more than 2.5 times more likely to have EGUS ≥ 2/5 than horses with constant access to water.24 This was the case for both ESGD or EGUS in all parts of the stomach. Fasting is a well described as a risk factor for ESGD and intermittent starvation causes and increases the severity of ESGD.32 Using this effect, an experimental model has been developed to produce ESGD; however, it is the opinion of the committee that the model's ability to replicate the multifactorial nature of clinical disease is limited.
Recommendation: Given that the fasting model appears to poorly reflect the multifactorial nature of gastric disease the committee believes that its use as an experimental model is not justified. Instead, the committee recommends that trials investigating therapeutic or prophylactic efficacy should focus on naturally occurring disease.
Clinical Signs
In the formative years of equine gastric ulcer research, the prevalence and severity of ESGD in horses with clinical signs (poor appetite, poor bodily condition, and abdominal discomfort) was demonstrated to be significantly greater than in horses without clinical signs.11 Gastric ulcers have been loosely associated with a range of clinical signs in adult horses, despite a paucity of strong epidemiological evidence to support these associations. Reported clinical signs include poor appetite or ‘picky eating’,7, 11, 33, 34 poor body condition, or weight loss,9, 11, 33 chronic diarrhea,11, 33 poor coat condition,7 bruxism,35 behavioral changes (including an aggressive or nervous disposition),12, 33, 36 acute or recurrent colic7, 11, 33, 37, 38, 39, and poor performance.7, 10, 33, 40, 41
Colic
There is some evidence to suggest that gastric ulcers are associated with an increased incidence of colic and, in particular, recurrent postprandial abdominal discomfort.7, 11, 22, 37, 39 Gastric ulcers were reported in 83% of horses with recurrent colic in one study of which 28% had colic attributable to gastric ulceration as documented by a response to acid suppressive treatment.37 There is an association between signs of colic and ESGD with 3.5% of horses with ESGD exhibiting colic over the preceding month.7 Forty‐nine percent of horses presenting with acute colic had ESGD in another study and horses that were surgically managed had a lower incidence of ESGD than horses that had been medically managed.38 The reason for this is unclear, but might be because medically treated horses are generally fasted for longer periods of time, placing them at an increased risk of ESGD.4, 32 Alternatively, the presence of ESGD might predispose horses to altered gastrointestinal motility and subsequent medical colic.
Inappetence, Poor Body Condition, and Weight Loss
Several authors have reported an association between inappetence or ‘fussy’ eating and gastric ulceration.7, 11, 34 Signs of reduced appetite in horses with gastric ulcers can vary from mild to severe, and consequently might go unrecognized. Owners often refer to decreased appetite as “fussy” eating, without actually considering it a clinical sign of gastric ulceration.14 Poor body condition is associated with a high prevalence of gastric ulcers in racehorses in active training.9
Poor Coat Condition
Poor coat condition is usually listed as a vague clinical sign of gastric ulceration. In a cross‐sectional study in Thoroughbred horses, a statistical association between gastric ulceration and rough hair coat was identified.7 In contrast, other studies have failed to identify an association between poor hair coat and EGUS.9, 42
Diarrhea
Diarrhea has been reported as a clinical sign of gastric ulceration in adult horses.11 However, there is no evidence of a cause‐and‐effect relationship; and furthermore, it is anatomically and physiologically implausible, except in the situation where gastric ulceration is part of a wider disease process.
Changes in Behavior
It is a common perception that horses demonstrating stereotypic or altered behavior are more likely to have ulcers.11, 33 Reported behavioral changes include nervousness, aggression, and self mutilation.43 Show horses with a nervous disposition are more likely to have ESGD than are quiet or behaviorally normal horses.12 In contrast, no effect of nervousness has been shown in racehorses, and in fact in this population, aggression might have an effect in limiting ESGD.23 There is an association between crib‐biting and ESGD, although the mechanism is unclear.36
Poor Performance
The potential for EGUS to cause poor performance is of particular importance, yet, surprisingly, to date few studies have investigated the potential relationship between poor performance and the presence of EGUS. Any one of the aforementioned clinical signs can potentially have an indirect effect on performance, for example, through reduced appetite or interruption in training, but the question remains whether gastric ulcers themselves, in the absence of other clinical signs, have an effect on performance.
The mechanism by which gastric ulceration might affect performance has not been established, but it has been proposed that reduced performance might arise as a direct consequence of gastric pain.44 Ulcers and erosions in the squamous region of the stomach are similar to the lesions causing heart burn or gastroesophageal reflux disease (GERD) which is common among elite human athletes, with 58% of athletes complaining of upper gastrointestinal pain during exercise that is proportional to increasing exercise intensity.45 Furthermore, human runners with frequent GERD have a significantly decreased time to exhaustion compared to runners without reflux.46
To date, there are only a few published studies that have attempted to examine an association between the presence of ulcers and performance in racehorses. Some used trainer expectation as a surrogate measure of performance while others used objective physiologic responses to incremental treadmill exercise testing. There is a significant association between the presence of ESGD and decreased performance in Thoroughbred racehorses; with poor performance associated with the presence of gastric ulcers, independent of their severity or the number of ulcers.7 Similarly, there is a significant association between the presence of ESGD and performance below expectations in Standardbred racehorses.10 A small case series reported 4 Thoroughbred racehorses that presented with poor performance and gastric ulceration as the only abnormal finding, where improved performance after treatment with omeprazole was observed.41 The effect of ESGD, induced by intermittent feed deprivation, upon physiologic responses to incremental treadmill exercise testing has been examined. Half of the study population was treated with omeprazole (4 mg/kg PO q24h) and half received no treatment. Significantly reduced time to fatigue, significantly lower increase in maximal specific oxygen uptake and stride length were found in untreated horses, when compared to treated animals. The reason for these differences is not clear but the authors postulated that increased abdominal pain could be affecting stride length and ventilation.47
Comment: The committee concludes that a wide range of clinical signs might be present in individual cases of EGUS with varying degrees of reduced appetite and poor body condition the most prevalent at a population level. Although inconsistent, effects on behavior (including stereotypical behaviors) are not uncommon. Likewise, it is recognized that EGUS might result in poor performance; however, given the numerous factors that potentially contribute to poor performance other causes need to be considered. Differences in clinical signs between ESGD versus EGGD are unknown but warrant investigation.
Recommendation: Although a wide variety of clinical signs might be present in individual cases of EGUS, they are nonspecific and are poorly associated with the presence of EGUS. The committee therefore does not support the practice of diagnosing EGUS based on ‘characteristic’ clinical signs and recommends that EGUS be confirmed by performing gastroscopy as discussed below.
Diagnosis
The committee considers that gastroscopy is the only reliable antemortem method for definitively identifying gastric ulceration. The technique has been described elsewhere.48 When performing gastroscopy, it is essential to examine the entire stomach, including the pylorus and proximal duodenum, as lesions in these regions are easily missed. There is no relationship between the presence of ESGD and EGGD6, 14, 49; as such the presence or absence of one cannot be used as predictor for the presence or absence of the other.
