Abstract
Background
Canine osteosarcoma (OS) is associated with localized pain as a result of tissue injury from tumor infiltration and peritumoral inflammation. Malignant bone pain is caused by stimulation of peripheral pain receptors, termed nociceptors, which reside in the localized tumor microenvironment, including the periosteal and intramedullary bone cavities. Several nociceptive ligands have been determined to participate directly or indirectly in generating bone pain associated with diverse skeletal abnormalities.
Hypothesis
Canine OS cells actively produce nociceptive ligands with the capacity to directly or indirectly activate peripheral pain receptors residing in the bone tumor microenvironment.
Animals
Ten dogs with appendicular OS.
Methods
Expression of nerve growth factor, endothelin‐1, and microsomal prostaglandin E synthase‐1 was characterized in OS cell lines and naturally occurring OS samples. In 10 dogs with OS, circulating concentrations of nociceptive ligands were quantified and correlated with subjective pain scores and tumor volume in patients treated with standardized palliative therapies.
Results
Canine OS cells express and secrete nerve growth factor, endothelin‐1, and prostaglandin E2. Naturally occurring OS samples uniformly express nociceptive ligands. In a subset of OS‐bearing dogs, circulating nociceptive ligand concentrations were detectable but failed to correlate with pain status. Localized foci of nerve terminal proliferation were identified in a minority of primary bone tumor samples.
Conclusions and Clinical Importance
Canine OS cells express nociceptive ligands, potentially permitting active participation of OS cells in the generation of malignant bone pain. Specific inhibitors of nociceptive ligand signaling pathways might improve pain control in dogs with OS.
Keywords: Nociception, Osteoblast cytokines, Painful malignant osteolysis
Abbreviations
- ET‐1
endothelin‐1
- mPGES‐1
microsomal prostaglandin E synthase‐1
- NGF
nerve growth factor
- PGE2
prostaglandin E2
Under normal physiologic conditions, pain serves as a protective mechanism and is generated by depolarization of specialized peripheral neurons called nociceptors.1 Anatomically, nociceptors densely innervate tissues of cutaneous, musculoskeletal, and visceral origin, providing a comprehensive afferent neuronal network for the detection of noxious stimuli and early adaptive responses to avert severe tissue damage. Nociceptors can be stimulated by mechanical, chemical, and thermal activating pathways with the molecular drivers of painful sensations being categorized as nociceptive, neuropathic, or inflammatory in nature.2
In diseases such as cancer, the protective function of pain can become maladaptive, resulting in aberrant and dysregulated afferent nociceptor stimulation.3 Direct infiltration of tumor cells into connective and neuronal tissues can generate sensations of nociceptive and neuropathic pain by secretion of chemical mediators and spatial compressive effects, respectively. In addition, cancer cells can promote immune cell chemotaxis into the local microenvironment with consequent secretion of cytokines and degradative proteases to generate inflammatory pain. By virtue of chronic and pathologic nociceptor activation, pain is a common clinical manifestation in cancer patients and has been reported to affect up to 85% of people diagnosed with advanced stage cancers.4
Although the actual incidence of cancer pain in companion animals is unreported, painful behaviors can be observed in dogs and cats diagnosed with tumors involving the oronasal cavity, urogenital tract, alimentary tract, skin, and musculoskeletal system. Bone cancers, such as canine appendicular osteosarcoma (OS), frequently are associated with pain and lameness given the role of the skeleton for withstanding cyclical compressive forces during weight‐bearing activities. Specifically for bone tumors such as OS, nociceptors residing principally within the periosteum and medullary cavity can be pathologically activated by both mechanical and chemical stimulation.5 As a consequence of malignant osteolysis, regional skeletal integrity is compromised and physical distortion of bone can induce pain by activating skeletal mechanotransducers of the transient receptor potential vanilloid receptor family.6 Furthermore, a variety of chemical mediators produced and secreted by cancer, stromal, and migratory immune cells within the immediate bone tumor microenvironment have the capacity to directly activate nociceptors and induce pain.7
Although several chemical nociceptive ligands likely participate in the generation of bone cancer pain, arguably the most biologically relevant for OS progression would be ligands that not only have the capacity to activate nociceptors but also confer potential protumorigenic activities for malignantly transformed osteoblasts. Nerve growth factor (NGF), endothelin‐1 (ET‐1), and prostaglandin E2 (PGE2) are 3 well‐documented nociceptive ligands,8, 9, 10 which act as drivers of bone cancer pain and also participate in normal osteoblast activities including proliferation, migration, and survival.11, 12, 13 Given the potential duality for specific mediators to orchestrate both nociception and osteoblast biology, as well as the clinical need for confirming the presence of drug targets to improve analgesia in cancer‐bearing dogs, the purpose of this study was to investigate the expressions of NGF, ET‐1, and PGE2 in canine OS. The specific aims of this study were (1) to characterize the expression and secretion of nociceptive ligands in OS cell lines and tumor samples; (2) to study the relationships between subjective pain scores and circulating nociceptive ligand concentrations in dogs with OS; and, (3) to identify and characterize the existence of disorganized peripheral neuronal proliferation networks in primary OS bone lesions.
