Abstract
Background
The adrenocorticotropic hormone (ACTH) stimulation test is the gold standard for diagnosing hypoadrenocorticism (HA) in dogs. However, problems with the availability of synthetic ACTH (tetracosactrin/cosyntropin) and increased costs have prompted the need for alternative methods.
Objectives
To prospectively evaluate the cortisol‐to‐ACTH ratio (CAR) as a screening test for diagnosing canine HA.
Animals
Twenty three dogs with newly diagnosed HA; 79 dogs with diseases mimicking HA; 30 healthy dogs.
Methods
Plasma ACTH and baseline cortisol concentrations were measured before IV administration of 5 μg/kg ACTH in all dogs. CAR was calculated and the diagnostic performance of ACTH, baseline cortisol, CAR and sodium‐to‐potassium ratios (SPRs) was assessed based on receiver operating characteristics (ROC) curves calculating the area under the ROC curve.
Results
The CAR was significantly lower in dogs with HA compared to that in healthy dogs and in those with diseases mimicking HA (P < .0001). There was an overlap between HA dogs and those with HA mimicking diseases, but CAR still was the best parameter for diagnosing HA (ROC AUC 0.998), followed by the ACTH concentration (ROC AUC 0.97), baseline cortisol concentration (ROC AUC 0.96), and SPR (ROC AUC 0.86). With a CAR of >0.01 the diagnostic sensitivity and specificity were 100% and 99%, respectively.
Conclusion and Clinical Importance
Calculation of the CAR is a useful screening test for diagnosing primary HA. As a consequence of the observed overlap between the groups, however, misdiagnosis cannot be completely excluded. Moreover, additional studies are needed to evaluate the diagnostic reliability of CAR in more dogs with secondary HA.
Keywords: Adrenal insufficiency, Canine, Hormone, Cosyntropin, Adrenocorticotropic
Abbreviations
- ACTH
adrenocorticotropic hormone
- SPR
sodium‐to‐potassium ratio
- CAR
cortisol‐to‐ACTH ratio
- ROC
receiver operating characteristics
- AUC
area under the curves
- HA
hypoadrenocorticism
Hypoadrenocorticism (HA) is an uncommon disease in which adrenocortical steroid hormone secretion falls below the physiological requirement of the animal. Most affected dogs suffer from primary HA. Usually, primary HA results from immune‐mediated destruction of the adrenal cortex, which terminates in absolute deficiency of glucocorticoids and mineralocorticoids. Due to the lack of negative feedback, high serum concentrations of endogenous adrenocorticotropic hormone (ACTH) accompany glucocorticoid deficiency. Secondary HA in dogs is rare and characterized by ACTH deficiency with normal mineralocorticoid, but a lack of glucocorticoid secretion.1
Dogs with HA can present with a range of nonspecific signs.1, 2, 3 The most consistent biochemical abnormalities include azotemia and electrolyte disturbances such as hyponatremia, hyperkalemia, and a low sodium‐to‐potassium ratio (SPR). Hyponatremia and hyperkalemia, although highly suspicious for HA, are neither always present nor pathognomonic for the condition.4, 5, 6, 7, 8
Definitive diagnosis of HA requires an ACTH stimulation test with synthetic ACTH (tetracosactrin or cosyntropin). Recently, the availability of tetracosactrin1 has become limited in Europe and, in some countries, costs of synthetic ACTH have dramatically increased. This situation has prompted a need for alternative tests with which to diagnose HA. Measurement of baseline cortisol concentration has been shown to be a useful screening test to rule out HA.9, 10 The disease is unlikely with a baseline serum cortisol >2 μg/dL (>55 nmol/L).9, 10 However, due to that test's rather low specificity, an ACTH stimulation test must be performed in dogs with a baseline cortisol ≤2 μg/dL to safely exclude HA.9, 10
Calculation of the cortisol‐to‐ACTH ratio (CAR) has been evaluated in previous studies and seems to be a promising tool to diagnose HA.11, 12 The only study that included a group of dogs with nonadrenal disease, however, had a retrospective design, the number of animals per group was relatively low, and no dog with secondary HA was included.12
The purpose of this study was to prospectively evaluate the CAR in a larger group of dogs with the main focus on the comparison of the CAR between dogs with HA and dogs with diseases mimicking HA. Our hypothesis was that CAR would prove to have good diagnostic value in differentiating dogs with HA from those with diseases mimicking HA, but that overlaps would occur. In addition, receiver operating characteristic (ROC) curves of the CAR, plasma ACTH concentration, baseline cortisol concentration and SPR were determined and the area under the curve (AUC) calculated to evaluate which would be the best single laboratory test for diagnosing HA in dogs.