There are currently no reliable hematological or biomechanical markers available to aid in diagnosis of gastric ulceration. A sucrose permeability test has shown promise for noninvasive detection of gastric ulcers,50, 51 but to date, the diagnostic accuracy of the test has not been reported in clinical cases. Contrary to initial reports, there is no association between the presence of gastric ulcers and the detection of either fecal albumin or hemoglobin.52, 53 Empiric treatment is common where gastroscopy is not available. The committee believes that, given the potential costs of treatment and the importance of distinguishing ESGD from EGGD, the initiation of treatment without prior gastroscopy is not recommended. It is noted that if empirical treatment is attempted and the horse fails to respond to treatment gastroscopy remains indicated to definitively rule out gastric disease as some animals do not appear to show resolution of clinical signs until complete healing of lesions has occurred.
Ulcer Grading
Once identified on gastroscopy, assessment of the severity of lesions is most commonly achieved by assigning a grade that describes the mucosal appearance at different anatomic sites. A variety of different systems have been published for the horse, with scales ranging from 0–354 to 0–105 described. A separate 2‐part system that describes lesion number and severity has also been proposed.55 In 1999 the Equine Gastric Ulcer Council proposed a 0–4 grading system designed to assign severity based upon lesion depth, size and number1 and recommended that the system should be adopted for both clinical and research use.
Despite widespread use, few of the scoring systems have been validated for intra‐, or inter‐, observer repeatability. When performed for a system describing number and severity, significant interobserver variability was found for the number of squamous lesions.55 Subsequently, this system was compared with necropsy examination and it was found that it underestimated the number of squamous lesions present, while a simplified 0–3 practitioner scale did not.56 A further study recommended that the 0–4 Equine Gastric Ulcer Council system be adopted as the standard EGUS scoring system because of its ease of use, and the repeatability and correlation of grades between examiners.57 Despite this validation, and the original recommendations of the EGUS council, many researchers continue to report their own systems. This lack of uniformity restricts comparison between studies, and hampers the assessment of clinical cases by different endoscopists.
Recommendation: The committee recommends that the existing Equine Gastric Ulcer Council 0–4 scoring system (shown in Table 1) be used for ESGD.
Table 1.
Grading system for equine squamous gastric disease (adapted from 1999 EGUS Council1)
| Grade | Squamous Mucosa |
|---|---|
| 0 | The epithelium is intact and there is no appearance of hyperkeratosis |
| I | The mucosa is intact, but there are areas of hyperkeratosis |
| II | Small, single or multifocal lesions |
| III | Large single or extensive superficial lesions |
| IV | Extensive lesions with areas of apparent deep ulceration |
There is minimal data on the validity of grading glandular lesions. The clinical relevance of the different manifestations of glandular disease are yet to be well evaluated although there is variation in the histologic appearance of glandular lesions, that can also be appreciated endoscopically.58, 59 Lesions can differ in their epithelial appearance (hyperemic, hemorrhagic, fibrinosuppurative, ulcerated) and in their mucosal contour (depressed, flat, raised). Furthermore, separate evaluation of the epithelial and mucosal appearance is important in developing a better appreciation of glandular healing, as epithelial restitution might not lead to the normal mucosal appearance that typifies squamous healing. Last, it appears that subjective visual assessment of severity and the histopathological appearance of the epithelium and mucosa might correlate poorly.58 Considering this; at present, it is not recommended that these different appearances be reflected in a hierarchical grading system such as that used for ESGD.
Recommendation: Until better defined, the use of a hierarchical grading system for EGGD is not recommended. In the absence of a grading system, terminology describing the presence/absence, anatomical location, distribution, and appearance of lesions as outlined in Figure 1 should be used.
The main challenge facing the endoscopist is assigning clinical, rather than just endoscopic, importance to individual lesions. It has been suggested that there is a correlation between the severity of gastric ulceration and the severity of clinical signs35, 39, 48 and it is intuitive to believe that more severe lesions are more likely to result in clinically important disease. The use of hierarchical systems further implies more severe disease with higher grades. However, this relationship might not be linear or temporally consistent and there is currently a paucity of information in the literature demonstrating a direct cause‐and‐effect relationship between clinical signs and the presence, severity, or location of gastric ulcers in adult horses. Furthermore, we know that many horses with EGUS will not demonstrate clinical signs, and are considered to have ‘silent’ or non‐clinical gastric ulceration.14, 25, 33 Whether these horses are truly without clinical signs, or simply subclinical, warrants consideration as there is improved behavior after treatment in some patients.33
Furthermore, it has been assumed that a loss of mucosal integrity is required for clinical importance. However, in humans hyperemia of the glandular mucosa is considered to reflect acidification of the mucosal surface that, in turn, results in activation of the sensory nerves and pain.60 Whether a similar effect is present in the horse is not known but anecdotally it appears that some horses with hyperkeratosis or hyperemia alone respond to treatment, suggesting that such an effect might be present in a subset of the population. In contrast, some horses with endoscopically severe disease do not have clinical signs and fail to change in response to treatment.
Comment: There is little evidence to support the notion that lesion grade (as assessed visually) correlates with clinical signs. Considering this the committee advises that the assessment of clinical relevance should not be made on endoscopic appearance alone. Instead the clinician should assess the relevance of an individual's lesions in light of the horse's recent usage, its history and presenting clinical signs. Future research on EGUS should focus on reporting both clinical and endoscopic outcomes.
Pathophysiology
A variety of management factors contribute to the development of ESGD. All of these factors share the common trait that they increase the exposure of the squamous mucosa to acid. In vitro experiments clearly show that squamous mucosal cells are susceptible to hydrochloric acid (HCl) and volatile fatty acid (VFA) injury in a pH, dose and time dependent manner.61 Damage of the outer cell barrier is induced by HCl, later followed by diffusion into the squamous cells of the stratum spinosum ultimately resulting in ulceration.62 Byproducts of bacterial fermentation of sugars in concentrate diets not only like VFAs and lactic acid, but also bile acids, have been shown to act synergistically with HCL.4, 62
There is a well‐described relationship between exposure of squamous mucosa to acidic content and training. Excessive exposure of the squamous mucosa results from the acidic gastric contents being pushed up by the increased intra‐abdominal pressure associated with gaits faster than a walk.63 Consistent with this racehorses have an increase in prevalence, lesion severity and number of lesion sites within the squamous mucosa significantly associated with increasing intensity of long‐duration training.5, 64, 65 The severity of ESGD in high level endurance horses is directly related to the distance of the ride.17
In contrast, the pathophysiology of EGGD is poorly understood. The glandular mucosa differs fundamentally from the squamous mucosa in that under normal physiological conditions it is exposed to highly acidic gastric contents with the pH in the ventral portion of the stomach relatively stable at between 1 and 3.66 As such, whereas ESGD results from exposure of mucosa unaccustomed to acidity, EGGD is believed to result from a breakdown of the normal defense mechanisms that protect the mucosa from acidic gastric contents. The factors that contribute to breakdown of this protective layer are yet to be elucidated in the horse, but in humans Helicobacter pylori and NSAIDs are the predominant causes of gastric ulceration.3 As such, research in the horse has focused primarily on these 2 mechanisms.