Materials and Methods
Cell Lines
Four dog (Abrams, D17, HMPOS, and POS), 1 human (143B), and 1 murine (K7M2) OS cell lines, 1 human prostate carcinoma cell line (DU145), 1 human pulmonary carcinoma cell line (A549), and 1 rat pheochromocytoma cell line (PC12) were used in this study. The HMPOS and POS cell lines were provided by James Farese, University of Florida; the Abrams cell line was provided by Doug Thamm, Colorado State University; and all other cell lines were purchased commercially.1
Antibodies
Antibodies used in this investigation were rabbit monoclonal NGF antibody (Anti‐NGF antibody2 ), rabbit polyclonal ET‐1 antibody (Anti‐Endothelin 1 antibody2), rabbit polyclonal mPGES‐1 antibody (Prostaglandin E Synthase‐1 antibody3 ), rabbit polyclonal peripherin antibody (Peripherin antibody2), mouse monoclonal βIII tubulin (Anti‐βIII Tubulin antibody4 ) and mouse monoclonal β‐actin antibody (Anti‐beta Actin antibody2).
Protein Detection of NGF, ET‐1, and mPGES‐1
Protein from 6 OS cell lines (4 canine OS, 1 human OS, and 1 murine OS) were extracted with a standard reagent (M‐PER5 ) and quantified using a commercial kit (BCA Assay).5 In addition to OS cell lines, proteins from positive control cell lines were processed similarly. For detection of NGF, ET‐1, or mPGES‐1, membranes were incubated with a rabbit monoclonal NGF antibody (1 : 500), rabbit polyclonal ET‐1 antibody (1 : 1,000), or rabbit polyclonal mPGES‐1 antibody (1 : 50), respectively, in 5% nonfat dry milk TBS‐Tween overnight at 4°C. Subsequently, membranes were stripped and reprobed with a rabbit polyclonal β‐actin antibody (1 : 5,000) and HRP‐conjugated secondary antibody (1 : 1,000).
Naturally Occurring OS Samples
Sixteen canine appendicular OS tissue blocks were provided by Dr Luke Borst (North Carolina State University) for immunohistochemical assessment. Blocked slides were incubated with rabbit primary antibodies against NGF (1 : 400), ET‐1 (1 : 2,000), or mPGES‐1 (1 : 250) for 1 hour at room temperature. Slides were incubated with a biotinylated secondary antibody for 20 minutes at room temperature, then washed in buffer before incubation for 20 minutes with a streptavidin–biotinylated horseradish peroxidase complex, and developed with DAB substrate for 5 minutes and counterstained with hematoxylin. Staining intensity for each naturally occurring OS sample was scored by a single investigator (KLW) based on a previously published methodology.14
Enzyme Linked Immunosorbent Assays
Cell Culture Secretion of NGF, ET‐1, and PGE2
Five OS cell lines and respective positive control cell lines for NGF, ET‐1, and PGE2 were incubated in 6‐well plates at a cell density of 2.5 × 105 per well in 1% FBS DMEM for 48 hours. After 48 hours, cell culture supernatants were assessed for NGF, ET‐1, and PGE2 concentrations using NGF,6 ET‐1,7 and PGE2 (Prostaglandin E2 EIA3) ELISA kits, previously described for the assessment of canine ligands.15, 16, 17 Concentrations of secreted nociceptive ligands were normalized for in vitro cell metabolic activity assessed by a colorimetric MTS assay (CellTiter 96 Aqueous One Solution4), and expressed as an arbitrary ratio (concentration/optical density).
Circulating NGF, ET‐1, and PGE2 in Dogs with OS
In 10 untreated dogs with appendicular OS, serial plasma and serum were collected at day 0 (pretreatment), day 28, day 84, and at the time of localized pain progression (failure) after institution of standardized palliative treatment including ionizing radiation (10 Gy on days 1 and 2), IV zoledronate (0.1 mg/kg IV as 15‐minute constant rate infusion q28d), and a combination of PO analgesics including deracoxib (2 mg/kg q24h), tramadol (2 mg/kg evq8h), and gabapentin (5 mg/kg q24h). Concentrations of circulating NGF, ET‐1, and PGE2 were quantified using commercially available NGF,6 ET‐1,8 and PGE23 ELISA kits.
Subjective Pain Assessment in Dogs with OS
In 10 untreated dogs with appendicular OS, serial assessment of pain was subjectively characterized by pet owners by use of the visual analogue scale (VAS) pain score questionnaire18 at day 0 (pretreatment), day 28, day 84, and at the time of localized pain progression (failure) after institution of standardized palliative treatment.
