Abstract
Background
Cardiac biomarkers provide objective data that augments clinical assessment of heart disease (HD).
Hypothesis/Objectives
Determine the utility of plasma N‐terminal pro‐brain natriuretic peptide concentration [NT‐proBNP] measured by a 2nd generation canine ELISA assay to discriminate cardiac from noncardiac respiratory distress and evaluate HD severity.
Animals
Client‐owned dogs (n = 291).
Methods
Multicenter, cross‐sectional, prospective investigation. Medical history, physical examination, echocardiography, and thoracic radiography classified 113 asymptomatic dogs (group 1, n = 39 without HD; group 2, n = 74 with HD), and 178 with respiratory distress (group 3, n = 104 respiratory disease, either with or without concurrent HD; group 4, n = 74 with congestive heart failure [CHF]). HD severity was graded using International Small Animal Cardiac Health Council (ISACHC) and ACVIM Consensus (ACVIM‐HD) schemes without knowledge of [NT‐proBNP] results. Receiver‐operating characteristic curve analysis assessed the capacity of [NT‐proBNP] to discriminate between dogs with cardiac and noncardiac respiratory distress. Multivariate general linear models containing key clinical variables tested associations between [NT‐proBNP] and HD severity.
Results
Plasma [NT‐proBNP] (median; IQR) was higher in CHF dogs (5,110; 2,769–8,466 pmol/L) compared to those with noncardiac respiratory distress (1,287; 672–2,704 pmol/L; P < .0001). A cut‐off >2,447 pmol/L discriminated CHF from noncardiac respiratory distress (81.1% sensitivity; 73.1% specificity; area under curve, 0.84). A multivariate model comprising left atrial to aortic ratio, heart rate, left ventricular diameter, end‐systole, and ACVIM‐HD scheme most accurately associated average plasma [NT‐proBNP] with HD severity.
Conclusions and Clinical Importance
Plasma [NT‐proBNP] was useful for discriminating CHF from noncardiac respiratory distress. Average plasma [NT‐BNP] increased significantly as a function of HD severity using the ACVIM‐HD classification scheme.
Keywords: Biomarkers, Canine, Heart disease, Respiratory distress
Abbreviations
- ACVIM‐HD
American College of Veterinary Internal Medicine consensus statement, heart disease severity grading scheme
- AUC
area under the curve
- BCS
body condition score
- BNP
brain natriuretic peptide
- CHF
congestive heart failure
- DCM
dilated cardiomyopathy
- %FS
percent fractional shortening
- HD
heart disease
- HR
heart rate
- IQR
interquartile range
- ISACHC
International Small Animal Cardiac Health Council classification scheme
- LA : Ao
left atrial to aortic root ratio
- LAd
left atrial diameter, end‐diastole
- LVIDd
left ventricular diameter, end‐diastole
- LVIDs
left ventricular diameter, end‐systole
- MMVD
chronic myxomatous mitral valve disease
- [NT‐proBNP]
N‐terminal pro‐brain natriuretic peptide concentration
- ROC
receiver operating characteristic curve
- TR
tricuspid regurgitation
- VHS
vertebral heart size
Investigation of cardiac biomarkers has been a subject of growing interest.1, 2, 3 Measurement of natriuretic peptides including canine N‐terminal pro‐brain natriuretic peptide concentration ([NT‐proBNP]) provides insights to distinguish cardiac from noncardiac respiratory distress,3, 4, 5, 6, 7, 8 assess heart disease (HD) severity,7, 9, 10, 11 predict onset of CHF,11 provide prognostic information in myxomatous mitral valve disease (MMVD),10, 11, 12, 13, 14 and dilated cardiomyopathy (DCM),15, 16 and help with treatment decisions.17
Semiquantitative schemes intended to classify HD severity provide guideline‐driven approaches for diagnosis and management. These can be hampered, however, by the complexity and variability in heart failure. The International Small Animal Cardiac Health Council (ISACHC) scheme,18 which uses assessment of clinical signs and permits reclassification of disease severity based upon degree of cardiac compensation, is prone to subjective interpretation. The American College of Veterinary Internal Medicine (ACVIM) scheme for heart disease (ACVIM‐HD) emphasizes structural prerequisites and risk factors associated with heart failure,19 but amalgamates current and previous heart failure patients into a single category (stage C). Thus, assessing whether these schemes can be augmented by taking into consideration [NT‐proBNP] and other clinical variables is germane to patient evaluation.
Marked increases in [NT‐proBNP] have been recorded in some human patients with renal disease,20 whereas in others, markedly increased [NT‐proBNP] was not necessarily correlated with the presence of heart failure, myocardial disease, or renal dysfunction.21 Previous reports in dogs that studied [NT‐proBNP] utilized a commercial ELISA assay1 with an upper limit of detection of 3,000 pmol/L, and the prevalence and extent of higher [NT‐proBNP] is uncertain. Recently, this assay was modified to increase its upper limit of quantification from ≥3,000 to ≥10,000 pmol/L.2 , 3 Conceivably, an expanded upper limit of detection might help differentiate cardiac versus noncardiac causes of dyspnea or influence cut‐off values to distinguish between stages of HD severity. This study aimed to evaluate the utility of this new, 2nd generation assay to distinguish dogs with and without compensated HD, assess its sensitivity and specificity to differentiate cardiac from noncardiac causes of respiratory distress, and identify an optimal model that considers average [NT‐proBNP], ISACHC, and ACVIM‐HD classification schemes, physical examination findings, as well as radiographic, and echocardiographic assessment.