Material and Methods
Animals and Study Design
Twenty three client‐owned dogs with newly diagnosed HA were prospectively enrolled between December 2008 and September 2014. Diagnostic evaluation included CBC, serum biochemical profile, urinalysis, ACTH stimulation test, abdominal ultrasonography with particular attention to the adrenal glands, and measurement of endogenous plasma ACTH concentration in all dogs. Dogs previously treated with glucocorticoids, mineralocorticoids, progestagens, or trilostane were excluded from the study. Hypoadrenocorticism was confirmed by a subnormal ACTH‐stimulated serum cortisol concentration after 60 minutes (<2 μg/dL) in 22 dogs. One dog had a baseline serum cortisol concentration of 2.0 μg/dL and a post‐ACTH cortisol concentration of 2.6 μg/dL. The endogenous ACTH concentration was >1250 pg/mL and the sodium and potassium concentrations were 114 and 8.3 mmol/L, respectively (reference range: sodium, 151–160 mmol/L; potassium, 4.2–5.4 mmol/L). Cross‐reactivity due to prior steroid treatment could be definitively excluded.
Seventy nine dogs with diseases mimicking HA diagnosed between October 2008 and October 2014 were prospectively enrolled in the study. All dogs initially had been suspected of having HA on the basis of clinical signs or laboratory findings routinely seen in dogs with HA, such as vomiting, diarrhea, weakness, lethargy, hyperkalemia, hyponatremia, or some combination of these. An ACTH stimulation test was performed after blood collection to determine plasma ACTH concentration in all dogs and a different final diagnosis was made in each dog. All dogs had an ACTH‐stimulated serum cortisol concentration ≥5 μg/dL.
Ten privately owned dogs and 20 purpose‐bred research dogs (12 mixed breed and 8 Beagles) were used as controls. The dogs were considered to be healthy on the basis of a normal history and physical examination, as well as hematology and serum biochemistry profile results within the normal reference interval. An abdominal ultrasound examination was not included in the diagnostic evaluation of the healthy dogs. Baseline serum cortisol and plasma ACTH concentrations were measured in all dogs. An ACTH stimulation test was performed in all privately owned and all mixed breed research dogs, but not in the 8 research Beagles. Hypoadrenocorticism was excluded either by a post‐ACTH serum cortisol concentration ≥5 μg/dL (in the privately owned dogs and the mixed breed research dogs) or a baseline serum cortisol concentration >2 μg/dL (in the research Beagles).
All procedures were approved by the Cantonal Veterinary Office of Zurich (permission number: 133/2013) and conducted in accordance with guidelines established by the Animal Welfare Act of Switzerland. In addition, informed consent was obtained from the owners of the healthy dogs.
Analytical Procedures
For the ACTH stimulation test, blood samples were taken before and 60 min after IV injection of 5 μg/kg synthetic ACTH.1 Serum cortisol concentrations were measured by chemiluminescence assay.2 Sensitivity of the cortisol assay was 0.2 μg/dL. Plasma endogenous ACTH concentration before ACTH stimulation was determined by a chemiluminescence assay.2 Blood was collected into chilled EDTA‐coated tubes, placed on ice, and centrifuged at 4°C. Cortisol and endogenous ACTH measurements were performed in house twice a week; plasma was stored either at −20°C (cortisol) or at −80°C (ACTH) until assayed.
Statistical Analysis
Statistical analysis was performed using commercial software by using nonparametric tests.3 , 4 , 5 Data are expressed as median and range. Differences between groups were tested by use of the Kruskal–Wallis H‐test and Dunn's post test. The cut‐off for various variables to discriminate between dogs with and without HA was determined by positive/negative likelihood ratios based on ROC curves. Diagnostic efficacy was assessed by calculating the area under the ROC curve (AUC). For results below the detection limit, the mean between 0 and the detection limit was entered for statistical analysis; for ACTH concentrations >1250, 1251 pg/mL was entered for statistical analysis. The level of significance was set at P < .05.