Absolute Tumor Volume Assessment
Absolute tumor volume (ATV) of primary bone OS tumors was calculated using a previously described methodology.19
Characterization of Periosteal Nerve End Sprouting in Archived Primary OS Lesions
Ten primary bone OS and 2 normal bone samples were histologically sectioned at 5 μm thickness, placed onto pretreated glass slides, dewaxed, rehydrated in water, and then used for immunohistochemical analyses as previously described.20 Each section was incubated with primary antibodies recognizing either βIII tubulin (1 : 200) or peripherin (1 : 1,000). A secondary biotinylated antibody9 (1 : 500) and avidin–biotin–peroxidase complex system10 were used. Slides were scanned for “hot spots” consistent with disorganized nerve endings sprouting within periosteal tissues immediately adjacent to viable OS bone lesions and compared to normal bone sections, and the number of nerve endings was counted in a total of 5 fields (135,000 μm2/field) per sample.
Statistical Analysis
In dogs with OS, changes in an experimental variable (VAS, NGF, ET‐1, and PGE2) with respect to time either were analyzed by a repeated measures 1‐ANOVA and posthoc Dunnet's test or by a Friedman test and posthoc Dunn test. Significant changes in either subjective pain scores or circulating nociceptor mediators were compared with day 0 (pretreatment). Spearman rank correlation was performed to analyze the existence of any relationship between VAS and ET‐1 or PGE2 concentrations, as well as relationships between ATV and VAS, ET‐1, or PGE2. Significance was defined as P < .05.
Results
Nociceptive Ligand Expression and Secretion by OS Cell Lines
Osteosarcoma cells derived from human, murine, and canine origin expressed NGF and ET‐1 (Fig 1A,B). In addition, all OS cell lines expressed the rate‐limiting enzyme, mPGES‐1, required for production of PGE2 (Fig 1C). Unlike the relatively uniform protein expressions detected by western blot analysis, the active secretions of nociceptive ligands were divergent. For NGF (Fig 2A), the average concentration secreted by positive control human prostatic carcinoma cells was 29.8 ± 1.5 pg/mL/OD, which was within the limits of analyte detection. In addition, the 143B human OS cell line released low but measurable concentrations of NGF (15.9 ± 1.1 pg/mL/OD). Of the 4 canine OS cell lines, only HMPOS actively secreted higher concentrations of NGF (44.0 ± 6.7 pg/mL/OD), whereas the remaining 3 canine OS cell lines questionably secreted NGF at concentrations approaching or below the manufacturer reported level of analyte detection, being 5.3 ± 1.1 pg/mL/OD, 3.1 ± 0.4 pg/mL/OD, and 11.7 ± 2.7 pg/mL/OD, respectively. For ET‐1 (Fig 2B), all cell lines released measurable quantities of ET‐1 within the sensitivity limits of the assay, with the positive control cell line DU145 producing 23.7 ± 0.4 pg/mL/OD. For the 5 OS cell lines, ET‐1 secretion varied with the HMPOS cell line producing the lowest quantity of 1.4 ± 0.1 pg/mL/OD, whereas the Abrams cell line actively secreted ET‐1 at a concentration of 100.3 ± 0.8 pg/mL/OD. The release of PGE2 was relatively uniform among 4 of the 5 OS cell lines screened, ranging from 111.2 ± 12.1 pg/mL/OD to 2,676.2 ± 93.1 pg/mL/OD. The HMPOS cell line secreted substantially greater concentrations of PGE2 at 89,112.0 ± 4,577.0 pg/mL/OD.
Figure 1.
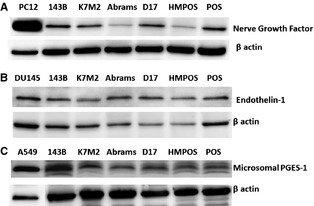
In a panel of cell lines inclusive of known positive controls (far left lane) and 6 osteosarcoma cell lines (human, murine, and canine), cellular proteins for (A) NGF, (B) ET‐1, and (C) mPGES‐1 are detected by western blot analysis. Species of origin: Human‐143B; Murine‐K7M2; Canine‐Abrams, D17, HMPOS, and POS.
Figure 2.
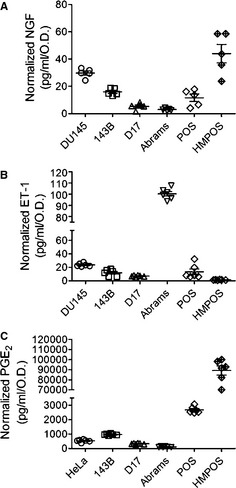
In a panel of cell lines inclusive of known positive secretory controls (far left) and 5 osteosarcoma cell lines (human and dog), active secretions of (A) NGF, (B) ET‐1, and (C) PGE2 into the cell culture supernatants are quantified by ELISA after 48 hours of incubation and normalized for differences in metabolic activity.
