Abstract
Background
The effect of feeding a limited iodine diet on radioactive iodine uptake in the thyroid glands of hyperthyroid cats is unknown.
Objectives
To determine how feeding limited dietary iodine affects radioactive iodine uptake by the thyroid glands of hyperthyroid cats.
Animals
Eight geriatric cats with spontaneous hyperthyroidism.
Methods
Prospective study of eight client owned hyperthyroid cats fed a commercially available iodine limited diet for 6 months. Clinical signs were evaluated and TT4 and fT4 were measured during consumption of the diet. Uptake of 123I was determined before and 8–16 weeks after exclusive consumption of the diet.
Results
Clinical signs of hyperthyroidism resolved in all cats, but there was no significant increase in body weight. TT4 and fT4 decreased into the reference range by 8–16 weeks in all cats. Mean TT4 before consumption of the diet was 9.7 μg/dL (SD 5.2) and after consumption of the diet was 3.1 μg/dL (SD 0.9). Scintigraphy revealed unilateral uptake of isotope in 5 cats and bilateral uptake in 3 cats. Mean percentage uptake of 123I by the thyroid gland at 8 hours after isotope administration was 16.2 (SD 11.8) before diet consumption and 34.6 (SD 11.7) 8–16 weeks after exclusive consumption of the diet. The percentage increase was variable between cats (38–639%).
Conclusions and clinical importance
Limited iodine diets increase iodine uptake in the autonomous thyroid glands of hyperthyroid cats. Further studies are necessary to determine if consumption of a limited iodine diet changes sensitivity of the thyroid gland to 131I treatment.
Keywords: 123Iodine, 131Iodine, Nuclear scintigraphy, Thyroid hormone, Thyroxine
Abbreviations
- 131I
131iodine
- 123I
123iodine
- TT4
total thyroxine
- fT4
free thyroxine
- RAIU
radioactive iodine uptake
Hyperthyroidism in cats is a common endocrine disease of geriatric cats and is the result of autonomous secretion of thyroxine (T4) and triiodothyronine (T3) by the thyroid gland.1 Treatment options include administration of oral antithyroid drugs (methimazole or carbimazole), thyroidectomy, or administration of 131iodine (131I). Exclusive consumption of a diet containing less than 0.3 mg/kg of iodine (or iodide in its iodized form) decreases thyroid hormone concentrations into the reference range and improve clinical signs in hyperthyroid cats.2, 3, 4, 5, 6 A commercial diet containing 0.2 mg iodine/kg is now marketed as an alternative therapeutic approach to management of hyperthyroidism in cats; however, the physiologic and endocrine changes that occur in hyperthyroid cats fed limited iodine diets have not been reported in detail. Diets containing less than 0.46 mg iodine/kg of diet increase iodine uptake into the thyroid gland of healthy cats,7 but the effect of limited dietary iodine on radioactive iodine uptake (RAIU) in the thyroid gland of hyperthyroid cats is unknown. If limited iodine diets also increase iodine uptake into an autonomous thyroid gland, this could change the sensitivity of the thyroid gland to treatment with 131I and increase the risk of hypothyroidism after treatment. The hypothesis of this study was that exclusive feeding of a diet containing 0.2 mg iodine/kg diet decreases thyroid hormone concentrations and increases iodine update into the thyroid gland in hyperthyroid cats. The specific aim of the study was to determine thyroidal 123iodine (123I) uptake of hyperthyroid cats before and after feeding an iodine limited diet.
Materials and Methods
Study Population
Adult cats with spontaneous hyperthyroidism that presented to Purdue University Veterinary Teaching Hospital (PUVTH) between June 2012 and May 2013 were enrolled in the study. The study was approved by the Purdue University Animal Care and Use Committee.
Cats were included in the study if they had spontaneous hyperthyroidism, had a total thyroxine (TT4) concentration of >4.6 μg/dL, and if informed consent had been obtained from the owner. Cats that had been previously treated for hyperthyroidism, cats with serious concurrent illness, and cats suspected to have thyroid carcinoma were excluded from the study.