Materials and Methods
This multicenter trial included 15 cardiologists from 4 cardiology specialty practices and 5 veterinary medical colleges. Study protocol was approved by Institutional Animal Use and Care Committees or related committees of investigators. Informed owner consent to participate was obtained for all cases.
Animals
Dogs that were presented for cardiology evaluation between April–May 2009 and May–August 2013 were prospectively recruited. Entry criteria included a history interview, complete physical examination, thoracic radiography, and echocardiography at the time of presentation. Additional tests were performed as necessary to establish a definitive diagnosis. Dogs were excluded if serum creatinine concentration exceeded 2.0 mg/dL, if systemic blood pressure exceeded 180 mmHg, if they had heartworm disease, or if they had congenital HD.
Classification of Cardiac Status
Data were recorded on standardized forms that included: age, body weight, body condition score, sex, presence or absence of respiratory signs, presence and grade of heart murmur, serum creatinine and l‐thyroxine concentrations, heart rate (HR), and clinical diagnosis. Thoracic radiographs helped identify CHF and respiratory disease. Cardiac size was quantified using the vertebral heart size (VHS) method.22 Two‐dimensional echo‐Doppler and M‐mode echocardiography were performed by a board‐certified cardiologist or cardiology resident under direct supervision of a board‐certified cardiologist. Echocardiographic variables (averaging at least 3 heart beats) included left ventricular posterior wall thickness at end‐diastole, interventricular septal thickness at end‐diastole, left ventricular internal dimension at end‐diastole (LVIDd), left ventricular internal dimension at end‐systole (LVIDs);23 and left atrial dimension at end‐diastole (LAd) and left atrial to aortic root ratio (LA : Ao ratio) using LAd and aortic root (Ao) from the right parasternal, short axis, basilar view.24 Maximal transtricuspid peak systolic tricuspid regurgitation (TR) velocity was measured by Doppler echocardiography to estimate systolic pulmonary hypertension, graded as mild, moderate, or severe.25, 26 Accordingly, each dog was classified into 1 of 4 clinical cohorts (Fig 1, Table 1): group 1—asymptomatic, healthy dogs without HD; group 2—asymptomatic, healthy dogs with HD but not CHF; group 3—dogs with respiratory distress attributable to respiratory disease; group 4—dogs with respiratory distress from pulmonary edema and CHF. HD severity was graded using ISACHC18 and ACVIM‐HD19, 26 schemes. Briefly, these schemes were characterized as follows: The ISACHC cardiac disease grading system specified: class IA—subclinical HD disease without radiographic or echocardiographic evidence of cardiac enlargement; class IB—subclinical HD with radiographic or echocardiographic evidence of cardiac enlargement; class II—mild signs of CHF (eg, increased respiratory rate and effort, exercise intolerance) because of cardiogenic pulmonary edema, and associated with cardiomegaly; class IIIA—severe, overt signs of CHF that could be treated on an outpatient basis; class IIIB—severe signs of CHF requiring hospitalization. ACVIM cardiac disease guidelines specified: stage A—patients at high risk for developing HD, but without identifiable structural cardiac changes; stage B—HD present, but patient has never developed clinical signs of CHF (stage B is subdivided into stage BI—asymptomatic patients without radiographic or echocardiographic evidence of cardiac remodeling and stage B2—asymptomatic patients that demonstrate radiographic or echocardiographic evidence of left‐sided heart enlargement); stage C—patients with current or previous CHF with associated structural HD; stage D—end‐stage HD with clinical signs of CHF that are refractory to standard treatment. In this study, no dogs had end‐stage HD, and ACVIM‐HD classification was confined to stages A, B1, B2, and C. Clinicians were blinded to [NT‐proBNP] results.
Figure 1.
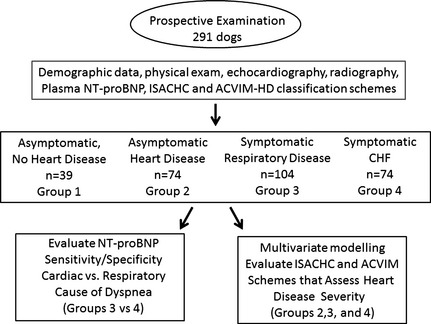
Flow diagram describing clinical cohorts and testing objectives (see text for more details).
Table 1.