Nociceptive Ligand Expression in Canine OS
All tumor samples demonstrated diffuse cytoplasmic staining for all 3 nociceptive ligands (Fig 3A). Among the 3 nociceptive ligands, ET‐1 was most robustly and consistently expressed with 16/16 samples demonstrating a 3+ staining score, whereas NGF and mPGES‐1 staining was 3+ positive in 9 and 10 samples, respectively (Fig 3B). Although all nociceptive ligands demonstrated cytoplasmic subcellular localization, immunohistochemical detection of mPGES‐1 also was identified within the nucleus in approximately 10–15% of malignant osteoblast cells.
Figure 3.
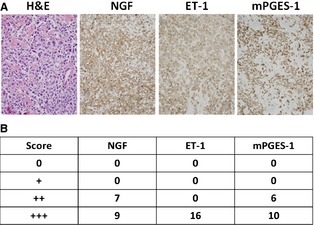
(A) Microscopic appearance of a representative spontaneous canine OS sample by H&E staining and confirmed protein expressions of NGF, ET‐1, and mPGES‐1 by immunohistochemistry. (B) Tabulated immunohistochemical staining summary for NGF, ET‐1, and mPGES‐1 expression in 16 spontaneously arising OS samples.
Cancer Pain and Circulating Nociceptive Ligands in Canine OS
In 10 untreated dogs with OS (Table S1), serial changes in VAS pain scores and circulating nociceptive ligand concentrations were characterized after effective pain alleviation. The median pretreatment (day 0) VAS pain score was 3.5 (range, 0.0–7.0), which decreased after the institution of treatment to 1.5 (range, 0.0–6.0) at day 28 and 2.5 (range, 0.0–6.0) at day 84, and subsequently increased to 6.0 (range, 1.0–9.0) at time of bone pain progression (Fig 4A). Median VAS pain scores on days 28 (P < .05) and 84 (P < .05) were significantly decreased in comparison with day 0.
Figure 4.
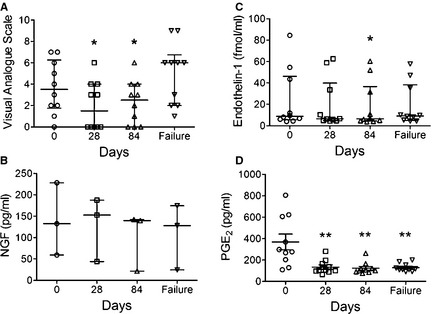
Serial changes as a function of time in (A) VAS pain scores and plasma concentrations of (B) NGF, (C) ET‐1, and (D) PGE2 derived from 10 OS‐bearing dogs treated with standardized palliative therapies. Data expressed as median and interquartile range for (A–C) and mean ± SEM for (D). Significant differences relative to Day 0 denoted with either *P < .05 or **P < .01.
In the same 10 dogs, circulating NGF, ET‐1, and PGE2 concentrations were serially quantified. In 7 dogs, NGF concentrations were below the limits of detection (Table S1), but the remaining 3 dogs had quantifiable NGF concentrations with median values of 132.6 pg/mL (range, 59.3–228.2), 152.6 pg/mL (range, 44.0–187.4), 139.6 pg/mL (range, 21.6–142.1), and 128.0 pg/mL (range, 24.6–174.4) at days 0, 28, 84, and failure, respectively (Fig 4B). Based on these 3 dogs, NGF concentrations did not appear to change after institution of effective palliative treatment, but statistical analysis was not performed because of the limited sample size. For ET‐1, 7 dogs had ET‐1 concentrations between 4.0 and 12.0 fmol/mL, whereas 3 dogs had higher concentrations of ET‐1 ranging from 45.0 to 84.5 fmol/mL (Table S1). The median concentrations of ET‐1 at days 0, 28, 84, and failure was 8.8 fmol/mL (range, 4.0–84.5), 6.6 fmol/ml (range, 4.4–62.6), 6.5 fmol/mL (range, 4.0–60.3), and 9.2 fmol/mL (range, 4.3–57.3), respectively. Compared to pretreatment (day 0), ET‐1 concentrations were significantly decreased on day 84 (P < .05; Fig 4C). The average plasma concentrations of PGE2 were 368.7 ± 73.5 pg/mL, 134.1 ± 20.5 pg/mL, 122.7 ± 17.5 pg/mL, and 129.7 ± 11.8 pg/mL on days 0, 28, 84, and failure, respectively (Fig 4D). Decreases in plasma PGE2 concentrations in comparison with day 0 were significant for all time points: day 28 (P < .01), day 84 (P < .01), and failure (P < .01).