Study Design
At the time of enrollment, a history and physical examination were obtained for all cats, and blood was collected to obtain a complete blood count (CBC), biochemical profile, urinalysis, TT4, and free thyroxine (fT4) concentrations. Thoracic radiographs were performed before study enrollment, to rule out intrathoracic neoplasia or other concurrent illness that could impact anesthetic safety. After study enrollment, nuclear scintigraphy was performed in each cat after oral administration of 300 μCi of 123I. The pulse height analyzer of the gamma camera was centered over the 159 keV photopeak of 123I. Before oral administration, the two capsules containing 123I (one capsule containing 200 μCi and one capsule containing 100 μCi) were placed on the gamma camera and an image of 200,000 counts was acquired. Isocontour regions of interest over the capsules were acquired to determine the activity of 123I administered expressed as counts per minute.
Each cat was anesthetized 8 and 24 hours after administration of the isotope for nuclear imaging. Cats were premedicated with butorphenol 0.2 mg/kg IM and isoflurane was used for chamber induction and maintenance of general anesthesia during scintigraphy. RAIU was measured 8 and 24 hours after 123I administration using a low energy high resolution collimator to acquire a static planar ventral image of 200,000 counts with the cat in sternal recumbency over the gamma camera. Isocontour regions of interest were drawn around the thyroid gland (one or two lobes depending upon whether uptake was unilateral or bilateral) of the ventral images acquired by the gamma camera. The time taken to acquire 200,000 counts for each image was recorded by the nuclear imaging software and used to calculate the counts per minute. RAIU for each scan was calculated as the percentage of the administered dose present in the thyroid gland(s) corrected for physical decay (see below). Background correction was performed using background regions of interest drawn in the soft tissue adjacent to the thyroid gland. Soft tissue attenuation correction was not performed because of the superficial location of the thyroid gland.
After recovery from anesthesia, each cat was transitioned to an iodine limited diet (0.2 mg/kg iodine) over 7 days.1 Owners were informed of the necessity for complete compliance with exclusive feeding of the diet. Cats were reevaluated 4 and 8 weeks after complete diet transition and a history and physical examination were performed. Blood samples were collected to obtain a CBC, serum chemistry profile, urinalysis, and TT4 and fT4 concentrations to assess response to the diet. If TT4 and fT4 concentrations were both within the reference range at 8 weeks after diet transition, nuclear scintigraphy was repeated as described above. If the TT4 and fT4 concentrations were above the reference range at 8 weeks, the reason for the poor response was evaluated and thyroid testing was repeated in an additional 4 weeks of the cats consuming the iodine limited diet. The second scintigraphic procedure was performed after the euthyroid state had been established for at least 4 weeks in all cats. In three cats that were transitioned to a different treatment for their hyperthyroidism after completion of the study, scintigraphy was repeated 2 weeks after diet withdrawal.
Calculation of Radioactive iodine uptake
The percentage uptake of 123I into the thyroid gland at 8 and 24 hours after administration was calculated using the following formula:
Hormone Assays
TT4 and fT4, were measured using chemiluminescent enzyme immunoassays validated for use in the cat.1 , 2 , 3 , 4
Statistical Analysis
Variables that were compared between study entry and 4 weeks after normalization of the thyroid hormone concentrations included TT4, fT4, blood urea nitrogen (BUN), and creatinine concentrations, body weight, RAIU at 8 hours and RAIU at 24 hours. The normality assumption was evaluated using the Kolmogorov–Smirnov test. If the assumption was not violated, paired t‐tests were used for comparisons. If the normality assumption was violated, Wilcoxon tests were used. Pearson's correlation coefficient was used to assess the association between TT4 at time of entry into the study and uptake at 8 and 24 hours before and after diet, if the association was linear (visually inspected). If not, Spearman's correlation coefficient was used. For fT4 values that were reported as >6 ng/dL, the value of 6 was used for statistical calculations, and for values reported as <0.01 ng/dL, the value of 0.005 was used. Statistical significance was set at P < .05.