To date there remains conflict in the literature as to the role of bacteria in EGGD. Both gastric‐adapted bacteria and opportunistic pathogens might play a role in squamous ulceration67 but whether the situation is similar in the glandular mucosa is unknown. Furthermore, although such bacteria are present in ESGD their role appears to be secondary as the response to acid suppression alone is good. Helicobacter‐like organisms have been identified in horses affected with EGGD in some studies,68, 69, 70 whereas other studies have failed to identify such organisms.21, 58
The potential for NSAIDs to cause EGGD under clinical conditions is equally controversial. An ulcerogenic capacity has been demonstrated for flunixin, phenylbutazone, and ketoprofen at doses 50% higher than typically recommended,71 while at clinical doses phenylbutazone and suxibuzone do not induce gastric ulceration when administered for 15 days.72 As previously stated, high prevalences of EGGD have been observed in many populations with disease rates disproportionate to the number of animals likely to receive NSAIDs under such conditions.
Comment: Based on the current knowledge evidence for bacteria as a direct, causative agent is lacking. Similarly, although NSAIDs have the potential to cause EGGD in individual animals they are unlikely to be an important contributor to the prevalence of disease at the population level. Similar to human medicine where a large number of different diseases with separate pathophysiologies result in PUD, 3 it is likely that multiple different mechanisms contribute to the development of EGGD in the horse.
Treatment and Prevention
Pharmaceutical Treatment
The mantra “no acid, no ulcer’ acid suppression is considered a cornerstone of gastric ulcer management in humans, regardless of the inciting cause.3 Consistent with this, it is the committee's opinion that appropriate acid suppressor treatment is indicated in the management of both ESGD, for which its efficacy is well documented, and EGGD, regardless of the failure to identify an underlying cause as of yet. Proton pump inhibitors and H2‐receptor antagonists are the most commonly used classes of drugs in the horse. Proton pumps inhibitors, of which omeprazole is the best studied in the horse, irreversibly impair the H+, K+ ATPase (proton) pump that secretes HCl with new pumps needing to be made before acid production resumes.73 In contrast, H2‐receptor antagonists work via competitively blocking the H2 receptor on the parietal cell and their efficacy is dependent on maintaining plasma concentrations of the drug. Omeprazole is superior to ranitidine in the treatment of naturally occurring disease74 and, in the opinion of the committee, omeprazole remains the drug of choice for treatment of EGUS.
A variety of factors including the formulation used, dose and duration of treatment influence the expected outcome of treatment. The recent expiration of the GastroGard1 patent is expected to result in an influx of different formulations onto the market and discussion of the method of protection used to protect the omeprazole in different formulations is warranted. Omeprazole is acid labile and it is generally regarded that some form of protection is necessary to prevent degradation of the drug within the acidic environment of the stomach.66 GastroGard1 and its true generic equivalents utilize a buffered paste formulation to achieve this protection.66 Other formulations utilize enteric coated granules suspended within a paste to achieve the same objective.19
To date, little work has been done on the relative pharmacokinetics of different formulations of omeprazole. A study directly comparing the bioavailability of 4 formulations (2 enteric coated granule formulations and 2 buffered formulations) to GastroGard1 as a reference standard did not find statistically significant differences in bioavailability.75 Similarly, a, clinical study found no significant difference in the bioavailability of an enteric coated granule formulation (Gastrozol2;) when compared with the reference buffered formulation (GastroGard1).76 In contrast, an enteric coated omeprazole formulation (Gastrozol2) has been shown to have approximately twice the bioavailability of a compounded paste formulation of plain, unprotected omeprazole.77
These findings are interesting in light of outcome of recent clinical studies. In a direct comparison between formulations, no difference in endoscopic outcome was observed despite the enteric coated granule formulation (Gastrozol2) being used at 1 mg/kg PO once daily versus 4 mg/kg PO once daily for the buffered formulation (GastroGard1).76 In another study, no difference in endoscopic outcome was noted between 1, 2, and 4 mg/kg PO once daily of enteric coated granule omeprazole formulation.19 Together these findings suggest that lower doses of omeprazole, either buffered or enteric coated, might be efficacious and warrant consideration.
The duration of intra‐day acid suppression required for healing of ESGD and EGGD has not been documented. In human, maintenance of a pH above 3 and 4 for a minimum of 16 hours is required for healing of gastric ulceration and reflux esophagitis, respectively.78 Initial studies suggested that once daily administration of omeprazole results in 24 hours of acid suppression,79, 80 however, the duration of acid suppression after PO dosing at 4 mg/kg might be as short as 12 hours in some animals.66 The results of clinical studies suggest that, even if the duration of acid suppression is only 12 hours, this is sufficient for the treatment of ESGD as the treatment response with doses ranging from 1.0 to 4.0 mg/kg PO once daily is well documented.19, 54, 76, 81, 82, 83 In line with this the following dosing recommendations are made based largely on the results of clinical trials and comparative pharmacokinetic work while acknowledging that further investigation into the actual duration of acid suppression achieved with a range of doses and formulations is needed.
GastroGard1 has been well studied at its registered dose of 4 mg/kg PO once daily for 28 days with ESGD healing rates of 70–77% consistently reported.54, 74, 81, 83, 84 As discussed above, the investigation of lower doses warrants investigation but in the absence of specific evidence to justify lower doses the continued use of buffered formulations at 4 mg/kg PO once daily appears justified when finances permit. However, it is the committee's opinion that, based on currently available evidence, the use of buffered formulations at 2 mg/kg PO once daily warrants consideration. Similarly, based on the reported clinical trials19, 76 the use of enteric coated granule formulations at 1.0 mg/kg PO once daily appears justified. In the committee's opinion, dose reduction in plain formulations cannot be recommended at this time because of the lack of published data on the use of these formulations in a clinical setting.