No correlations between individual VAS pain score and ET‐1 or PGE2 concentrations at each respective time point (days 0, 28, 84, and failure) were identified. The ability to assess for any correlation between individual VAS pain score and NGF concentrations was precluded given the limited number of dogs (n = 3) with measurable NGF concentrations. Relative primary bone tumor burden in dogs with OS was expressed as ATV, and among the 10 OS‐bearing dogs was calculated to be 107.0 ± 13.6 cm3. No correlation between ATV and any individual pain parameter at baseline was identified.
Disorganized Foci of Nerve Ending Proliferations in Canine OS
To investigate if sensory nerve fiber sprouting occurs in canine OS, 10 archived primary OS samples were stained for 2 neuronal markers: class III β tubulin and peripherin (Fig 5A,B). Systematic evaluation of 5 microscopic fields (135,000 μm2/field) containing periosteal tissues adjacent to viable malignant osteoblasts identified positive staining for both class III β tubulin and peripherin in 20% of tumor samples (Fig 5C,D), whereas periosteum from normal bone (data not shown) and the majority of OS samples did not show any significant immunolabeling (Fig 5E,F). Quantitatively, in 2 of the 10 OS samples, the number of positively labelled nerve foci per microscopic field ranged from 0 to 25, with a median value of 3 nerve termini/field. In the remaining 10 bone samples (8 OS and 2 normal bone), no obvious detectable nerve termini were identified within the periosteum.
Figure 5.
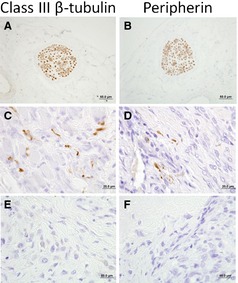
Microscopic appearance of a peripheral sensory nerve traversing through control tissue as indicated by positive immunohistochemical staining for (A) class III β tubulin and (B) peripherin. Haphazard and disorganized proliferation of neuronal endings identified within OS‐associated periosteum as determine by positive immunohistochemical staining for (C) class III β tubulin and (D) peripherin. Representative OS‐associated periosteum which is devoid of excessive neuronal end proliferations based upon the absence of (E) class III β tubulin and (F) peripherin immunohistochemical staining.
Discussion
Although various nociceptive ligands likely participate in generating OS bone cancer pain, the current investigation focused on NGF, ET‐1, and PGE2 given their potential roles in not only malignant osteolytic nociception but also OS protumorigenic biology. In particular, NGF and ET‐1 have been intensively evaluated as drug targets for the alleviation of bone cancer pain in murine preclinical models. In these studies, the neutralization of NGF with blocking antibodies or small molecule inhibitors decreased nociceptive guarding behaviors in mice implanted with prostatic carcinoma or osteolytic sarcoma cells within the bone medullary cavity.21, 22, 23 Similarly, ET‐1 signaling blockade with small molecule inhibitors in tumor‐bearing mice alleviated painful sensations induced by the osteolytic growth of prostatic carcinoma or bone sarcoma cells.24, 25 Although inflammatory eicosanoids, particularly PGE2, have long been recognized to participate in nociceptor sensitization associated with inflammatory disorders, recent investigations have directly linked PGE2 with tumor‐induced osteolysis and bone cancer pain.26, 27, 28 Importantly, the rate limiting enzyme involved in PGE2 generation (mPGES‐1) has been demonstrated to enhance bone tumor growth and malignant osteolytic pain behaviors in preclinical murine models,29 and provides a biologic rationale for inhibiting mPGES‐1 enzymatic activities to ameliorate pain associated with malignant osteolysis. Despite many studies defining the drivers of malignant bone pain in murine preclinical models, canine OS‐associated bone pain remains incompletely defined and warrants studies to identify pathways involved in the development and maintenance of bone cancer pain in dogs.
Although localized pain is a salient hallmark in dogs diagnosed with OS, the clinical spectrum of pain symptomatology is heterogeneous among affected patients, and likely reflects the complex interplay among several tumor‐ and host‐related factors including degree of osteolysis, tumor volume and location, skeletal mass and physical activity. Findings in this study suggest that additional tumor‐specific factors also could contribute to the variable spectrum of malignant bone pain, specifically the inherent capacity of OS cells to express and actively secrete nociceptive ligands. Malignant osteoblast cell lines demonstrated a wide variability in the secretion of nociceptive ligands, with the magnitude of difference between the highest and lowest secreted concentrations ranging approximately 10‐fold, 100‐fold, and 800‐fold for NGF, ET‐1, and PGE2, respectively. In addition, all naturally occurring OS samples demonstrated uniform and positive staining for NGF, ET‐1, and mPGES‐1, and if malignant osteoblasts retain divergent capacities for localized nociceptive ligand secretion within the bone tumor microenvironment, it is plausible that the severity of bone pain in dogs could be partially mediated by the existence of different clonal populations with variable secretory capacities.