Results
Eight cats were enrolled in the study. The cats ranged in age from 12 to 19 years (median 13 years). Six cats were domestic shorthairs, one was a domestic longhair, and one was a Maine Coon. Three were castrated males and 5 were spayed females. Clinical signs of hyperthyroidism included weight loss in 7 cats, polyphagia in 4 cats, polyuria–polydipsia in 2 cats, alopecia in 2 cats, vomiting in 2 cats, increased activity in 2 cats and decreased activity in 1 cat. Physical examination findings included an enlarged thyroid lobe or lobes in all 8 cats, otitis externa in 2 cats, a healed aural hematoma in one cat, an unkempt hair coat in 2 cats, tachycardia in one cat, a systolic heart murmur in one cat and a gallop rhythm in one cat. One cat had hypertension which was being treated with amlodipine at the time of entry into the study. Laboratory abnormalities were consistent with hyperthyroidism. Scintigraphy revealed unilateral uptake in 5 cats and bilateral 123I thyroidal uptake in three cats.
All evaluated variables were normally distributed, so paired t‐tests were used for all comparisons. TT4 and fT4 concentrations before and after feeding the iodine limited diet are shown in Table 1.
Table 1.
TT4, fT4, BUN, creatinine, and body weight in 8 hyperthyroid cats before and after exclusive feeding of an iodine limited diet (mean, SD)
| Before | After | Reference Range | P | |
|---|---|---|---|---|
| TT4 (μg/dL) | 9.7 (5.2) | 3.1 (0.9) | 2.5–4.6 | .008 |
| fT4 (ng/dL) | 4.7 (1.2) | 1.7 (0.5) | 0.7–2.6 | <.001 |
| BUN (mg/dL) | 22.3 (6.6) | 24.0 (6.3) | 15–35 | .31 |
| Creatinine (mg/dL) | 0.9 (0.3) | 0.9 (0.2) | 0.9–2.3 | .52 |
| Body weight (kg) | 4.4 (1.1) | 4.5 (1.0) | .45 |
TT4, Total thyroxine; fT4, Free thyroxine; BUN, blood urea nitrogen.
After 4 weeks of consuming the limited iodine diet, 6 of 8 cats had thyroid hormone concentrations within the reference range. After 8 weeks, 7 of 8 cats had thyroid hormone concentrations within the reference range. Clinical signs of hyperthyroidism had resolved in all cats, but there was no significant gain in body weight (Table 1). In 6 of the 7 cats that had thyroid hormone concentrations within the reference range at 8 weeks, nuclear imaging was performed at this time. In the cat that had increased thyroid hormone concentrations at 8 weeks and in another cat that relapsed because of poor owner compliance before scheduling of the second nuclear scan, imaging was performed at 12 and 16 weeks after entry into the study, respectively, once the TT4 and fT4 concentrations had normalized.
Before consumption of the iodine limited diet, percentage uptake of 123I, 8 hours after isotope administration, ranged from 3.1 to 36.4%. Percentage uptake at 8 hours during consumption of the iodine limited diet ranged from 22.5 to 57.8%. This was significantly higher (P < .001) than before consumption of the diet (Table 2, Fig. 1). Before consumption of the iodine limited diet, percentage uptake of 123I, 24 hours after isotope administration, ranged from 5.2 to 41.9%. Percentage uptake during consumption of the iodine limited diet ranged from 17.1 to 66.3%. This was not statistically higher than before consumption of the diet (P = .151, Table 2, Fig. 2). The mean within‐pair difference for 8‐hour and 24‐hour uptake were 18% (13–24%) and 12% (95% CI −6 to 30%), respectively. Five of the 8 cats had an increase in uptake 24 hours after isotope administration during consumption of the iodine limited diet whereas 3 cats had decreased iodine uptake 24 hours after isotope administration during consumption of the diet. These cats had the highest pre‐diet uptake (>30% in all 3 cats, Fig. 2).
Table 2.
Percentage uptake of 123iodine at 8 and 24 hours in 8 hyperthyroid cats before and 8–16 weeks after exclusive consumption of an iodine limited diet (mean, SD)
| Before | After | Mean Change and 95% CI | P Value | |
|---|---|---|---|---|
| Percentage 123iodine uptake at 8 hour scan | 16.2 (11.8) | 34.6 (11.7) | 18 (95% CI 13–24) | <.001 |
| Percentage 123iodine uptake at 24 hour scan | 22.5 (13.2) | 34.5 (14.2) | 12 (95% CI −6–30) | .15 |
Figure 1.