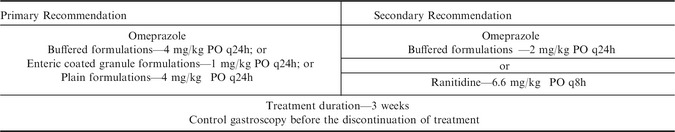
The duration of treatment also warrants consideration. Few studies have compared the rate of healing within the standard 28 day treatment period but an early study suggested that if healing of ESGD is going to occur that it is typically complete by 21 days.81 As such, a reduction in the standard treatment period for ESGD to 3 weeks appears justified. Regardless of the formulation or dose used it is important to recognize at best only approximately 70–80% of ESGD lesions will heal within a 28 day treatment period54, 81, 82, 83 and repeat gastroscopy is recommended before the cessation of treatment to ensure that healing has occurred. Adjunctive therapies do not appear to be justified in the treatment of ESGD but ranitidine could be considered as an alternative when omeprazole is not available or has been shown to be ineffective. Ranitidine has been shown to effectively suppress gastric acidity in experimental studies85, 86 and is most commonly used at 6.6 mg/kg PO q8h. Treatment recommendations for ESGD are summarized in Table 2.
Table 2.
Treatment recommendations for Equine Squamous Gastric Disease (ESGD)
Until recently, treatment recommendations for EGUS have not differentiated between squamous and glandular disease. However, in a series of recent studies only 25% of EGGD lesions healed with 28–35 days of omeprazole treatment at 4.0 mg/kg PO once daily in direct contrast to an ESGD lesion healing rate of 78%.19, 82, 87 The reasons for the poor response of EGGD to omeprazole treatment is not understood but 3 factors warrant consideration namely; the duration of intra‐day acid suppression achieved with current dosing regimens (discussed above), the duration of treatment required and the use of adjunctive therapies.
In humans, the duration of treatment for glandular ulceration is dependent on primary cause of the lesion3 and the possibility exists that, simply, longer durations of treatment might be needed for EGGD. In humans, 8 and 12 weeks duration of acid suppression treatment are required for 84% and 100% healing rates to be observed in NSAID induced ulceration.88 The authors have consistently observed healing in the majority of EGGD cases over a similar time frame, however, it is recognized that clinical trials specifically examination this effect over time are lacking.
An alternative explanation for the failure of EGGD to respond to omeprazole monotherapy is that bacteria might play a role in the development or perpetuation of glandular ulceration and that acid suppression treatment alone might be inadequate. As discussed above, the role of bacteria in the pathogenesis of EGGD is unclear. In H. pylori‐positive ulceration in human healing rates of >80% are consistently observed with 7–14 days of triple treatment, including antimicrobials.3 Extrapolating from this; antimicrobials are anecdotally popular for the treatment of EGGD in the horse. However, antimicrobials do not improve healing of non H. pylori‐associated ulceration in humans.3 Furthermore, no evidence exists to support their use in the horse with a single, clinical trial documenting no benefit of the addition of trimethoprim–sulphadimidine over omeprazole monotherapy.89
Recommendation: In line with the profession's obligations for responsible stewardship of antimicrobials, and given A) the failure of bacterial agents to be definitely established as a contributory factor to EGGD and B) the absence of clinical trials supporting their use, it is the committee's opinion that the routine use of antimicrobials in the treatment of EGGD is not justified until their efficacy is appropriately documented.
Alternatively, given the proposed role of failed mucosal defenses in the pathogenesis of EGGD, the use of mucosal protectants as a component of treatment is logical. Furthermore, their use is not complicated by ethical considerations as antimicrobials are. Sucralfate is the best studied. Its mechanism of action is likely a combination of adherence to ulcerated mucosa, stimulation of mucous secretion, prostaglandin E synthesis and enhanced blood flow all of which are likely to be beneficial in EGGD.90 Supporting its use, a recent study reported a 67.5% healing rate for EGGD of the pyloric antrum using omeprazole (GastroGard1) at 4 mg/kg PO once daily and sucralfate at 12 mg/kg PO twice daily.91 Further studies investigating the role of sucralfate and the potential for interactions between it and omeprazole, as reported in human medicine, are required.
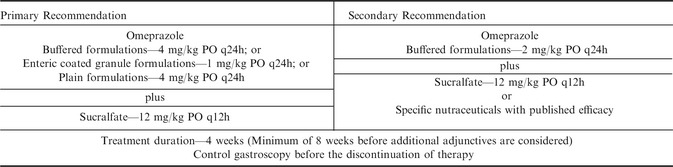
Considering the above factors the authors’ recommendation for the treatment of EGGD include the use of omeprazole as outlined for ESGD plus the addition of sucralfate at 12 mg/kg PO twice daily as summarized in Table 3. Given the positive response recently reported to this combination91 control examination at 4 weeks appears logical. It is the committee's opinion that a minimum of 8 weeks of combination therapy should be completed before additional therapies are considered. Further it is recommended that in such cases, further investigation into the underlying cause of disease, such a mucosal biopsy, appears justified before empirical treatment is continued.
Table 3.
Treatment recommendations for Equine Glandular Gastric Disease (EGGD)
Pharmaceutical Prevention
The pharmacological approach to prevention of ESGD is similar to treatment. Prevention should be approached on a case by case basis, wherein the greater the ability to impact on risk factors (as discussed below), the lower the need for additional treatment. Omeprazole in the form of buffered and enteric coated formulations is typically used at 1.0 mg/kg PO once daily for prevention.92, 93
To date, specific guidelines for the prevention of EGGD have not been developed although the efficacy of omeprazole as a prophylactic for EGGD is unclear with 23% of horses experiencing worsening of their EGGD grade in a series of recent studies, despite omeprazole treatment at doses ranging from 1–4 mg/kg PO once daily.19, 82, 87 Interestingly, in humans, the efficacy of long‐term acid suppression treatment in the prevention of non H. pylori, non‐NSAID associated ulceration has also been questioned.94 At the time of writing, the exact role of omeprazole for the prevention of EGGD is unclear; however, its use as per the recommendations for ESGD is logical until further defined.
Nutraceuticals
Nutraceuticals are appealing because of their ease of use and availability. Pectin‐lecithin complexes have been studied experimentally and increase the total mucus concentration in gastric juice.95 Two studies failed to demonstrate a protective effect in fed/fasting models of ESGD, despite initially promising results from a small case series.96 More recently, the combination of an antacid (magnesium hydroxide), a pectin‐lecithin complex and Saccharomyces cerevisiae has shown promise as a prophylactic agent for both ESGD and EGGD.97 Similarly, a feed supplement consisting of salts of organic acids in combination with B‐vitamins might be beneficial in the management of ESGD98 and a preparation containing sea buckthorn berries appeared to have protective effects against the development of EGGD in a fasting model of disease.99 Antacids appear to give some symptomatic relieve, however, their effect is short‐lived (≤2 hours)85, 100 and their use as a sole therapeutic agent is not justified.
Nutritional Management
In providing the following recommendations it is recognized that, as discussed above, strong evidence supporting some nutritional recommendations is lacking. Considering this, the following recommendations are based on the committee's interpretation of the available literature and their collective opinion as to what represents the current best practice. Furthermore, to date little evidence exists for the role of diet in EGGD and as such the following recommendations are based primarily on risk factors identified for ESGD.