Descriptive data from 291 dogs
| Variable | Group 1: Asymptomatic No Heart Disease | Group 2: Asymptomatic Heart Disease | Group 3: Respiratory Distress—Primary Respiratory or Airway Disease | Group 4: Respiratory Distress—Congestive Heart Failure |
|---|---|---|---|---|
| Number of dogs | 39 | 74 | 104 | 74 |
| Age (years), median (IQR) | 5.5 (3–7) | 10 (7.7–12) | 10.5 (8–13) | 10 (8–12) |
| Weight (kg), median (IQR) | 32.4 (17.9–36.8) | 11.4 (7.5–29.5) | 10.2 (6.5–15.8) | 10 (5.1–25) |
| Sex M, n (%) | 18 (46.1) | 39 (52.7) | 60 (57.7) | 51 (68.9) |
| Body condition score, median (IQR) | 4 (4–4) | 4.5 (4–5) | 5.3 (5–6) | 5 (4.5–6.5) |
| MMVD, n (%) | 0 | 68 (91.9) | 90 (86.5) | 62 (83.8) |
| DCM, n (%) | 0 | 6 (8.2) | 2 (1.9) | 13 (16.2) |
| Heart murmur grade, median (IQR) | 0 | 2 (2–2) | 4 (3.75–4.25) | 4 (2.5–4.75) |
| VHS, median (IQR) | 10.5 (10–11) | 11.2 (10.5–12) | 11 (10.1–12) | 12.5 (11.6–13.2) |
| HR, median (IQR) | 100 (85.7–120) | 120 (100–140) | 120 (100–138.5) | 140 (120–160) |
| LAd (cm), median (IQR) | 2.7 (2.5–3.7) | 2.9 (2.6–3.8) | 2.5 (2.1–3.4) | 3.5 (2.7–4.3) |
| LA : Ao ratio, median (IQR) | 1.3 (1.2–1.4) | 1.7 (1.4–2.0) | 1.6 (1.4–2.0) | 2.3 (1.9–2.7) |
| LVIDd (cm), median (IQR) | 3.9 (3.6–4.4) | 3.9 (3.3–4.3) | 3.3 (2.8–4.1) | 4.3 (3.6–5.1) |
| LVIDs (cm), median (IQR) | 2.8 (2.4–3.1) | 2.3 (1.8–3.0) | 1.9 (1.3–2.3) | 2.4 (1.8–3.0) |
| %FS, median (IQR) | 29.8 (23.3–34.1) | 38.8 (30.1–45.0) | 43.5 (37.8–52.6) | 43.7 (33.2–49.8) |
| [NT‐proBNP] (pmol/L), median (IQR) | 573 (471–866) | 1,287 (700–2,027) | 1,287 (672–2,704) | 5,110 (2,769–8,466) |
| % Dogs with [NT‐proBNP] >3,000 pmol/L, median (IQR) | 0 | 14.9 4,538 (3,803–4,761) |
18.3 4,944 (3,763–7,462) |
68.9 6,925 (4,917–9,752) |
MMVD, myxomatous mitral valve disease; DCM, dilated cardiomyopathy; VHS, vertebral heart size; HR, heart rate; LAd, left atrial diameter, end‐diastole; LA : Ao, left atrial to aortic root ratio; LVIDd, left ventricular diameter, end‐diastole; LVIDs, left ventricular diameter, end‐systole; %FS, percent fractional shortening; [NT‐proBNP], N‐terminal pro‐brain natriuretic peptide concentration. IQR, interquartile range; M, male.
Blood Sampling and Handling
Blood samples were collected at presentation into EDTA to measure plasma [NT‐proBNP]. Tubes were centrifuged and plasma separated, and frozen within 60 minutes of collection. For 2009 samples, plasma was transferred to a protease inhibitor tube before freezing according to manufacturer instructions. For 2013 samples, plasma was transferred to a secondary sample tube containing no additive before freezing, in keeping with updated manufacturer instructions. All plasma samples were shipped overnight on dry ice to IDEXX Laboratories, Inc. (Westbrook, ME) and stored at −80°C.
Measurement of Plasma [NT‐proBNP]
All samples were tested in 2013 using a commercially available, 2nd generation canine assay (Cardiopet proBNP2) which utilized a sandwich ELISA format with colorimetric end‐point detection at 450 nm for quantitative determination of [NT‐proBNP]. The assay contained unique anti‐canine NT‐proBNP capture and detection antibodies specific to distinct canine NT‐proBNP epitopes. Capture antibody was immobilized on 96‐well microtiter plates and detection antibody was conjugated to horseradish peroxidase. This antibody pair recognizes different epitopes in more stable regions of the molecule as compared to the antibodies utilized in the 1st generation assay, eliminating the need for protease inhibitor to facilitate sample collection. Increased dynamic range achieved 10,000 pmol/L as an upper reporting limit; 1 : 4 dilution in neutral pH buffer enabled further quantification up to 40,000 pmol/L. Three canine control samples with assigned [NT‐proBNP] at low, medium, and high concentrations were included in study sample runs to evaluate accuracy and precision. Controls with assigned concentrations of 757, 2,634, and 7,315 pmol/L were analyzed and measured in duplicate across 4 runs, totaling 8 replicates per control. Measured [NT‐proBNP] in this study were determined as 720, 2,549, and 7,123 pmol/L. Mean recovery was 96.4%. Intra‐assay coefficients of variation were 2.2, 4.2, and 4.0%; interassay coefficients of variation were 5.6, 4.4, and 2.8%, respectively, for the low (720 pmol/L), medium (2,549 pmol/L), and high (7,123 pmol/L) control samples. The limit of detection was 77 pmol/L based on the +3 standard deviation response from 8 replicate measurements of the zero standard.