Results
Animals
In the dogs with HA, age ranged from 0.8 to 10 years (median, 5 years) and body weight from 1.9 to 30.4 kg (median, 10.4 kg). There were 10 males (4 castrated) and 13 females (11 spayed). The HA group consisted of 17 purebred dogs and 6 mixed breed dogs. Twenty two dogs were diagnosed with primary HA and 1 dog was diagnosed with secondary HA.
In the dogs with diseases mimicking HA, age ranged from 0.3 to 15 years (median, 4 years) and body weight from 1.7 to 67 kg (median, 24 kg). There were 46 males (23 castrated) and 33 females (20 spayed). This group consisted of 71 purebred dogs and 8 mixed breed dogs. The final diagnoses were acute gastroenteritis (28), chronic intermittent gastroenteritis (9), urinary disease (5), colitis (3), hypothyroidism (3), megaesophagus (2), psychogenic polydipsia (2), pancreatic disease (1), intestinal perforation and septic abdomen (1), stomach overload (1), myasthenia gravis (1), laryngeal paralysis (1), insulinoma (1), muscular dystrophy (1), acetaminophen intoxication (1), and other ambiguous diseases (19).
In the healthy dogs, age ranged from 0.8 to 12 years (median, 1.75 years) and bodyweight from 6.9 to 27 kg (median, 15 kg). There were 13 males (0 castrated) and 17 females (5 spayed). In all, 16 purebred dogs and 14 mixed breed dogs were included.
Baseline Serum Cortisol Concentrations
Results of baseline serum cortisol concentrations in dogs with HA, in those with diseases mimicking HA and in healthy dogs are summarized in Table 1. Baseline serum cortisol concentrations were significantly lower in dogs with HA than in dogs with diseases mimicking HA and in healthy dogs (P < .0001 and P < .0001). There was distinct overlap among the 3 groups (Fig 1). There was no difference between the baseline serum cortisol concentrations of dogs with diseases mimicking HA and healthy dogs (Fig 1).
Table 1.
Median an range of cortisol‐to‐adrenocorticotropic hormone (ACTH)‐ratio (CAR), endogenous ACTH (ACTH), baseline cortisol, and sodium‐to‐potassium ratio (SPR) of 23 dogs with HA, 79 dogs with diseases mimicking HA and in 30 healthy dogs
| Dogs with Hypoadrenocorticism | Dogs with Diseases Mimicking HA | Healthy Dogs | ||||
|---|---|---|---|---|---|---|
| Range | Median | Range | Median | Range | Median | |
| CAR | 0.00016–0.009 | 0.00028 | 0.001–0.37 | 0.076 | 0.03–0.38 | 0.078 |
| ACTH (pg/mL) | 22–>1250 | 945 | <10–1069 | 20 | <10–38 | 18 |
| Baseline cortisol (μg/dL) | <0.2–2 | <0.2 | <0.2–9.3 | 1.5 | 0.6–12 | 1.35 |
| SPR | 13.3–34.6 | 23.6 | 20.7–47.6 | 32.6 | 31.9–37.3 | 34.1 |
Figure 1.
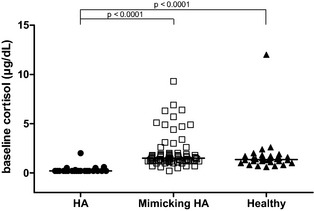
Scatter scale plot comparing baseline serum cortisol concentrations of dogs with hypoadrenocorticism (HA, n = 23), dogs with diseases mimicking HA (mimicking HA, n = 79) and healthy dogs (healthy, n = 30). The horizontal bars represent the median of each group.
Endogenous Plasma Concentrations ACTH (ACTH)
Results of plasma ACTH concentrations in dogs with HA, in those with diseases mimicking HA and in healthy dogs are summarized in Table 1. Plasma ACTH concentrations were significantly higher in dogs with HA than in dogs with diseases mimicking HA and in healthy dogs (P < .0001 and P < .0001). There was no difference between the plasma ACTH concentrations of dogs with diseases mimicking HA and those of healthy dogs (Fig 2).
Figure 2.

Scatter log scale plot comparing plasma adrenocorticotropic hormone (ACTH) concentrations of dogs with hypoadrenocorticism (HA, n = 23), dogs with diseases mimicking HA (mimicking HA, n = 79) and healthy dogs (healthy, n = 30). The horizontal bars represent the median of each group.