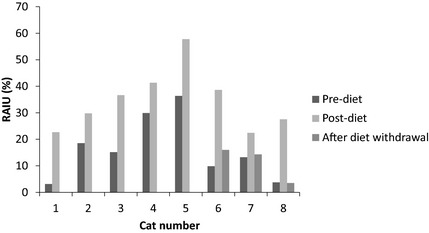
Eight hour 123iodine scan. Uptake of 123iodine (RAIU) 8 hours after radioisotope administration in 8 cats before and 8–16 weeks after exclusive consumption of a diet containing 0.2 mg/kg iodine. In three cats, uptake of 123iodine is also shown 2 weeks after diet withdrawal.
Figure 2.
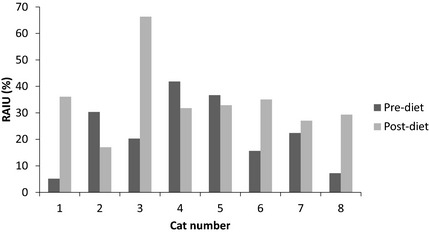
Twenty four hour 123iodine scan. Uptake of 123iodine (RAIU) 24 hours after radioisotope administration in 8 cats before and 8–16 weeks after exclusive consumption of a diet containing 0.2 mg/kg iodine.
The percentage change in 123I uptake at 8 hours ranged from 38 to 639% (median 106%) and the percentage change at 24 hours ranged from −44 to 600% (median 73%). There was no significant correlation between baseline TT4 concentration and change in percentage uptake of 123I before or after the diet at either 8 hours (Spearman's rho = −0.31, P = .46) or 24 hours (Spearman's rho = −0.05, P = .91).
All cats in the study were maintained on the iodine limited diet for at least 6 months and owners reported a good quality of life during this time. Four cats were managed long term with the diet. Definitive treatment (thyroidectomy in 3 cats, radioactive iodine treatment in one cat) was performed 7–17 months after starting the diet in 4 cats. In 3 of these cats, scintigraphy was repeated 2 weeks after diet withdrawal (8 hour scans only) before thyroidectomy or radioactive iodine treatment. Iodine uptake in these three cats after diet withdrawal decreased close to the pre‐diet uptake (Fig. 1). The one cat in which the uptake after diet withdrawal was still slightly higher than baseline, had been consuming the iodine limited diet for 17 months before diet withdrawal (percentage uptake at 8 hours pre‐diet 9.9%, post‐diet 39%, after diet withdrawal 16%).
Discussion
In this study, it was demonstrated that in hyperthyroid cats exclusively fed an iodine limited diet, mean percentage uptake of 123I by the thyroid gland at 8 hours after isotope administration significantly increased from baseline values after consumption of the iodine limited diet for 8–16 weeks; however, the percentage increase at 24 hours after isotope administration was not significantly different before and after consumption of the diet. Clinical signs of hyperthyroidism improved, and thyroid hormone concentrations (TT4 and fT4) normalized during consumption of the diet.