In this study, the relationship between VAS pain scores and plasma concentrations of NGF, ET‐1, and PGE2 were explored in OS‐bearing dogs receiving standardized palliative treatment. As expected, the alleviation of pain after effective palliative treatment decreased the median VAS pain scores on days 28 and 84 in comparison with day 0 and, upon recurrence of breakthrough pain, VAS pain scores rebounded back to pretreatment levels. Plasma concentrations of NGF, ET‐1, and PGE2 tended to decrease after palliative treatment, but strong correlations between VAS pain scores and circulating nociceptive ligand concentrations were not identified in the limited population of dogs evaluated. As such, the preliminary data generated from the current investigation do not support the utility of plasma NGF, ET‐1, and PGE2 concentrations as surrogates of bone pain or absolute tumor volume in dogs with OS. Corroborating the imperfect association between pain intensity and circulating nociceptive ligands identified in this study, correlations between plasma NGF concentrations and pain intensity in people suffering from various pathologic conditions including chronic migraine, interstitial cystitis, and temporomandibular joint and myofascial pain also have yielded conflicting results.30, 31, 32
Although circulating concentrations of NGF, ET‐1, and PGE2 were not correlated with pain status in dogs with OS, ours is the first report to serially characterize pre‐ and posttreatment plasma concentrations of nociceptive ligands in dogs with OS. For NGF and ET‐1, it is noteworthy that systemic concentrations of both ligands could be dichotomously categorized as being either marginally or robustly detectable, and the observed pattern could be consistent with differing secretory capacities of canine OS cells as demonstrated in vitro. Among the 3 ligands, plasma PGE2 concentrations were most consistently detectable in dogs with OS, and demonstrated expected decreases after administration of nonsteroidal anti‐inflammatory drugs (NSAIDs), findings consistent with historical studies in tumor‐bearing dogs treated with NSAIDs.33
Gradual intensification of bone pain is a frequent clinical observation in dogs diagnosed with OS, and is a consequence of progressive mechanical, chemical, and neuronal perturbations within the immediate bone tumor microenvironment. In murine preclinical models of malignant bone pain, aberrant nerve sprouting has been identified as a driver of pathologic pain intensification,34, 35 yet whether an analogous process is operative in naturally occurring bone tumors such as canine OS has not been reported previously. Of 10 primary OS samples, excessive proliferation of nerve endings within tumor‐associated periosteum was definitively observed in 2 cases, suggesting that aberrant nerve sprouting might contribute to progressive pain intensification in some affected dogs. Given that only a small fraction of the periosteal surface area was microscopically evaluated in this study, the percentage of bone samples recognized to exhibit aberrant nerve sprouting likely underestimates the true frequency of neuropathologic alterations induced by OS‐associated chronic bone pain. Based upon these findings, it would be reasonable to suggest that treatment strategies that inhibit pathologic neuronal sprouting, such as NGF blockade, might attenuate the severity of bone pain in dogs with OS.
Despite the novel findings of this study, there are several investigative limitations that should be recognized. First, the mere demonstration of nociceptive ligand expression by OS cells does not provide evidence for the direct or indirect participation of OS cells in pain generation, rather it can only be stated that OS cells are capable of producing ligands with recognized nociceptive functions. Second, although NGF, ET‐1, and PGE2 were chosen for investigation based on their known involvement in nociception, these molecules can be produced by a broad range of cell types and also exert diverse effects completely unrelated to pain sensation. In fact, within the bone tumor microenvironment, nonmalignant resident cells including osteoclasts, mature osteocytes, and trafficking immune cells were identified to stain positively for some of the nociceptive ligands characterized. Hence, their evaluation in dogs with OS as exclusive biomarkers of bone pain would be erroneous given their involvement in diverse homeostatic processes including cardiovascular, neuronal, and cell membrane physiology. Third, rather than measuring systemic circulating nociceptive ligands, assessment of their intratumoral concentrations would have been expected to correlate better with localized bone pain status, as supported by preclinical murine tumor models.28
In summary, the descriptive findings reported here provide incremental advancements in identifying potential driver pathways that might participate in malignant bone pain generation in dogs with OS. Improving treatment outcomes in people suffering from bone cancer pain has been possible only by the initial identification of key cellular processes involved in malignant osteolysis and pathologic nociceptor activation, with several novel analgesic targets being validated and consequently translated into clinical practice.24, 36, 37 The rational development and clinical institution of superior analgesic regimens for dogs suffering from OS‐associated bone pain will require a similar discovery path. The findings of thist study might serve as an initial catalyst to stimulate future research into the functional pathways responsible for pathologic nociception in dogs suffering from malignant bone pain.
Supporting information
Table S1. Patient characteristics and comparison of baseline parameters relative to tumor volume.
Acknowledgment
Conflict of Interest Declaration: Dr Timothy Fan is an associate editor at the Journal of Veterinary Internal Medicine.
Off‐label Antimicrobial Declaration: The authors declare no off‐label use of antimicrobials.