Two dogs with HA had plasma ACTH concentrations in the range of healthy dogs or in the range of those with diseases mimicking HA (ACTH concentrations, 22 and 30 pg/mL; Fig 2).
In 1 of the dogs, serum sodium and potassium concentrations were 122 and 7.3 mmol/L, respectively (reference range: sodium, 151–160 mmol/L; potassium, 4.2–5.4 mmol/L). Serum cortisol and aldosterone concentrations before and 1 hour after ACTH stimulation were below the detection limits of the assays. Due to the severe electrolyte abnormalities and undetectable aldosterone concentrations, this dog was diagnosed with primary HA and treated with mineralocorticoid and glucocorticoid supplementation.
In the other dog, serum sodium and potassium concentrations were 147 and 4.3 mmol/L, respectively. Cortisol concentrations (before and 1 hour after ACTH stimulation) were undetectable, baseline aldosterone concentration was low (3.2 pg/mL) and serum renin activity was in the low reference range (0.45 ng/mL/h), which seemed consistent with secondary HA.13 This dog was treated with glucocorticoids only; 1.5 years later the dog was completely stable on glucocorticoid treatment alone.
One dog with disease mimicking HA had a plasma ACTH concentration in the range of dogs with HA (1069 pg/mL, Fig 2). In this dog, serum sodium and potassium concentrations were 130 and 3.5 mmol/L, respectively. The dog finally was diagnosed with acute kidney injury and HA was excluded on the basis of a post‐ACTH serum cortisol concentration of 8.6 μg/dL.
Cortisol‐to‐ACTH Ratio
Results of the CAR of dogs with HA, those with diseases mimicking HA and all healthy dogs are summarized in Table 1. The CAR was significantly lower in dogs with HA compared to dogs with diseases mimicking HA and healthy dogs (P < .0001 and P < .0001). There was no difference between the CAR of dogs with diseases mimicking HA and healthy dogs (Fig 3). One dog in the group with diseases mimicking HA had a CAR in the range of the HA dogs (Fig 3). This dog was the 1 described above with the markedly increased plasma ACTH concentration and a final diagnosis of acute kidney injury.
Figure 3.
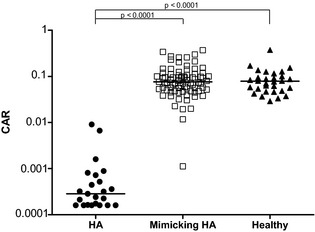
Scatter log scale plot comparing cortisol‐to‐adrenocorticotropic hormone (ACTH) ratios of dogs with hypoadrenocorticism (HA, n = 23), dogs with diseases mimicking HA (mimicking HA, n = 79) and healthy dogs (healthy, n = 30). The horizontal bars represent the median of each group.
Sodium‐to‐Potassium Ratio
Results of the SPR ratio in dogs with HA, those with diseases mimicking HA and healthy dogs are summarized in Table 1. The SPR ratio was significantly lower in dogs with HA than in dogs with diseases mimicking HA and healthy dogs (P < .0001 and P < .0001), but considerable overlap was observed. There was no difference between the SPR of dogs with diseases mimicking HA and healthy dogs (Fig 4).
Figure 4.
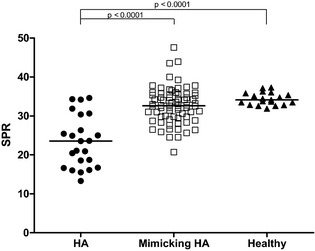
Scatter scale plot comparing sodium‐to‐potassium ratios of dogs with hypoadrenocorticism (HA, n = 23), dogs with diseases mimicking HA (mimicking HA, n = 79) and healthy dogs (healthy, n = 30). The horizontal bars represent the median of each group.
Of the 23 dogs with HA, 3 dogs had both serum sodium and potassium concentrations within the normal reference range, 16 dogs had hyponatremia combined with hyperkalemia and 4 dogs had hyponatremia (1 with secondary HA).
Serum sodium and potassium concentrations at initial presentation were available in 71 dogs with diseases mimicking HA. Forty one dogs had both electrolytes within the normal reference range, 3 dogs had hyponatremia combined with hyperkalemia, 20 dogs had hyponatremia, and 7 dogs hyperkalemia.