To determine whether plasma samples collected in 2009 and stored frozen degraded over time, 102 of these 2009‐collected samples were remeasured in 2013 using the same, 1st generation commercial assay.1 NT‐proBNP concentrations (mean, IQR; 2009 versus 2013) were: 468; 379–824 pmol/L versus 516; 269–1,075 pmol/L, respectively (P = .747) for normal dogs; 551; 371–889 pmol/L versus 590; 418–938 pmol/L, respectively (P = .304) for ISACHC class 1a dogs; and 2,314; 876–3001 pmol/L versus 2,680; 1,844–3,001 pmol/L, respectively (P = .03) for class II dogs. In general, NT‐proBNP recoveries from the 1st generation assay were higher in 2013 than 2009 indicating that samples did not degrade during 4 years of frozen storage. This bias observed between 2009 and 2013 is within the normal lot‐to‐lot variability for the 1st generation Cardiopet kit.1
Statistical Analysis
Descriptive statistics summarized demographic and clinical data. Pearson's correlation assessed for potential associations. Mann‐Whitney rank‐sum test was used to compare 2 groups. One‐way analysis of variance on ranks analyzed differences in medians among clinical groups for continuous variables. Chi‐square analysis compared categorical variables. Receiver operator characteristic (ROC) curve analysis identified optimal [NT‐proBNP] cut‐off values to discriminate CHF versus noncardiac causes of dyspnea. The natural log of [NT‐proBNP] was used to achieve normality and analyzed by Kolmogorov‐Smirnoff testing (P < .05) for descriptive and multivariate models. Transformed data were used for formal analyses, whereas actual values were used for geometric means. Multivariate general linear models were built testing main effects and interactions between variables. Variables analyzed included age, body weight, sex, body condition score, murmur grade, presence or absence of respiratory distress, HR, echocardiographic indices (LA dimension and LA : Ao ratio in short axis, LVIDd and LVIDs; and maximal TR velocity), VHS, clinical cohort; and ISACHC and ACVIM schemes. Body condition score recorded on a 1–9 scale was collapsed to 3 levels (1–3, 4–6, and 7–9). Murmur grade recorded on a 1–6 scale was collapsed to 3 levels (1–2, 3–4, and 5–6). Because of potential collinearity of VHS and LA : Ao, these covariates were analyzed in separate models. Final models were selected based upon model goodness‐of‐fit criteria and statistical significance of covariates. Analyses were considered significant at P < .05 and multiple comparisons were adjusted with Fisher's least significant difference test (P < .05). Analyses were made using SAS 9.3.4
Results
Animals
Two hundred and ninety‐one recruited dogs represented 65 breeds (Table 1). The most common breeds with MMVD included: Cavalier King Charles Spaniel (n = 38; 13%), mixed breeds (n = 30; 10.3%), Beagle (n = 15; 5.1%), Chihuahua (n = 13; 4.5%), Miniature Poodle (n = 13; 4.5%), Yorkshire Terrier (n = 11; 3.8%), and Shi Tzu (n = 10; 3.4%). Twenty dogs had DCM most commonly involving the Doberman Pinscher (n = 5), Labrador Retriever (n = 3), Weimaraner (n = 2), and Boxer (n = 2). There were 168 males (23 [13.7%] intact; 145 [86.3%] neutered) and 123 females (13 [10.6%] intact; 110 [89.4%] neutered).
Clinical Status
All dogs were categorized into 1 of 4 cohorts (Table 1). The proportions of dogs with MMVD in groups 2, 3, and 4 were not different (P = .319). Group 1 was younger and heavier versus groups 2, 3, and 4 (P < .001). Pairwise comparisons for age and body weight among groups 2, 3, and 4 did not differ (all P > .05). Body condition score (P = .481) or proportion of males (P = .082) did not differ among groups. VHS was higher in group 4 versus all other groups (pairwise comparisons, P < .05). Heart murmur grade was not different among groups 2, 3, and 4 (P = .134). HR was lower in group 1 versus groups 2, 3, and 4, and higher in group 4 versus all other groups (all P < .05). LA : Ao ratio was higher in group 4 versus group 1 (P < .05), but not versus groups 2 or 3 (P > .05). LAd was larger in group 4 versus group 3 (P < .05). Tricuspid regurgitation was detected in 56% of the 291 dogs. The proportions with maximal TR velocities ≥2.8 m/s were 18.9, 40.5, and 43.4% in groups 2, 3, and 4, respectively. Estimated systolic pulmonary hypertension was moderate in 17.3% (group 3) and 9.5% (group 4); and severe in 8.6% (group 3) and 12.2% (group 4; Table 2).
Table 2.
Number and percentage of dogs with elevated systolic pulmonary artery pressure estimated from peak tricuspid regurgitation velocity
| Peak Systolic TR Velocity (m/s) Used to Designate Severity of Estimated Systolic Pulmonary Hypertension | Group 1: Asymptomatic No Heart Disease | Group 2: Asymptomatic Heart Disease | Group 3: Respiratory Distress, Primary Respiratory, or Airway Disease | Group 4: Respiratory Distress, Congestive Heart Failure |
|---|---|---|---|---|
| 2.8–3.5 (mild) | 0 | 12 (14.8) | 15 (14.4) | 20 (27) |
| >3.5–4.3 (moderate) | 1 (1.2) | 18 (17.3) | 7 (9.5) | |
| >4.3 (severe) | 1 (1.2) | 9 (8.6) | 9 (12.2) |
Plasma [NT‐proBNP] and HD Severity
Univariate analysis of plasma [NT‐proBNP] (median, IQR) differed significantly among cohorts (P < .001; Table 1; Fig 2). CHF dogs had higher [NT‐proBNP] than dogs with noncardiac dyspnea (5,110 pmol/L; 2,769–8,466 pmol/L versus 1,287 pmol/L; 672–2,704 pmol/L, respectively; pairwise comparisons, P < .05). Asymptomatic dogs without HD had lower [NT‐proBNP] versus all other groups (P < .05). Median [NT‐proBNP] did not differ between noncardiac respiratory distress dogs versus asymptomatic dogs with HD (P > .05). In the CHF cohort, [NT‐proBNP] did not differ between DCM (6,784; 5,097–10,618 pmol/L) and MMVD (4,893; 2,749–8,465 pmol/L; P = .1).
Figure 2.
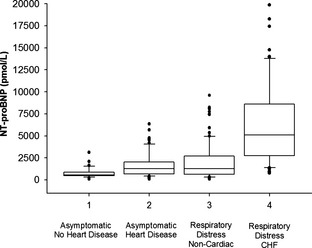
Plasma NT‐proBNP concentrations from 4 clinical cohorts (total = 291 dogs). Boxes show lowest, median, and upper quartiles. Whiskers denote the 90th and 10th percentile, respectively; black symbols represent outliers. Median plasma [NT‐proBNP] in congestive heart failure (CHF) dogs (group 4) was significantly higher (P < .05) than dogs with respiratory distress from noncardiac diseases (group 3), dogs with asymptomatic heart disease (group 2), and asymptomatic dogs without heart disease (group 1); median plasma [NT‐proBNP] was significantly higher in group 3 versus group 1 (P < .05).