Although the evidence supporting such a recommendation is conflicting; continuous access to good quality grass pasture is considered ideal. Free choice, or at least frequent feedings (4–6 meals/day), of hay might be a suitable replacement. Horses fed hay should receive a minimum of 1.5 kg (DM)/100 kg bodyweight per day.39 Overweight horses and ponies at risk of EGUS should receive a minimum amount of high quality forage (1.5 kg (DM)/100 kg bodyweight per day) that is mature and has low energy content. If low energy forage is not available then a mixture of high quality forage and straw divided into a minimum of 4 feedings might be a suitable alternative. Straw should not be the only forage provided but can be safely included in the ration at <0.25 kg (DM)/100 kg BW.
Horses should be fed grain and concentrates as sparingly as possible. Feeding of sweet feed should be avoided as a large quantity of VFAs could be produced if greater than 1 to 2 kg of sweet feed is fed per meal. Grains like barley and oats can be substituted to decrease fermentation to VFAs. The diet should not exceed 2 g/kg bodyweight of starch intake per day or more than 1 g/kg bodyweight of starch per meal.24 Concentrate meals should not be fed less than 6 hours apart.39
Vegetable oils such as corn oil might help reduce the risk of EGGD. Ponies with gastric cannulas fed 45 mL corn oil PO once daily by dose syringe had significantly lower gastric acid output and increased prostaglandin concentration in their gastric juice compared to the non oil dosed animals.101 Studies evaluating the use of oil in the management of naturally occurring EGGD are needed to document if these effects are clinically relevant. Water should be provided continuously. An increased risk of ESGD has been shown with electrolyte pastes or hypertonic solutions given PO,102 but not when electrolytes were mixed in feed or given in lower doses in water. As such the committee considers the use of electrolytes with feed to be safe.
Acknowledgment
Conflict of Interest Declaration: The authors of this statement have received support from the following companies in the form of speaker honorarias, research funding, consulting or employment; Merial, Boehringher Ingelheim, Virbac, Abler, Bova Compounding. Boehringher Ingelheim supported the development of this consensus statement by supporting a meeting of the authors held in Helsinki during February, 2014. None of the aforementioned companies had any involvement, direct or indirect, in the development of the manuscript and the works reflects the opinions solely of the authors.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Consensus Statements of the European College of Equine Internal Medicine (ECEIM) provide veterinarians with guidelines regarding the pathophysiology, diagnosis, or treatment of equine internal medicine diseases. The foundation of the Consensus Statement is evidence‐based medicine, but if such evidence is conflicting or lacking the panel provides interpretive recommendations based on their collective expertise. The Consensus Statement panel is chosen by the board of the ECEIM.
Footnotes
Merial, Duluth, GA
Virbac, Milperrra, NSW, Australia
References
- 1. Andrews F, Bernard W, Byars D, et al. Recommendations for the diagnosis and treatment of equine gastric ulcer syndrome (EGUS). Equine Vet Educ 1999;11:262–272. [Google Scholar]
- 2. Merritt AM. Appeal for proper usage of the term “EGUS”: Equine gastric ulcer syndrome. Equine Vet J 2009;41:616. [DOI] [PubMed] [Google Scholar]
- 3. Malfertheiner P, Chan FFKL, McColl KEL, Leung W. Peptic‐ulcer disease. Lancet 2009;374:1449–1461. [DOI] [PubMed] [Google Scholar]
- 4. Berschneider HM, Blikslager AT, Roberts MC. Role of duodenal reflux in nonglandular gastric ulcer disease of the mature horse. Equine Vet J Suppl 1999;29(Suppl 29):24–29. [DOI] [PubMed] [Google Scholar]
- 5. Murray MJ, Schusser GRF, Pipers FS, Gross SJ. Factors associated with gastric lesions in thoroughbred racehorses. Equine Vet J 1996;28:368–374. [DOI] [PubMed] [Google Scholar]
- 6. Begg LM, O'Sullivan CB. The prevalence and distribution of gastric ulceration in 345 racehorses. Aust Vet J 2003;81:199–201. [DOI] [PubMed] [Google Scholar]
- 7. Vatistas NJ, Snyder JR, Carlson G, et al. Cross‐sectional study of gastric ulcers of the squamous mucosa in Thoroughbred racehorses. Equine Vet J 1999;31(Suppl 29):34–39. [DOI] [PubMed] [Google Scholar]
- 8. Rabuffo TS, Orsini JA, Sullivan E, et al. Associations between age or sex and prevalence of gastric ulceration in Standardbred racehorses in training. J Am Vet Assoc 2002;221:1156–1159. [DOI] [PubMed] [Google Scholar]
- 9. Dionne R, Vrins A, Doucet M. Gastric ulcers in Standardbred racehorses: Prevalence, lesion description, and risk factors. J Vet Int Med 2003;17:218–222. [DOI] [PubMed] [Google Scholar]
- 10. Jonsson H, Egenvall A. Prevalence of gastric ulceration in Swedish Standardbreds in race training. Equine Vet J 2006;38:209–213. [DOI] [PubMed] [Google Scholar]
- 11. Murray MJ, Grodinsky C, Anderson CW, et al. Gastric ulcers in horses: A comparison of endoscopic findings in horses with and without clinical signs. Equine Vet J Suppl 1989;21(Suppl 7):68–72. [DOI] [PubMed] [Google Scholar]
- 12. McClure SR, Glickman LT, Glickman NW. Prevalence of gastric ulcers in show horses. J Am Vet Med Assoc 1999;215:1130–1133. [PubMed] [Google Scholar]
- 13. Hartmann AM, Frankeny RL. A preliminary investigation into the association between competition and gastric ulcer formation in non‐racing performance horses. J Equine Vet Sci 2003;23:560–561. [Google Scholar]
- 14. Luthersson N, Nielsen KH, Harris P, Parkin TDH. The prevalence and anatomical distribution of equine gastric ulceration syndrome (EGUS) in 201 horses in Denmark. Equine Vet J 2009;41:619–624. [DOI] [PubMed] [Google Scholar]
- 15. Niedźwiedź A, Kubiak K, Nicpoń J. Endoscopic findings of the stomach in pleasure horses in Poland. Acta Vet Scand 2013;55:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nieto JE, Snyder JR, Beldomenico P, et al. Prevalence of gastric ulcers in endurance horses – a preliminary report. Vet J. 2004;167:33–37. [DOI] [PubMed] [Google Scholar]
- 17. Tamzali Y, Marguet C, Priymenko N, Lyazrhi F. Prevalence of gastric ulcer syndrome in high‐level endurance horses. Equine Vet J 2011. Mar;43:141–144. [DOI] [PubMed] [Google Scholar]
- 18. Chameroy KA, Nadeau JA, Bushmich SL, et al. Prevalence of non‐glandular gastric ulcers in horses involved in a university riding program. J Equine Vet Sci 2006;26:207–211. [Google Scholar]
- 19. Sykes BW, Sykes KM, Hallowell GD. A comparison of three doses of omeprazole in the treatment of gastric ulceration in the horse: A randomised, blinded clinical trial. Equine Vet J 2015;47:285–290. [DOI] [PubMed] [Google Scholar]
- 20. Hepburn RJ. Endoscopic examination of the squamous and glandular gastric mucosa in sport and leisure horses: 684 horses (2005–2011) [abstract]. Proc 11th International Equine Colic Research Symposium. 2014. 5.