ROC Analysis
The AUC of the ROC was largest for the CAR, followed by ACTH, baseline cortisol and SPR (Fig 5). Table 2 shows the AUCs and their 95% confidence intervals. The AUC of CAR and ACTH were significantly larger than the AUC of SPR (P = .006 and P = .04) (Table 2). There was no significant difference between the AUC of baseline cortisol and SPR. In addition, the AUC of CAR, ACTH, and baseline cortisol did not significantly differ from 1 another.
Figure 5.
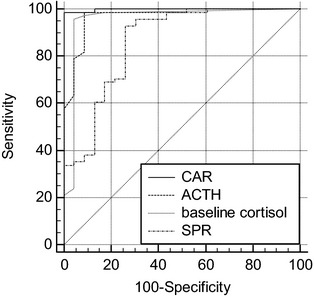
Receiver operating characteristic curves of cortisol‐to‐adrenocorticotropic hormone (ACTH) ratio (CAR), ACTH, baseline cortisol and sodium‐to‐potassium ratio (SPR) for the prediction of hypoadrenocorticism (HA). The more closely the curve follows the left and top borders of the receiver operating characteristic (ROC) space, the more accurate is the test.
Table 2.
Area under the receiver operating characteristic curves (AUC) and 95 per cent confidence interval of the cortisol‐to‐ACTH ratio (CAR), ACTH concentration, baseline cortisol and sodium‐to‐potassium ratio (SPR)
| Value | AUC | 95% CI |
|---|---|---|
| CARa | 0.998 | 0.958–1.000 |
| ACTHa | 0.97 | 0.910–0.993 |
| Baseline cortisola , b | 0.96 | 0.894–0.988 |
| SPRb | 0.86 | 0.775–0.924 |
a,bDifferent letters indicate statistically significant difference between groups (P < .05).
Maximizing both the sensitivity and the specificity of the variables yielded cut‐off values of >0.01 for the CAR and >2 μg/dL for baseline cortisol to exclude HA and >178 pg/mL for ACTH and ≤24 for SPR to confirm HA. Table 3 shows the cut‐off values for each of the variables as well as their diagnostic sensitivity and specificity.
Table 3.
Cut‐off values and maximized sensitivity and specificity to exclude (CAR, baseline cortisol) or confirm (ACTH, SPR) HA derived from the two‐graph receiver operating characteristic analysis
| Value | Cut‐off Value | Sensitivity (%) | 95% CI | Specificity (%) | 95% CI |
|---|---|---|---|---|---|
| CAR | >0.01 | 100 | 85–100 | 99 | 93–100 |
| Baseline cortisol (μg/dL) | >2 | 100 | 85–100 | 20 | 13–32 |
| ACTH (pg/mL) | >178 | 91 | 72–99 | 99 | 93–100 |
| SPR | ≤24 | 56 | 35–77 | 99 | 93–100 |
Discussion
Dogs with HA had a significantly lower CAR than did dogs with diseases mimicking HA and healthy dogs. This result is in accordance with previous observations.11, 12 However, in those 2 previous studies, there was no overlap between the different groups, making CAR a more than ideal test for diagnosing HA. In contrast, our study showed an overlap of CAR values among the different groups. In a previous study, dogs with HA only were compared to healthy dogs (ie, a group of dogs with nonadrenal disease was not included).11 Another study included dogs with nonadrenal disease, but the number of animals per group was lower than in our study.12 In addition, no dog with secondary HA was included, although many of their dogs with HA did not have electrolyte abnormalities. In one‐third of the dogs in their study, plasma ACTH concentration was not determined at the same time as baseline serum cortisol concentration or ACTH stimulation test. Instead, plasma was collected 12–24 hours after stimulation. Our study was designed prospectively and standardization of sample collection was a prerequisite (inclusion criteria).
Despite some overlap of the CAR among the different groups in this study, CAR still was the single best test for diagnosing HA with a diagnostic sensitivity of 100% and a specificity of 99%, using a CAR cut‐off ratio of >0.01. With this cut‐off, only 1 dog would have been falsely diagnosed with HA (CAR, 0.001). This dog had a very high plasma ACTH concentration (1069 pg/mL) and without results of the ACTH stimulation test would have been misdiagnosed with HA and have received lifelong treatment. However, HA was reliably excluded based on a normal ACTH stimulation test and on follow‐up of 1.5 years without any clinical signs compatible with HA. Explaining the markedly increased plasma ACTH concentration in this dog is difficult. The dog appeared normal at presentation and did not seem overtly stressed. One speculative explanation could be the administration of a short‐acting corticosteroid (eg, prednisolone succinate, hydrocortisone) just days before referral by a private veterinarian. Cessation of cortico‐steroids can lead to a rebound increase in ACTH. Although plasma ACTH concentrations observed in experimental Beagle dogs treated with hydrocortisone were increased after cessation of treatment, their concentrations did not reach the magnitude of ACTH concentration observed in our dog (Schellenberg S, Glaus T.M., Reusch C. E. unpublished data).