Plasma [NT‐proBNP] >3,000 pmol/L was recorded in 68.9% of CHF (group 4) dogs (Table 1, Fig 2). The highest [NT‐proBNP] recorded in noncardiac respiratory dogs (group 3) was 9,603 pmol/L, whereas 17.6% of CHF dogs exceeded this concentration (highest concentration was 19,873 pmol/L). In comparison, 14.9% of group 2 dogs had [NT‐proBNP] >3,000 pmol/L (range, 3,378–6,349 pmol/L).
A 2,447 pmol/L [NT‐proBNP] cut‐off value conferred 81.1% sensitivity and 73.1% specificity to differentiate dogs with cardiac from noncardiac cause of respiratory distress. The ROC curve area under the curve (AUC) was 0.84 (Fig 3). Lower and higher cut‐off values affected sensitivity and specificity (Table 3). For example, a lower cut‐off of 1,492 pmol/L increased sensitivity (87.8%) but decreased specificity (59%); a 4,247 pmol/L cut‐off decreased sensitivity (59.5%) but increased specificity (87.5%).
Figure 3.
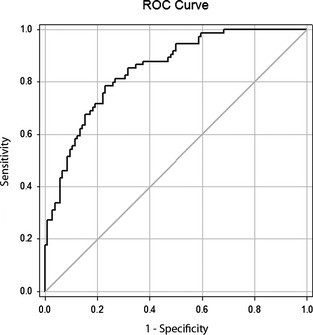
Receiver‐operating characteristic (ROC) curve illustrating the sensitivity and specificity of plasma [NT‐proBNP] to distinguish dogs with respiratory distress associated with congestive heart failure from dogs with respiratory distress because of noncardiac diseases. Area under the ROC curve is 0.84.
Table 3.
Receiver operating curve (ROC) analyses showing selected plasma [NT‐proBNP] cut‐off values that distinguish cardiac from noncardiac cause of respiratory distress
| BNP Cut‐off Value (pmol/L) | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|
| 1,492 | 0.878 (0.782–0.943) | 0.590 (0.428–0.627) |
| 2,201 | 0.824 (0.718–0.903) | 0.692 (0.594–0.779) |
| 2,402 | 0.811 (0.703–0.892) | 0.712 (0.614–0.796) |
| 2,418 | 0.811 (0.703–0.892) | 0.721 (0.625–0.805) |
| 2,447 | 0.811 (0.703–0.892) | 0.731 (0.635–0.813) |
| 2,483 | 0.797 (0.688–0.882) | 0.731 (0.635–0.813) |
| 3,021 | 0.716 (0.599–0.815) | 0.808 (0.719–0.878) |
| 4,247 | 0.595 (0.474–0.707) | 0.875 (0.796–0.932) |
The ability of plasma [NT‐proBNP] to discriminate levels of HD severity differed between ISACHC and ACVIM‐HD schemes (P < .0001; Table 4). Using the ISACHC scheme, [NT‐proBNP] was significantly higher in class 3 versus class 1, and class 2 versus class 1 (all P < .05), but not between classes 3 and 2 (P > .05). With the ACVIM‐HD scheme, plasma [NT‐proBNP] was higher in stage C versus B2, B1, and A; stage B2 was higher than stages B1 and A (all P < .05). Only stages B1 and A did not differ significantly (P > .05).
Table 4.
Plasma [NT‐proBNP] concentrations grouped in accordance to ISACHC and ACVIM‐HD classification schemes
| ISACHC System | ACVIM System | ||||
|---|---|---|---|---|---|
| Class | [NT‐proBNP] (pmol/L), median (IQR) | P < .05 | Stage | [NT‐proBNP] (pmol/L), median (IQR) | P < .05 |
| A | 535 (373–723) | *, ** | |||
| 1A | 639 (399–1,010) | *, **, ***, † | B1 | 665 (401–1,024) | *, ** |
| 1B | 1,627 (941–2,972) | *, **, ***, † | B2 | 1,405 (789–2,375) | *, ** |
| 2 | 4,942 (2,762–8,488) | *** | C | 3,922 (2091–7,111) | * |
| 3A | 5,313 (4,067–7,581) | ** | D‡ | ||
| 3B | 5,252 (1,954–13,418) | *, *** | |||
Symbols designate statistically different pairwise comparisons (P < .05).
*3B > 1B, 3B > 1A; **3A > 1B, 3A > 1A;***2 > 1B, 2 > 1A; †1B > 1A; *C > B2, C > B1, C > A; **B2 > B1, B2 > A; ‡No cases represented stage D.
Multivariate modeling compared HD classification schemes and cohort based upon average differences from a single plasma [NT‐proBNP] measurement, and taking into account other clinical variables (Table 5). Plasma [NT‐proBNP] was not statistically different between ISACHC classes 2 and 3 that denote mild CHF and moderate to severe CHF, respectively. Therefore, this scheme was not evaluated further. Plasma [NT‐proBNP] was significantly different among all ACVIM‐HD stages except between B1 and A, which were combined for modeling purposes. Multivariate models that included sex, BCS, murmur grade, pulmonary hypertension, age, body weight, and TR maximal velocity were nonsignificant and were excluded from the final models. Although LVIDd, LVIDs, and LA2d were significant in the presence of LA : Ao and VHS, LAd was collinear with other explanatory variables, as were the covariates LVIDd, LVIDs, and %FS. LVIDs exhibited better fit statistics and was retained for modeling, whereas LVIDd, %FS, and LAd were removed.