- 21. Husted L, Jensen TK, Olsen SN, Mølbak L. Examination of equine glandular stomach lesions for bacteria, including Helicobacter spp by fluorescence in situ hybridisation. BMC Microbiol 2010;10:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sandin A, Skidell J, Häggström J, et al. Post‐mortem findings of gastric ulcers in Swedish horses up to 1 year of age: A retrospective study 1924–1996. Acta Vet Scand 1999;40:109–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lester GD, Robinson I, Secombe C. Risk Factors for Gastric Ulceration in Thoroughbred Racehorses. Canberra: Australian Government: Rural Industries Research and Development Corporation; 2008. 1–42. [Google Scholar]
- 24. Luthersson N, Nielsen KH, Harris P, Parkin TDH. Risk factors associated with equine gastric ulceration syndrome (EGUS) in 201 horses in Denmark. Equine Vet J 2009;41:625–630. [DOI] [PubMed] [Google Scholar]
- 25. Bell RJW, Kingston JK, Mogg TD, Perkins NR. The prevalence of gastric ulceration in racehorses in New Zealand. N Z Vet J 2007;55:13–18. [DOI] [PubMed] [Google Scholar]
- 26. Husted L, Sanchez LC, Olsen SN, et al. Effect of paddock vs. stall housing on 24 hour gastric pH within the proximal and ventral equine stomach. Equine Vet J 2008;40:337–341. [DOI] [PubMed] [Google Scholar]
- 27. Nadeau J, Andrews F, Mathew A. Evaluation of diet as a cause of gastric ulcers in horses. Am J Vet Res 2000;61:784–790. [DOI] [PubMed] [Google Scholar]
- 28. Lybbert T, Gibbs P, Cohen N. Feeding alfalfa hay to exercising horses reduces the severity of gastric squamous ulceration. Proc 53rd Am Assoc Eq Pract Conv. 2007. 525–526.
- 29. Boswinkel M, Ellis AD, Van Oldruitenborgh‐oosterbaan MMS. The influence of low versus high fibre haylage diets in combination with training or pasture rest on equine gastric ulceration syndrome (EGUS). Pferdeheilkunde 2007;23:123–129. [Google Scholar]
- 30. Frank N, Andrews FM, Elliott SB, Lew J. Effects of dietary oils on the development of gastric ulcers in mares. Am J Vet Res 2005;66:2006–2011. [DOI] [PubMed] [Google Scholar]
- 31. Vatistas NJ, Sifferman RL, Holste J. Induction and maintenance of gastric ulceration in horses in simulated race training. Equine Vet J 1999;29:40–44. [DOI] [PubMed] [Google Scholar]
- 32. Murray MJ, Eichorn ES. Effects of intermittent feed deprivation, intermittent feed deprivation with ranitidine administration, and stall confinement with ad libitum access to hay on gastric ulceration in horses. Am J Vet Res 1996;57:1599–1603. [PubMed] [Google Scholar]
- 33. Andrews FM, Nadeau J. Clinical syndromes of gastric ulceration in foals and mature horses. Equine Vet J 1999;31(Suppl 29):30–33. [DOI] [PubMed] [Google Scholar]
- 34. Bezděková B, Jahn P, Vyskočil M. Gastric Ulceration, Appetite and feeding practices in Standardbred racehorses in the Czech Republic. Acta Vet Brno 2008;77:603–607. [Google Scholar]
- 35. Bell RJW, Mogg TD, Kingston JK. Equine gastric ulcer syndrome in adult horses: A review. N Z Vet J 2007;55:1–12. [DOI] [PubMed] [Google Scholar]
- 36. Nicol CJC, Davidson HPD, Harris PA, et al. Study of crib‐biting and gastric inflammation and ulceration in young horses. Vet Rec 2002;151:658–662. [DOI] [PubMed] [Google Scholar]
- 37. Murray MJ. Gastric ulceration in horses: 91 cases (1987–1990). J Am Vet Med Assoc 1992;201:117–120. [PubMed] [Google Scholar]
- 38. Dukti S, Perkins S, Murphy J, et al. Prevalence of gastric squamous ulceration in horses with abdominal pain. Equine Vet J 2006;38:347–349. [DOI] [PubMed] [Google Scholar]
- 39. Videla R, Andrews FM. New perspectives in equine gastric uler syndrome. Vet Clin North Am Eq Pract 2009;25:283–301. [DOI] [PubMed] [Google Scholar]
- 40. Kollias‐Baker C, Cox K, Jones J. Evaluation of the effects of omeprazole on physiological indices of performance of horses during incremental treadmill exercise. Vet Ther 2001;2:361–369. [PubMed] [Google Scholar]
- 41. Franklin SH, Brazil TJ, Allen KJ. Poor performance associated with equine gastric ulceration syndrome in four Thoroughbred racehorses. Equine Vet Educ 2008;20:119–124. [Google Scholar]
- 42. Le Jeune SS, Nieto JE, Dechant JE, Snyder JR. Prevalence of gastric ulcers in Thoroughbred broodmares in pasture: A preliminary report. Vet J. 2009;181:251–255. [DOI] [PubMed] [Google Scholar]
- 43. McDonnell SM. Practical review of self‐mutilation in horses. Anim Reprod Sci 2008;107:219–228. [DOI] [PubMed] [Google Scholar]
- 44. Andrews FM. Poor performance: Can heartburn slow a horse? Equine Vet Educ 2008;20:125–126. [Google Scholar]
- 45. Worobetz LJ, Gerrard DF. Gastrointestinal symptoms during exercise in Enduro athletes: Prevalence and speculations on the aetiology. N Z Med J 1985;98:644–646. [PubMed] [Google Scholar]
- 46. Rodriguez‐Stanley S, Bemben D, Zubaidi S, et al. Effect of esophageal acid and prophylactic rabeprazole on performance in runners. Med Sci Sports Exerc 2006;38:1659–1665. [DOI] [PubMed] [Google Scholar]
- 47. Nieto JE, Snyder JR, Vatistas NJ, Jones JH. Effect of gastric ulceration on physiologic responses to exercise in horses. Am J Vet Res 2009;70:787–795. [DOI] [PubMed] [Google Scholar]
- 48. Sykes BW, Jokisalo JM. Rethinking equine gastric ulcer syndrome: Part 1 – Terminology, clinical signs and diagnosis. Equine Vet Educ 2014;26:543–547. [Google Scholar]
- 49. Murray MJ, Nout YS, Ward DL. Endoscopic findings of the gastric antrum and pylorus in horses: 162 cases (1996–2000). J Vet Int Med 2001;15:401–406. [PubMed] [Google Scholar]
- 50. O'Conner MS, Steiner JM, Roussel AJ, et al. Evaluation of urine sucrose concentration for detection of gastric ulcers in horses. Am J Vet Res 2004;65:31–39. [DOI] [PubMed] [Google Scholar]
- 51. Hewetson M, Cohen ND, Love S, et al. Sucrose concentration in blood: A new method for assessment of gastric permeability in horses with gastric ulceration. J Vet Int Med 2006;20:388–394. [DOI] [PubMed] [Google Scholar]
- 52. Pellegrini FL. Results of a large‐scale necroscopic study of equine colonic ulcers. J Equine Vet Sci 2005;25:113–117. [Google Scholar]
- 53. Sykes BW, Jokisalo J, Hallowell GD. Evaluation of a commercial faecal blood test for the diagnosis of gastric ulceration in Thoroughbred racehorses: A preliminary report [abstract]. Proc 11th International Equine Colic Research Symposium. 2014. 4.