Two dogs with HA had a high CAR (0.009 and 0.0067) due to rather low ACTH concentrations (22 and 30 pg/mL). One of these dogs was presented during an hypoadrenal crisis with severe electrolyte abnormalities (serum sodium concentration, 122 mmol/L; serum potassium concentration, 7.6 mmol/L). Approximately 5 months later, the dog was presented again, severely sick and with serum electrolyte abnormalities (serum sodium concentration, 143 mmol/L; serum potassium concentration, 10.8 mmol/L) because the owner had discontinued the dog's mineralocorticoid and glucocorticoid supplementation. The dog therefore was diagnosed with primary HA (undetectable serum cortisol and aldosterone concentrations). Glucocorticoid treatment with ongoing effect by the referring veterinarian could have suppressed the ACTH concentration, but this could be reliably excluded. Improper sample handling leading to degradation of ACTH could be another explanation. However, because our sample handling for ACTH measurement is standardized (eg, rapid processing, continuous cooling immediately after blood sampling) this possibility seems unlikely. The other dog with a high CAR was diagnosed with secondary HA. This dog had a mild hyponatremia (serum sodium concentration, 147 mmol/L; most likely caused by sodium loss as a result of progressive vomiting for 1 week) and 1.5 years later was completely stable on glucocorticoid treatment alone.
In a recent study, in which the CAR was evaluated as a diagnostic test for HA, no dog with secondary HA was included.12 The authors speculated that the CAR values would be expected to be much higher than those of dogs with primary HA. At least for the 1 dog of our study with secondary HA this speculation seems correct. However, the CAR of the 1 dog with secondary HA in our study still was lower than the CAR results of healthy dogs or dogs with diseases mimicking HA. Very few cases of secondary HA in which ACTH concentrations were measured could be identified in the veterinary literature.1, 14, 15, 16 In only 1 of these cases could the CAR be calculated, because in the others either baseline serum cortisol concentrations of 1 individual dog could not be extracted from their results or plasma ACTH concentration was reported to be 0. In the 1 other dog with secondary HA, baseline serum cortisol concentration was 0.21 μg/dL and plasma ACTH concentration was 9.9 pg/mL, resulting in a CAR of 0.02.16 In our study, this CAR value would fall into the CAR range of dogs with diseases mimicking HA. In the previous study, this CAR would be in the CAR range of dogs with HA.12 These findings emphasized 2 important points: first, overlap between CAR results of dogs with HA (especially dogs with secondary HA) and of dogs with diseases mimicking HA can occur, which decreases the reliability of the CAR to diagnose HA; and, second, care should be taken in adopting CAR results from the literature for the diagnosis of HA. Comparing CAR results from 1 study to another shows striking differences; Upper values of dogs with HA in the 2 published studies were 0.17 and 0.03111, 12 compared to 0.009 in our study. It might be reasonable to assume that the most likely explanation for the observed discrepancies is the different assay systems (radioimmunoassay versus chemiluminescence assay) used for the determination of ACTH and cortisol. Interestingly, the same chemiluminescence assay (Immulite) was used in 1 previous study as in our study and, even so, CAR results were approximately 3.5 times higher in the previous study.12 Adopting a published CAR value can lead to decreased sensitivity and a missed diagnosis, which can be potentially fatal in dogs with HA. Consequently, clinician should be very careful when comparing values among different studies. It would be advisable to establish reference values not only for each assay system (cortisol and ACTH) but also for each laboratory that offers the assays. Furthermore, the reliability of CAR to diagnose secondary HA must be evaluated in a larger group of dogs.