Table 5.
Comparison of 2 multivariate models that evaluate clinical variables, ACVIM HD classifications, and study cohorts
| Model Variables | VHS Model (Model 1) | LA : Ao Model (Model 2) |
|---|---|---|
| ACVIM classification | P < .0001 | P < .0001 |
| Cohort classifications | P = .0079 | P = .0024 |
| LVIDs | P = .0111 | P = .0177 |
| HR | P = .0061 | P = .0055 |
| VHS | P = .0018 | a |
| LA : Ao | a | P = .0182 |
| ACVIM classifications | (Pairwise Difference) | (Pairwise Difference) |
| Class: C versus B2 | P < .05 | P < .05 |
| Class: C versus AB1 (combined) | P < .05 | P < .05 |
| Class: B2 versus AB1 (combined) | P < .05 | P < .05 |
| Clinical cohorts | (Pairwise Difference) | (Pairwise Difference) |
| CHF versus respiratory | ||
|
(Group 4 versus group 3) CHF versus Asympt. HD |
P < .05 | P < .05 |
|
(Group 4 versus group 2) CHF versus Asympt. No HD |
P < .05 | P < .05 |
|
(Group 4 versus group 1) Respiratory versus Asympt. No HD |
NS | P < .05 |
|
(Group 3 versus group 1) Respiratory versus Asympt. HD |
NS | NS |
|
(Group 3 versus group 2) Asympt. HD versus Asympt. No HD (Group 2 versus group 1) |
NS | P < .05 |
LVIDs, left ventricular diameter, end‐systole; HR, heart rate; VHS, vertebral heart size; LA : Ao, left atrial to aortic root ratio; CHF, congestive heart failure; HD, heart disease; Asypmt., asymptomatic; NS, not statistically significant.
Variable not included from respective model.
Two multivariate models detected mean plasma [NT‐proBNP] differences (Tables 5, 6). Model 1 included ACVIM‐HD stage, clinical cohort, HR, LVIDs, and VHS. Model 2 was identical except that LA : Ao was substituted for VHS. HR and LVIDs persisted as significant predictors in both models. In model 1, ACVIM‐HD classification variables were significant (P < .0001), all pairwise comparisons were significant (P < .05), and cohort type was highly significant (P = .008); all pairwise comparisons with group 4 compared to groups 3, 2, and 1 were statistically significant. Model 2 behaved similarly to model 1; all pairwise comparisons for ACVIM‐HD classes were different (P < .0001); all pairwise comparisons for cohort also were different from each other (P = .0024), and also showed differences between groups 3 versus 1 and groups 2 versus 1, which model 1 using VHS did not detect.
Table 6.
Harmonic mean and standard deviation values for plasma [NT‐proBNP] concentrations recorded from 4 study cohorts. Harmonic means were the same for models 1 and 2
| Group | Clinical Cohort | |
|---|---|---|
| Description | Plasma [NT‐proBNP] (mean ± SD) | |
| 1 | Asymptomatic, no HD | 906.8 ± 1,173.4 |
| 2 | Asymptomatic, compensated HD | 1,593.8 ± 1,292.5 |
| 3 | Respiratory distress, non‐cardiogenic | 2,064.5 ± 2,064.0 |
| 4 | Respiratory distress, CHF | 6,143.3 ± 4,517.3 |
HD, heart disease; CHF, congestive heart failure.
Discussion
We evaluated the clinical utility of a validated, 2nd generation, commercial canine, ELISA test to compare plasma [NT‐proBNP] in a heterogeneous study population comprising asymptomatic dogs with and without HD, and dogs with respiratory distress. The predominantly small breed, middle to older age dogs in our study closely reflect the demographics of dogs at risk for these conditions.7, 18, 25, 27 The AUC for the ROC curve analysis to distinguish cardiac from noncardiac cause of respiratory distress was 0.84 (Fig 3). This indicated that plasma [NT‐proBNP] was useful and accurate within the context of 81% specificity and 73% sensitivity, using a 2,447 pmol/L cut‐off value. Other studies of dogs have reported relatively similar natriuretic peptide sensitivity and specificity with respective values ranging from 85 to 82.4%,28 86.4 to 80.8%,4 90 to 78%,5 and 85.5 to 81.3%.8 Our study confirmed the utility of measuring plasma [NT‐proBNP] to help discriminate cardiac from noncardiac causes of dyspnea, consistent with earlier reports.4, 5, 6, 7, 8, 27, 28
The extended upper range of this assay2 detected a substantial number of CHF dogs with plasma [NT‐proBNP] exceeding 3,000 pmol/L (68.9%) versus 18.3% of dogs with noncardiogenic dyspnea and 14.9% of asymptomatic dogs with HD (Table 1, Fig 2). Approximately, 28% of CHF dogs had [NT‐proBNP] >8,000 pmol/L, the highest reaching 19,873 pmol/L. In contrast, only 3% of dogs with noncardiac respiratory distress had concentrations >8,000 pmol/L, with the highest being 9,603 pmol/L. Extremely high BNP calculations have been reported in human patients with renal dysfunction, elderly status, and cancer,20, 21, 29 although this phenomenon remains poorly understood.30 Increased circulating natriuretic peptide concentrations in dogs have been associated with renal failure, high serum creatinine concentration,9, 28, 31, 32 and severe pulmonary hypertension,33 although in this study, [NT‐proBNP] was weakly associated with both serum creatinine concentration and pulmonary hypertension. Extremely high [NT‐proBNP] in CHF dogs exceeded the highest values recorded from all other cohorts. The potential clinical relevance of these findings requires further investigation.