- 54. Andrews FM, Sifferman RL, Bernard W, et al. Efficacy of omeprazole paste in the treatment and prevention of gastric ulcers in horses. Equine Vet J Suppl 1999;31:81–86. [DOI] [PubMed] [Google Scholar]
- 55. MacAllister CG, Andrews FM, Deegan E, et al. A scoring system for gastric ulcers in the horse. Equine Vet J 1997;29:430–433. [DOI] [PubMed] [Google Scholar]
- 56. Andrews FM, Reinemeyer CR, McCracken MD, et al. Comparison of endoscopic, necropsy and histology scoring of equine gastric ulcers. Equine Vet J 2002;34:475–478. [DOI] [PubMed] [Google Scholar]
- 57. Bell RJW, Kingston JK, Mogg TD. A comparison of two scoring systems for endoscopic grading of gastric ulceration in horses. N Z Vet J 2007;55:19–22. [DOI] [PubMed] [Google Scholar]
- 58. Martineau H, Thomson H, Taylor D. Pathology of gastritis and gastric ulceration in the horse. Part 1: Range of lesions present in 21 mature individuals. Equine Vet J 2009;41:638–644. [DOI] [PubMed] [Google Scholar]
- 59. Hepburn R. Equine glandular ulceration: Pathophysiology and epidemiology. Proc Am Coll Vet Int Med An Congr. 2012.
- 60. Raybould HE, Li DS, Guth PH. Calcitonin gene‐related peptide mediates the gastric hyperemic response to acid back‐diffusion. Ann N Y Acad Sci 1992;657:536–537. [DOI] [PubMed] [Google Scholar]
- 61. Andrews FM, Buchanan BR, Smith SH, et al. In vitro effects of hydrochloric acid and various concentrations of acetic, propionic, butyric, or valeric acids on bioelectric properties of equine gastric squamous mucosa. Am J Vet Res 2006;67:1873–1882. [DOI] [PubMed] [Google Scholar]
- 62. Nadeau JA, Andrews FM, Patton CS, et al. Effects of hydrochloric, valeric, and other volatile fatty acids on pathogenesis of ulcers in the nonglandular portion of the stomach of horses. Am J Vet Res 2003;64:413–417. [DOI] [PubMed] [Google Scholar]
- 63. Lorenzo‐Figueras M, Merritt A. Effects of exercise on gastric volume and pH in the proximal portion of the stomach of horses. Am J Vet Res 2002;63:1481–1487. [DOI] [PubMed] [Google Scholar]
- 64. Roy M‐A, Vrins A, Beauchamp G, Doucet MY. Prevalence of ulcers of the squamous gastric mucosa in standardbred horses. J Vet Intern Med 2005;19:744–750. [DOI] [PubMed] [Google Scholar]
- 65. Orsini J A, Hackett ES, Grenager N. The effect of exercise on equine gastric ulcer syndrome in the Thoroughbred and Standardbred athlete. J Equine Vet Sci 2009;29:167–171. [Google Scholar]
- 66. Merritt AM, Sanchez LC, Burrow JA, et al. Effect of GastroGard and three compounded oral omeprazole preparations on 24 h intragastric pH in gastrically cannulated mature horses. Equine Vet J 2003;35:691–695. [DOI] [PubMed] [Google Scholar]
- 67. Al JR, McGowan T, Andrews F, McGowan C Gastric Ulceration in Horses: The Role of Bacteria and Lactic Acid. Canberra: Australian Government: Rural Industries Research and Development Corporation; 2008. 1–26. [Google Scholar]
- 68. Contreras M, Morales A, García‐Amado Ma, et al. Detection of Helicobacter‐like DNA in the gastric mucosa of Thoroughbred horses. Lett Appl Microbiol 2007;45:553–557. [DOI] [PubMed] [Google Scholar]
- 69. Morales A, Garcia F, Bermudez V. Detection of Helicobacter‐like organisms in Thoroughbred horses from Venezuela. Br J Vet Path 2010;3:52–55. [Google Scholar]
- 70. Fox J. The non‐H pylori Helicobacters: Their expanding role in gastrointestinal and systemic diseases. Gut 2002;50:273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. MacAllister CG, Morgan SJ, Borne AT, Pollet RA. Comparison of adverse effects of phenylbutazone, flunixin meglumine, and ketoprofen in horses. J Am Vet Med Assoc 1993;202:71–77. [PubMed] [Google Scholar]
- 72. Andrews F, Reinemeyer CR, Longhofer SL. Effects of top‐dress formulations of suxibuzone and phenylbutazone on development of gastric ulcers in horses. Vet Ther 2009;10:113–120. [PubMed] [Google Scholar]
- 73. Fellenius E, Berglindh T, Sachs G, et al. Substituted benzimidazoles inhibit gastric acid secretion by blocking (H+ + K+) ATPase. Nature 1981;290:159–161. [DOI] [PubMed] [Google Scholar]
- 74. Lester GD, Smith RL, Robertson ID. Effects of treatment with omeprazole or ranitidine on gastric squamous ulceration in racing Thoroughbreds. J Am Vet Med Assoc 2005;227:1636–1639. [DOI] [PubMed] [Google Scholar]
- 75. Sykes BW, Underwood C, McGowan C, Mills P. Pharmacokinetics and bioavailability of five commercially available formulations of omeprazole. J Vet Pharm Ther 2015; DOI: 10.1111/jvp.12240. Early view:1–6. [DOI] [PubMed] [Google Scholar]
- 76. Birkmann K, Junge HK, Maischberger E, et al. Efficacy of omeprazole powder paste or enteric‐coated formulation in healing of gastric ulcers in horses. J Vet Int Med 2014;28:925–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sykes BW, Underwood C, McGowan C, Mills P. Pharmacokinetics of intravenous, plain oral and enteric‐coated oral omeprazole in the horse. J Vet Pharm Ther 2015;38:130–136. [DOI] [PubMed] [Google Scholar]
- 78. Bell NJV, Burget D, Howden CW, et al. Appropriate acid suppression for the management of gastro‐oesophageal reflux disease. Digestion 1992;51:59–67. [DOI] [PubMed] [Google Scholar]
- 79. Jenkins CC, Blackford JT, Andrews F, et al. Duration of antisecretory effects of oral omeprazole in horses with chronic gastric cannulae. Equine Vet J 1992;24(Suppl 13):89–92. [Google Scholar]