The increased uptake of 123I after iodine restriction in all 8 cats confirmed that autonomously functioning thyroid follicular cells of hyperthyroid cats respond to iodine restriction in a similar way to healthy cats. The increased uptake of iodine was only statistically significant 8 hours after isotope administration. Early thyroid uptake of iodine reflects iodine trapping and subsequent organic binding, whereas iodine uptake at 24 hours and thereafter is increasingly influenced by the rate of discharge of iodine from the thyroid gland.8 The lack of a statistically significant increase in isotope uptake at 24 hours after isotope administration may reflect type II error because the mean differences in uptake were not that different between 8‐hour uptake and 24‐hour uptake (Table 2). Whereas 5 of 8 cats in the study had increased iodine uptake 24 hours after isotope administration, a decrease in iodine uptake was observed in the other 3 cats. These 3 cats had the highest pre‐diet iodine uptake (Fig. 2). The reason for the decreased uptake at 24 hours in some cats is unknown, but may reflect an increased rate of discharge of iodine from the thyroid gland. Discharge rate of iodothyronines from the thyroid gland is increased in hyperthyroidism in humans and cats.8, 9 Accelerated iodine turn‐over has been attributed to a small thyroid iodine pool and possibly very high levels of thyroid stimulation.9 In a study of 20 hyperthyroid cats administered 20 μCi 131I, discrimination between euthyroid and hyperthyroid cats was better at 4 hours than at 24 hours after isotope administration and at 24 hours, there was some overlap with the uptake of healthy cats.10 In some individual cats, thyroidal uptake declined into the range observed in euthyroid individuals by 24 hours after isotope administration.8
The percentage increase in 123I uptake at 8 and at 24 hours, both before and after consumption of the iodine limited diet was highly variable between cats, and there was no correlation between the baseline TT4 concentration and percentage change in 123I uptake. Thus, it would be difficult to predict the effect of consumption of the iodine limited diet on the uptake of 131I in an individual hyperthyroid cat without performing nuclear scintigraphy in each patient.
Although clinical signs of hyperthyroidism improved and thyroid hormone concentrations normalized during consumption of the iodine limited diet, the body weight of the eight cats did not increase after starting the diet. This finding is similar to that of a larger study of 49 hyperthyroid cats managed with an iodine limited diet.10 There are two of potential explanations for this lack of change in body weight. The first is that despite normalization of serum TT4 concentrations, some of the cats in this study might have still been physiologically hyperthyroid. In humans, documentation of a low TSH in the face of a TT4 within reference range supports physiological hyperthyroidism, but this was not assessed in this study because of the poor sensitivity of currently available assays for TSH in cats. Another possibility is that undiagnosed concurrent illness in these geriatric cats might have also influenced their body weight.10 This seems less likely since none of the cats developed other signs of concurrent illness during the 6 months of follow‐up.
In human patients, low iodine diets are utilized before radioactive iodine scans or treatment of well‐differentiated thyroid cancer to optimize radioactive iodine uptake.11 The typical duration of restriction is 1–4 weeks.11 A longer duration of iodine restriction was used in this study, because normalization of TT4 was considered to be the best method of confirming owner compliance with feeding of the iodine limited diet. If the study had failed to show an increase in iodine uptake, this could have been blamed on poor compliance with the diet. In addition, in these cats, the diet was being used as a long‐term management strategy to control clinical signs of hyperthyroidism. For these reasons, follow‐up nuclear scintigraphy was performed only after the thyroid hormone concentrations had been documented to decrease into the reference range in each cat. This resulted in a variable time between the first and second nuclear scans, which is a weakness of the study design.
Treatment of hyperthyroidism with 131I depends upon uptake of the isotope by hyperplastic or neoplastic thyroid follicular cells. The 131I is then incorporated into thyroglobulin and the ionizing effects of beta emissions cause follicular cell death. Normal thyroid follicular cells are protected from the effects of 131I, because of suppression of TSH concentration and resultant follicular cell atrophy. The results of this study suggest that increased uptake of iodine after consumption of a diet with a limited iodine content could potentially be used as a strategy to decrease the dose of 131I necessary for treatment of hyperthyroidism in cats. This strategy is currently used for treatment of thyroid cancer in humans. Benefits of a lower dose of 131I include decreased radiation exposure, decreased cost, and decreased duration of isolation after treatment.12 A possible disadvantage of such an approach could be increased risk of hypothyroidism after 131I treatment, because of increased trapping of isotope by normal follicular cells that were previously atrophic. It is also possible that in cats with rapid thyroidal iodine turn‐over, an iodine limited diet could result in less 131I becoming incorporated into thyroglobulin, leading to decreased sensitivity to radioisotope.9 These questions emphasize the need for further studies to determine the clinical outcome of radioactive iodine treatment in cats being fed iodine limited diets. Until more information is known, iodine limited diets should be discontinued before treatment with 131I. In this study, radioactive iodine uptake after diet withdrawal was only evaluated in 3 of the 8 cats; based on this information it appears that a 2 week washout period may be long enough for iodine uptake to return to pre‐diet uptake, however, further studies are required to confirm this.