Schemes to characterize HD severity attempt to define tiered classes or stages of disease. Plasma [NT‐proBNP] was not statistically different between ISACHC stages 2 and 3, categories that denote mild versus moderate to severe CHF, respectively. Our data regarding this distinction were in keeping with findings from previous reports.5, 9 This study did reveal significant differences in plasma [NT‐proBNP] among all stages of ACVIM‐HD severity, except between stage B1 designating dogs with a heart murmur but no evidence of heart enlargement, and stage A describing genetic risk to develop HD (Table 4). Furthermore, [NT‐proBNP] was significantly higher in stage C, a category that encompasses previous or current CHF patients, from stage B2, a category restricted to dogs with heart enlargement but not CHF. Collectively, the ACVIM‐HD scheme demonstrated that dogs with more advanced stages of HD had higher [NT‐proBNP] than those with less advanced disease.
Multivariate modeling demonstrated differences among ACVIM‐HD stages based on a single plasma [NT‐proBNP] measurement. Significant variables included clinical cohort, HR, LVIDs, and VHS or LA : Ao. Age, sex, body weight, BCS, murmur grade, moderate or severe pulmonary hypertension, and TR maximal velocity were not statistically significant.
Model 2 which included LA : Ao instead of VHS, was slightly superior to model 1 (VHS instead of LA : Ao) as it additionally demonstrated statistical differences between dogs with respiratory distress because of noncardiac disease versus both asymptomatic dogs with HD and the normal cohort (Table 5).
This study had several limitations. NT‐proBNP variability has been reported in healthy dogs,34 heart failure patients,35 and among breeds,36 but these factors were not specifically considered in our analyses. Economic considerations precluded centralized evaluation of radiographic and echocardiographic data. Guidelines for characterizing HD severity are semiquantitative and subject to interpretation. Blood samples were collected over 2 time periods, but frozen storage did not appear to degrade [NT‐proBNP] from the earlier samples. We did not directly assess whether protease inhibition had an effect on plasma [NT‐proBNP] other than reducing its premature degradation. The cohort of healthy dogs without HD was not matched for age or weight with other cohorts, and cohorts were not matched with regard to breed.
Overall, plasma [NT‐proBNP] using this 2nd generation assay reliably discriminated CHF from noncardiac causes of respiratory distress. Concentrations were more substantially increased in dogs with CHF and with greater severity of HD. Severely increased [NT‐proBNP] was detected, but cause‐effect relationships require further examination. Using plasma [NT‐proBNP] with ACVIM‐HD classification scheme, clinical cohort, HR, LVIDs, and either VHS or LA : Ao provided complementary information that strengthened stratification of HD severity. Significant increases in plasma [NT‐proBNP] occurred with worse stages of HD using the ACVIM‐HD, but not the ISACHC scheme. NT‐proBNP measurement should be utilized in conjunction with medical history, physical examination, echocardiographic and radiographic findings, and other tests to optimize clinical diagnosis.
Acknowledgment
This study was supported by a grant from IDEXX Laboratories, Westbrook, ME.
Conflict of Interest Declaration: Drs Fox, Oyama, Rush, Stepien, Gordon, and Nguyenba have consulted for IDEXX; Dr Cunningham has received research funding from IDEXX.
Off‐label Antimicrobial Declaration: The authors declare no off‐label use of antimicrobials.
The work was done at institutions and practices listed for each author.
Preliminary study results were presented at the 20th ECVIM Congress in Toulouse, France, September, 2010, and 23rd ECVIM Congress, Liverpool, UK, September, 2013.
Footnotes
VETSIGN Canine CardioSCREEN NT‐proBNP Guildhay Ltd., Biomedica, Vienna, Austria (Manufactured and distributed by IDEXX Laboratories, Inc. as of 2008, including Canine Cardiopet proBNP in the US as of 2009)
Canine Cardiopet proBNP, IDEXX Laboratories, Westbrook, ME (launched 2013)
Cahill R, Pigeon K, Strong‐Townsend M, et al. Bioanalytical method validation of a 2nd generation immunoassay for the quantification of NT‐proBNP in canine blood. J Vet Intern Med 2013;3:638
SAS Institute, Cary, NC
References
- 1. Christenson ES, Collinson PO, Defilippi CR, et al. Heart failure biomarkers at point‐of‐care: Current utilization and future potential. Expert Rev Mol Diagn 2014;47:327–337. [DOI] [PubMed] [Google Scholar]
- 2. Troughton R, Michael Felker G, Januzzi JL Jr. Natriuretic peptide‐guided heart failure management. Eur Heart J 2014;35:16–24. [DOI] [PubMed] [Google Scholar]
- 3. Oyama MA, Boswood A, Connolly DJ, et al. Clinical usefulness of an assay for measurement of circulating N‐terminal pro‐B type natriuretic peptide concentration in dogs and cats with heart disease. J Am Vet Med Assoc 2013;243:71–82. [DOI] [PubMed] [Google Scholar]
- 4. Prosek R, Sisson DD, Oyama MA, et al. Distinguishing cardiac and noncardiac dyspnea in 48 dogs using plasma atrial natriuretic factor, B‐type natriuretic factor, endothelin, and cardiac troponin‐I. J Vet Intern Med 2007;21:238–242. [DOI] [PubMed] [Google Scholar]