- 80. Daurio CP, Holste JE, Andrews FM, et al. Effect of omeprazole paste on gastric acid secretion in horses. Equine Vet J 1999;31(Suppl 29):59–62. [DOI] [PubMed] [Google Scholar]
- 81. Murray MJ, Haven ML, Eichorn ES, et al. Effects of omeprazole on healing of naturally‐occurring gastric ulcers in Thoroughbred racehorses. Equine Vet J 1997;29:425–429. [DOI] [PubMed] [Google Scholar]
- 82. Sykes BW, Sykes KM, Hallowell GD. A comparison between pre‐ and post‐exercise administration of omeprazole in the treatment of EGUS: A randomised, blinded clinical trial. Equine Vet J 2014;46:422–426. [DOI] [PubMed] [Google Scholar]
- 83. Doucet MY, Vrins AA, Dionne R, et al. Efficacy of a paste formulation of omeprazole for the treatment of naturally occurring gastric ulcers in training Standardbred racehorses in Canada. Can Vet J 2003;44:581–585. [PMC free article] [PubMed] [Google Scholar]
- 84. MacAllister CG, Sifferman RL, McClure SR, et al. Effects of omeprazole paste on healing of spontaneous gastric ulcers in horses and foals: A field trial. Equine Vet J Suppl 1999;31(Suppl 29):77–80. [DOI] [PubMed] [Google Scholar]
- 85. Murray MJ, Grodinsky C. The effects of famotidine, ranitidine and magnesium hydroxide/aluminium hydroxide on gastric fluid pH in adult horses. Equine Vet J Suppl 1992;24(Suppl 11):52–55. [DOI] [PubMed] [Google Scholar]
- 86. Murray MJ, Schusser GF. Measurement of 24‐h gastric pH using an indwelling pH electrode in horses unfed, fed and treated with ranitidine. Equine Vet J 1993;25:417–421. [DOI] [PubMed] [Google Scholar]
- 87. Sykes BW, Sykes KM, Hallowell GD. A comparison of two doses of omeprazole in the treatment of EGUS: A blinded, randomised, clinical trial. Equine Vet J 2014;46:416–421. [DOI] [PubMed] [Google Scholar]
- 88. Lancaster‐Smith MJ, Jaderberg ME, Jackson DA. Ranitidine in the treatment of non‐steroidal anti‐inflammatory drug associated gastric and duodenal ulcers. Gut 1991;32:252–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Sykes BW, Sykes KM, Hallowell GD. Administration of once daily trimethoprim‐sulphadimidine does not improve healing of glandular gastric ulceration in horses receiving omeprazole: A randomised, blinded clinical trial. BMC Vet Res 2014;10:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Murray MJ. Diseases of the stomach In: Smith BP, ed. Large Animal Internal Medicine. 3 rd Missouri: Mosby Elsevier; 2009; 695–702. [Google Scholar]
- 91. Hepburn RJ, Proudman CJ Treatment of ulceration of the gastric glandular mucosa: Retrospective evaluation of omeprazole and sucralfate combination therapy in 204 sport and leisure horses [abstract]. Proc 11th International Equine Colic Research Symposium. 2014. 108.
- 92. McClure SR, White GW, Sifferman RL, et al. Efficacy of omeprazole paste for prevention of gastric ulcers in horses in race training. J Vet Int Med 2005;226:1681–1684. [DOI] [PubMed] [Google Scholar]
- 93. Tamzali Y, Desmaizieres IM, Marguet C, et al. Efficacy of omeprazole paste in the prevention of gastric ulcers in high level endurance horses. Proc Am Coll Vet Int Med An Congr. 2012.
- 94. Wong GL‐H, Au KW‐L, Lo AO‐S, et al. Gastroprotective therapy does not improve outcomes of patients with Helicobacter pylori‐negative idiopathic bleeding ulcers. Clin J Gastroenterol Hepatol 2012;10:1124–1129. [DOI] [PubMed] [Google Scholar]
- 95. Koller G, Recknagel S, Spallek A, et al. Magenschleimkonzentration und intragastraler pH‐wert adulter pferde wahrend der nahrungskarenz und nach oraler applikation von Pronutrin. Pferdeheilkunde 2010;26:186–190. [Google Scholar]
- 96. Venner M, Lauffs S. Treatment of gastric lesions in horses with pectin‐lecithin complex. Equine Vet J 1999;29(Suppl 29):91–96. [DOI] [PubMed] [Google Scholar]
- 97. Sykes BW, Sykes KM, Hallowell GD. Efficacy of a combination of Apolectol®, live yeast (CNCM I‐1077) and magnesium hydroxide in the management of Equine Gastric Ulcer Syndrome in thoroughbred racehorses: A randomised, blinded, placebo controlled clinical trial. J Eq Vet Sci 2014;34:1274–1278. [Google Scholar]
- 98. Hellings IR, Larsen S. ImproWin® in the treatment of gastric ulceration of the squamous mucosa in trotting racehorses. Acta Vet Scand 2014;56:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Huff NK, Auer AD, Garza F, et al. Effect of sea buckthorn berries and pulp in a liquid emulsion on gastric ulcer scores and gastric juice pH in horses. J Vet Int Med 2012;26:1186–1191. [DOI] [PubMed] [Google Scholar]
- 100. Clark CK, Merritt AM, Burrow JA, Steible CK. Effect of aluminium hydroxide/magnesium hydroxide antacid and bismuth subsalicylate on gastric pH in horses. J Am Vet Med Assoc 1996;208:1687–1691. [PubMed] [Google Scholar]
- 101. Cargile J, Burrow J, Kim I. Effect of dietary corn oil supplementation on equine gastric fluid acid, sodium, and prostaglandin E2 content before and during pentagastrin infusion. J Vet Int Med 2004;18:545–549. [DOI] [PubMed] [Google Scholar]
- 102. Holbrook TC, Simmons RD, Payton ME, MacAllister CG. Effect of repeated oral administration of hypertonic electrolyte solution on equine gastric mucosa. Equine Vet J 2005;37:501–504. [DOI] [PubMed] [Google Scholar]