Acknowledgment
This study was supported by a grant from the Purdue College of Veterinary Medicine Small Animal Disease Research Fund and by a gift from Hills Pet Nutrition.
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Footnotes
Hill's Prescription Diet y/d® Feline Thyroid Health, Topeka, KS, USA
Immulite® Total T4; Diagnostic Products Corp, Los Angeles, CA
Immulite® Veterinary Free T4 (VfT4); Siemens Healthcare Diagnostics Products Ltd, Llanberis, Gwynedd, UK
Peterson ME, et al.. Accuracy of serum free thyroxine concentrations determined by a new veterinary chemiluminescent immunoassay in euthyroid and hyperthyroid cats (abstract). Proceedings of the 21st ECVIM‐CA Congress.Seville (Spain), September 8–10, 2011. Poster No. EN‐P‐3. p. 239
References
- 1. Edinboro CH, Scott‐Moncrieff JC, Janovitz E, et al. Epidemiological study of relationships between consumption of commercial canned cat food and risk of hyperthyroidism in cats. J Am Vet Med Assoc 2004;224:879–886. [DOI] [PubMed] [Google Scholar]
- 2. Melendez LM, Yamka RM, Forrester SD. Titration of dietary iodine for reducing serum thyroxine concentrations in newly diagnosed hyperthyroid cats. J Vet Intern Med 2011;25:683 (abstract EN 16). [Google Scholar]
- 3. Melendez LM, Yamka RM, Burris PA. Titration of dietary iodine for maintaining serum thyroxine concentrations in hyperthyroid cats. J Vet Intern Med 2011;25:683 (abstract EN 17). [Google Scholar]
- 4. Yu S, Wedekind KJ, Burris PA, et al. Controlled level of dietary iodine normalizes serum total thyroxine in cats with naturally occurring hyperthyroidism. J Vet Intern Med 2011;25:683–684 (abstract EN 18). [Google Scholar]
- 5. Van der Kooij M, Becvárová I, Meyer HP, et al. Effects of an iodine‐restricted food on client‐owned cats with hyperthyroidism. J Feline Med Surg 2014;16:491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fritsch DA, Allen TA, Dodd CE, et al. A restricted iodine food reduces circulating thyroxine concentrations in cats with hyperthyroidism. Int J Appl Res Vet Med 2014;12:24–32. [Google Scholar]
- 7. Wedekind KJ, Blumer ME, Huntington CE, et al. The feline iodine requirement is lower than the 2006 NRC recommended allowance. J Anim Physiol Anim Nutr (Berl) 2010;94:527–539. [DOI] [PubMed] [Google Scholar]
- 8. Sjollema BE, Pollak YWEA, van den Brom WE, et al. Thyroidal radioiodine uptake in hyperthyroid cats. Vet Q 1989;11:165–170. [DOI] [PubMed] [Google Scholar]
- 9. Zanzonico PB, Becker DV, Hurley JR. Enhancement of radioiodine treatment of small‐pool hyperthyroidism with antithyroid drugs: Kinetics and dosimetry. J Nucl Med 2004;45:2102–2108. [PubMed] [Google Scholar]
- 10. Hui TY, Bruyette DS, Moore GE, Scott‐Moncrieff JC. Effect of feeding an iodine‐restricted diet in cats with spontaneous hyperthyroidism. J Vet Intern Med 2015. DOI: 10.1111/jvim.13368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sawka AM, Ibrahim‐Zada I, Galcgac P, et al. Dietary iodine restriction in preparation for radioactive iodine treatment or scanning in well‐differentiated thyroid cancer: A systematic review. Thyroid 2010;20:1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weichselbaum RC, Feeney DA, Jessen CR, et al. Evaluation of relationships between pretreatment patient variables and duration of isolation for radioiodine‐treated hyperthyroid cats. Am J Vet Res 2003;64:425–427. [DOI] [PubMed] [Google Scholar]