Footnotes
American Tissue Culture Collection, Manassas, VA
Abcam, Cambridge, MA
Cayman Chemical, Ann Arbor, MI
Promega, Madison, WI
Pierce, Rockford, IL
ChemiKine NGF, Sandwich ELISA, EMD Millipore, Billerica, MA
Endothelin‐1 ELISA, Immuno‐Biological Labs Co, Hamburg, Germany
Endothelin ELISA (1‐21), ALPCO, Salem, NH
Jackson Laboratories, Bar Harbor, MA
ABC Vector Laboratories, Burlingame, CA
References
- 1. Bowsher D. Pain pathways and mechanisms. Anaesthesia 1978;33:935–944. [DOI] [PubMed] [Google Scholar]
- 2. Millan MJ. The induction of pain: An integrative review. Prog Neurobiol 1999;57:1–164. [DOI] [PubMed] [Google Scholar]
- 3. Koltzenburg M. The changing sensitivity in the life of the nociceptor. Pain 1999;(Suppl 6):S93–S102. [DOI] [PubMed] [Google Scholar]
- 4. Sykes NP. Pain control in terminal cancer. Int Disabil Stud 1987;9:33–37. [DOI] [PubMed] [Google Scholar]
- 5. Mach DB, Rogers SD, Sabino MC, et al. Origins of skeletal pain: Sensory and sympathetic innervation of the mouse femur. Neuroscience 2002;113:155–166. [DOI] [PubMed] [Google Scholar]
- 6. Guilak F, Leddy HA, Liedtke W. Transient receptor potential vanilloid 4: The sixth sense of the musculoskeletal system? Ann N Y Acad Sci 2010;1192:404–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clohisy DR, Mantyh PW. Bone cancer pain. Cancer 2003;97:866–873. [DOI] [PubMed] [Google Scholar]
- 8. Hans G, Deseure K, Adriaensen H. Endothelin‐1‐induced pain and hyperalgesia: A review of pathophysiology, clinical manifestations and future therapeutic options. Neuropeptides 2008;42:119–132. [DOI] [PubMed] [Google Scholar]
- 9. Hori T, Oka T, Hosoi M, et al. Pain modulatory actions of cytokines and prostaglandin E2 in the brain. Ann N Y Acad Sci 1998;840:269–281. [DOI] [PubMed] [Google Scholar]
- 10. Lewin GR, Mendell LM. Nerve growth factor and nociception. Trends Neurosci 1993;16:353–359. [DOI] [PubMed] [Google Scholar]
- 11. Mogi M, Kondo A, Kinpara K, et al. Anti‐apoptotic action of nerve growth factor in mouse osteoblastic cell line. Life Sci 2000;67:1197–1206. [DOI] [PubMed] [Google Scholar]
- 12. von Schroeder HP, Veillette CJ, Payandeh J, et al. Endothelin‐1 promotes osteoprogenitor proliferation and differentiation in fetal rat calvarial cell cultures. Bone 2003;33:673–684. [DOI] [PubMed] [Google Scholar]
- 13. Igarashi K, Hirafuji M, Adachi H, et al. Role of endogenous PGE2 in osteoblastic functions of a clonal osteoblast‐like cell, MC3T3‐E1. Prostaglandins Leukot Essent Fatty Acids 1994;50:169–172. [DOI] [PubMed] [Google Scholar]
- 14. Ramos‐Vara JA, Miller MA. When tissue antigens and antibodies get along: Revisiting the technical aspects of immunohistochemistry—The red, brown, and blue technique. Vet Pathol 2014;51:42–87. [DOI] [PubMed] [Google Scholar]
- 15. Isola M, Ferrari V, Miolo A, et al. Nerve growth factor concentrations in the synovial fluid from healthy dogs and dogs with secondary osteoarthritis. Vet Comp Orthop Traumatol 2011;24:279–284. [DOI] [PubMed] [Google Scholar]
- 16. Mohammed SI, Coffman K, Glickman NW, et al. Prostaglandin E2 concentrations in naturally occurring canine cancer. Prostaglandins Leukot Essent Fatty Acids 2001;64:1–4. [DOI] [PubMed] [Google Scholar]
- 17. Fukumoto S, Hanazono K, Miyasho T, et al. Serum big endothelin‐1 as a clinical marker for cardiopulmonary and neoplastic diseases in dogs. Life Sci 2014; doi: 10.1016/j.lfs.2014.01.002. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18. Hudson JT, Slater MR, Taylor L, et al. Assessing repeatability and validity of a visual analogue scale questionnaire for use in assessing pain and lameness in dogs. Am J Vet Res 2004;65:1634–1643. [DOI] [PubMed] [Google Scholar]
- 19. Sternberg RA, Pondenis HC, Yang X, et al. Association between absolute tumor burden and serum bone‐specific alkaline phosphatase in canine appendicular osteosarcoma. J Vet Intern Med 2013;27:955–963. [DOI] [PubMed] [Google Scholar]
- 20. Lezmi S, Rokh N, Saint‐Macary G, et al. Chloroquine causes similar electroretinogram modifications, neuronal phospholipidosis and marked impairment of synaptic vesicle transport in albino and pigmented rats. Toxicology 2013;308:50–59. [DOI] [PubMed] [Google Scholar]