Determination of baseline serum cortisol concentration has a high sensitivity (100% if >2 μg/dL) to exclude HA, but a low specificity of only 63.3–78.2%.9, 10 In our study, although having the same sensitivity of 100%, its specificity was only 20%. Median baseline serum cortisol concentrations were 1.35 μg/dL in the healthy dogs and 1.5 μg/dL in those with disease mimicking HA, meaning that at least 50% of our control population (healthy dogs and dogs with diseases mimicking HA) had baseline serum cortisol concentrations below the suggested optimal cut‐off of 2 μg/dL. Several explanations must be taken into account. First, we had a rather high percentage of experimental dogs in the healthy controls. They were adapted to blood sampling and blood was not taken in the hospital but in their natural environment (research facility). The most plausible explanation for the observed rather low baseline serum cortisol concentrations in the healthy and nonadrenal disease group seems to be the difference in the assay system used, as discussed above. Wide variations in cortisol concentrations can occur for the same sample measured by different laboratories and even using the same commercially available assay (Extended Quality Assessment of the Society for Comparative Endocrinology [SCE] and the European Society for Veterinary Endocrinology [ESVE], unpublished data).
Determination of ACTH in the diagnosis of HA is mainly used in dogs with normal serum electrolyte concentrations to differentiate primary from secondary HA. Although in primary HA plasma ACTH concentration would be expected to be increased, in secondary HA concentrations within or even below the reference range would confirm the diagnosis. This variable also has been evaluated as a screening test for HA; but, overlap between the groups (HA, diseases mimicking HA) is more pronounced than with the CAR. One study, using plasma ACTH concentration of >50 pg/mL as the best calculated cut‐off, resulted in a sensitivity of 96% and a specificity of 100%.16 Evaluating plasma ACTH concentration as single variable in our study also showed overlap among the groups, resulting in a sensitivity of only 91% and a specificity of 99% using the best calculated cut‐off concentration of >178 pg/mL. The main limitation of ACTH measurement in practice is the instability of the hormone. To avoid degradation blood must be collected in precooled EDTA plastic tubes, processed immediately without cessation of cooling and frozen until analysis. This procedure is time‐consuming and cost‐intensive. However, depending on cost development and availability of synthetic ACTH, determination of ACTH may become practical for practitioners in the future.
Although a low SPR is highly suspicious for HA, there are several other conditions such as Trichuris vulpis infection, other gastrointestinal problems, renal, or cardiorespiratory problems and ascites that also are associated with hyperkalemia and concurrent hyponatremia.4, 5, 6, 7, 8 In our study, using the best calculated cut‐off ratio of ≤24 resulted in a specificity of 99% and a sensitivity of only 56% for diagnosing HA. Compared to a recent study16 that showed a sensitivity of 92% and a specificity of 91% with a best calculated cut‐off of ≤22, our sensitivity is markedly lower. Several earlier studies have evaluated SPR as a diagnostic test in dogs in which HA is suspected.1, 17, 18 In none of them was there convincing evidence that SPR could be a reliable test, either to confirm or exclude the diagnosis. Not all dogs with HA have electrolyte abnormalities and only a minority of dogs with decreased SPR do indeed have HA.1, 17
In conclusion, determination of CAR in dogs suspected of primary HA is a valuable and reliable tool with which to exclude or confirm the diagnosis. The greatest advantage is the need for only a single blood sample from which both serum cortisol and plasma ACTH concentrations can be measured. Unlike findings in a previous study, differentiation between dogs with HA and those with diseases mimicking HA was not 100%, based on the present results. In addition, the reliability of the CAR in diagnosing secondary HA needs to be evaluated in future.
Acknowledgments
The authors thank colleagues at the Clinic for Small Animal Internal Medicine, Vetsuisse Faculty University of Zurich for contributing cases.