- 21. Halvorson KG, Kubota K, Sevcik MA, et al. A blocking antibody to nerve growth factor attenuates skeletal pain induced by prostate tumor cells growing in bone. Cancer Res 2005;65:9426–9435. [DOI] [PubMed] [Google Scholar]
- 22. Sevcik MA, Ghilardi JR, Peters CM, et al. Anti‐NGF therapy profoundly reduces bone cancer pain and the accompanying increase in markers of peripheral and central sensitization. Pain 2005;115:128–141. [DOI] [PubMed] [Google Scholar]
- 23. Ghilardi JR, Freeman KT, Jimenez‐Andrade JM, et al. Administration of a tropomyosin receptor kinase inhibitor attenuates sarcoma‐induced nerve sprouting, neuroma formation and bone cancer pain. Mol Pain 2010;6:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Peters CM, Lindsay TH, Pomonis JD, et al. Endothelin and the tumorigenic component of bone cancer pain. Neuroscience 2004;126:1043–1052. [DOI] [PubMed] [Google Scholar]
- 25. Yuyama H, Koakutsu A, Fujiyasu N, et al. Effects of selective endothelin ET(A) receptor antagonists on endothelin‐1‐induced potentiation of cancer pain. Eur J Pharmacol 2004;492:177–182. [DOI] [PubMed] [Google Scholar]
- 26. Sabino MC, Ghilardi JR, Feia KJ, et al. The involvement of prostaglandins in tumorigenesis, tumor‐induced osteolysis and bone cancer pain. J Musculoskelet Neuronal Interact 2002;2:561–562. [PubMed] [Google Scholar]
- 27. Sabino MA, Ghilardi JR, Jongen JL, et al. Simultaneous reduction in cancer pain, bone destruction, and tumor growth by selective inhibition of cyclooxygenase‐2. Cancer Res 2002;62:7343–7349. [PubMed] [Google Scholar]
- 28. Smeester BA, Al‐Gizawiy M, Beitz AJ. Effects of different electroacupuncture scheduling regimens on murine bone tumor‐induced hyperalgesia: Sex differences and role of inflammation. Evid Based Complement Alternat Med 2012;2012:671386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Isono M, Suzuki T, Hosono K, et al. Microsomal prostaglandin E synthase‐1 enhances bone cancer growth and bone cancer‐related pain behaviors in mice. Life Sci 2011;88:693–700. [DOI] [PubMed] [Google Scholar]
- 30. Jang MU, Park JW, Kho HS, et al. Plasma and saliva levels of nerve growth factor and neuropeptides in chronic migraine patients. Oral Dis 2011;17:187–193. [DOI] [PubMed] [Google Scholar]
- 31. Liu HT, Kuo HC. Increased urine and serum nerve growth factor levels in interstitial cystitis suggest chronic inflammation is involved in the pathogenesis of disease. PLoS One 2012;7:e44687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Basi DL, Velly AM, Schiffman EL, et al. Human temporomandibular joint and myofascial pain biochemical profiles: A case‐control study. J Oral Rehabil 2012;39:326–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Knapp DW, Richardson RC, Chan TC, et al. Piroxicam therapy in 34 dogs with transitional cell carcinoma of the urinary bladder. J Vet Intern Med 1994;8:273–278. [DOI] [PubMed] [Google Scholar]
- 34. Jimenez‐Andrade JM, Bloom AP, Stake JI, et al. Pathological sprouting of adult nociceptors in chronic prostate cancer‐induced bone pain. J Neurosci 2010;30:14649–14656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mantyh WG, Jimenez‐Andrade JM, Stake JI, et al. Blockade of nerve sprouting and neuroma formation markedly attenuates the development of late stage cancer pain. Neuroscience 2010;171:588–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Carducci MA, Jimeno A. Targeting bone metastasis in prostate cancer with endothelin receptor antagonists. Clin Cancer Res 2006;12:6296s–6300s. [DOI] [PubMed] [Google Scholar]
- 37. James ND, Caty A, Payne H, et al. Final safety and efficacy analysis of the specific endothelin A receptor antagonist zibotentan (ZD4054) in patients with metastatic castration‐resistant prostate cancer and bone metastases who were pain‐free or mildly symptomatic for pain: A double‐blind, placebo‐controlled, randomized Phase II trial. BJU Int 2010;106:966–973. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Patient characteristics and comparison of baseline parameters relative to tumor volume.