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Footnotes
Synacthen®, Novartis Pharma Schweiz AG, Bern, Switzerland
DPC Immulite® 1000, Siemens Schweiz AG, Zurich, Switzerland
GraphPad Prism6, GraphPad Software, San Diego, CA, USA
SPSS 20.0 for Windows, SPSS Inc, Chicago, IL, USA
MedCalc Version 15.4, MedCalc Software bvba, Ostend, Belgium
References
- 1. Thompson AL, Scott‐Moncrieff JC, Anderson JD. Comparison of classic hypoadrenocorticism with glucocorticoid‐deficient hypoadrenocorticism in dogs: 46 cases (1985–2005). J Am Vet Med Assoc 2007;230:1190–1194. [DOI] [PubMed] [Google Scholar]
- 2. Melian C, Peterson ME. Diagnosis and treatment of naturally occurring hypoadrenocorticism in 42 dogs. J Small Anim Pract 1996;37:268–275. [DOI] [PubMed] [Google Scholar]
- 3. Peterson ME, Kintzer PP, Kass PH. Pretreatment clinical and laboratory findings in dogs with hypoadrenocorticism: 225 cases (1979–1993). J Am Vet Med Assoc 1996;208:85–91. [PubMed] [Google Scholar]
- 4. Bissett SA, Lamb M, Ward CR. Hyponatremia and hyperkalemia associated with peritoneal effusion in four cats. J Am Vet Med Assoc 2001;218:1590–1592, 1580. [DOI] [PubMed] [Google Scholar]
- 5. Pak SI. The clinical implication of sodium‐potassium ratios in dogs. J Vet Sci 2000;1:61–65. [PubMed] [Google Scholar]
- 6. Roth L, Tyler RD. Evaluation of low sodium:potassium ratios in dogs. J Vet Diagn Invest 1999;11:60–64. [DOI] [PubMed] [Google Scholar]
- 7. Ruckstuhl N, Hoerauf A, Tomsa K, et al. [Pseudohypoadrenocorticism in two Siberian huskies with gastrointestinal parasitoses]. Schweiz Arch Tierheilkd 2002;144:75–81. [DOI] [PubMed] [Google Scholar]
- 8. Schaer M, Halling KB, Collins KE, et al. Combined hyponatremia and hyperkalemia mimicking acute hypoadrenocorticism in three pregnant dogs. J Am Vet Med Assoc 2001;218:897–899. [DOI] [PubMed] [Google Scholar]
- 9. Bovens C, Tennant K, Reeve J, et al. Basal serum cortisol concentration as a screening test for hypoadrenocorticism in dogs. J Vet Intern Med 2014;28:1541–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lennon EM, Boyle TE, Hutchins RG, et al. Use of basal serum or plasma cortisol concentrations to rule out a diagnosis of hypoadrenocorticism in dogs: 123 cases (2000–2005). J Am Vet Med Assoc 2007;231:413–416. [DOI] [PubMed] [Google Scholar]
- 11. Javadi S, Galac S, Boer P, et al. Aldosterone‐to‐renin and cortisol‐to‐adrenocorticotropic hormone ratios in healthy dogs and dogs with primary hypoadrenocorticism. J Vet Intern Med 2006;20:556–561. [DOI] [PubMed] [Google Scholar]
- 12. Lathan P, Scott‐Moncrieff JC, Wills RW. Use of the cortisol‐to‐ACTH ratio for diagnosis of primary hypoadrenocorticism in dogs. J Vet Intern Med 2014;28:1546–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baumstark ME, Nussberger J, Boretti FS, et al. Use of plasma renin activity to monitor mineralocorticoid treatment in dogs with primary hypoadrenocorticism: desoxycorticosterone versus fludrocortisone. J Vet Intern Med 2014;28:1471–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Platt SR, Chrisman CL, Graham J, et al. Secondary hypoadrenocorticism associated with craniocerebral trauma in a dog. J Am Anim Hosp Assoc 1999;35:117–122. [DOI] [PubMed] [Google Scholar]
- 15. Scott‐Moncrieff JC. Hypoadrenocorticism In: Feldman EC, Nelson RW, Reusch CE, Scott‐Moncrieff JC, eds. Canine and Feline Endocrinology, 4th ed St. Louis, Missouri; Elsevier Saunders; 2015:485 ff. [Google Scholar]
- 16. Zeugswetter FK, Schwendenwein I. Diagnostic efficacy of the leukogram and the chemiluminometric ACTH measurement to diagnose canine hypoadrenocorticism. Tierarztl Prax Ausg K Kleintiere Heimtiere 2014;42:223–230. [PubMed] [Google Scholar]
- 17. Nielsen L, Bell R, Zoia A, et al. Low ratios of sodium to potassium in the serum of 238 dogs. Vet Rec 2008;162:431–435. [DOI] [PubMed] [Google Scholar]
- 18. Seth M, Drobatz KJ, Church DB, et al. White blood cell count and the sodium to potassium ratio to screen for hypoadrenocorticism in dogs. J Vet Intern Med 2011;25:1351–1356. [DOI] [PubMed] [Google Scholar]