- 5. DeFrancesco TC, Rush JE, Rozanski EA, et al. Prospective clinical evaluation of an ELISA B‐type natriuretic peptide assay in the diagnosis of congestive heart failure in dogs presenting with cough or dyspnea. J Vet Intern Med 2007;21:243–250. [DOI] [PubMed] [Google Scholar]
- 6. Fine DM, DeClue AE, Reinero CR. Evaluation of circulating amino terminal‐pro‐B‐type natriuretic peptide concentration in dogs with respiratory distress attributable to congestive heart failure or primary pulmonary disease. J Am Vet Med Assoc 2008;232:1674–1679. [DOI] [PubMed] [Google Scholar]
- 7. Ettinger SJ, Farace G, Forney SD, et al. Evaluation of plasma N‐terminal pro‐B‐type natriuretic peptide concentrations in dogs with and without cardiac disease. J Am Vet Med Assoc 2012;240:171–180. [DOI] [PubMed] [Google Scholar]
- 8. Oyama MA, Rush JE, Rozanski EA, et al. Assessment of serum N‐terminal pro‐B‐type natriuretic peptide concentration for differentiation of congestive heart failure from primary respiratory tract disease as the cause of respiratory signs in dogs. J Am Vet Med Assoc 2009;235:1319–1325. [DOI] [PubMed] [Google Scholar]
- 9. Oyama MA, Fox PR, Rush JE, et al. Clinical utility of serum N‐terminal pro‐B‐type natriuretic peptide concentration for identifying cardiac disease in dogs and assessing disease severity. J Am Vet Med Assoc 2008;232:1496–1503. [DOI] [PubMed] [Google Scholar]
- 10. Reynolds CA, Brown DC, Rush JE, et al. Prediction of first onset of congestive heart failure in dogs with degenerative mitral valve disease: The PREDICT cohort study. J Vet Cardiol 2012;14:193–202. [DOI] [PubMed] [Google Scholar]
- 11. Häggström J, Hansson K, Kvart C, et al. Relationship between different natriuretic peptides and severity of naturally acquired mitral regurgitation in dogs with chronic myxomatous valve disease. J Vet Cardiol 2000;2:7–16. [DOI] [PubMed] [Google Scholar]
- 12. Greco DS, Biller B, Van Liew CH. Measurement of plasma atrial natriuretic peptide as an indicator of prognosis in dogs with cardiac disease. Can Vet J 2003;44:293–297. [PMC free article] [PubMed] [Google Scholar]
- 13. Tarnow I, Olsen LH, Kvart C, et al. Predictive value of natriuretic peptides in dogs with mitral valve disease. Vet J 2009;180:195–201. [DOI] [PubMed] [Google Scholar]
- 14. Moonmart W, Boswood A, Luis Fuentes V, et al. N‐terminal pro B‐type natriuretic peptide and left ventricular diameter independently predict mortality in dogs with mitral valve disease. J Small Anim Pract 2010;51:84–96. [DOI] [PubMed] [Google Scholar]
- 15. Singletary GE, Morris NA, O'Sullivan LM, et al. Prospective evaluation of NT‐proBNP assay to detect occult dilated cardiomyopathy and predict survival in Doberman Pinschers. J Vet Intern Med 2012;26:1330–1336. [DOI] [PubMed] [Google Scholar]
- 16. Wess G, Butz V, Mahling M, et al. Evaluation of N‐terminal pro‐B type natriuretic peptide as a diagnostic marker of various stages of cardiomyopathy in Doberman Pinschers. Am J Vet Res 2011;72:642–649. [DOI] [PubMed] [Google Scholar]
- 17. Schober KE, Hart TM, Stern JA, et al. Effects of treatment on respiratory rate, serum natriuretic peptide concentration and Doppler echocardiographic indices of left ventricular filling pressure in dogs with congestive heart failure secondary to degenerative mitral valve disease and dilated cardiomyopathy. J Am Vet Med Assoc 2011;239:468–479. [DOI] [PubMed] [Google Scholar]
- 18. International Small Animal Cardiac Health Council . Recommendations for the Diagnosis and Treatment of Heart Failure in Small Animals. Woodbridge, NJ: ISACHC Publication; 1994:5. [Google Scholar]
- 19. Atkins C, Bonagura J, Ettinger S, et al. Guidelines for the diagnosis and treatment of canine chronic valvular heart disease. J Vet Intern Med 2009;23:1142–1150. [DOI] [PubMed] [Google Scholar]
- 20. Cui H, Huo G, Liu L, et al. Association of cardiac and renal function with extreme N‐terminal fragment pro‐B‐type natriuretic peptide levels in elderly patients. BMC Cardiovasc Disord 2012;12:57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Law C, Glover C, Benson K, et al. Extremely high brain natriuretic peptide does not reflect the severity of heart failure. Congest Heart Fail 2010;16:221–225. [DOI] [PubMed] [Google Scholar]
- 22. Nakayama H, Nakayama T, Hamlin RL. Correlation of cardiac enlargement as assessed by vertebral heart size and echocardiographic and electrocardiographic findings in dogs with evolving cardiomegaly due to rapid ventricular pacing. J Vet Intern Med 2001;15:217–221. [DOI] [PubMed] [Google Scholar]
- 23. Thomas WP, Gaber CE, Jacobs GJ, et al. Recommendations for standards in transthoracic two‐dimensional echocardiography in the dog and cat. Echocardiography Committee of the Specialty of Cardiology, American College of Veterinary Internal Medicine. J Vet Intern Med 1993;7:247–252. [DOI] [PubMed] [Google Scholar]
- 24. Rishniw M, Erb HN. Evaluation of four 2‐dimensional echocardiographic methods of assessing left atrial size in dogs. J Vet Intern Med 2000;14:429–435. [DOI] [PubMed] [Google Scholar]
- 25. Johnson L, Boon J, Orton EC. Clinical characteristics of 53 dogs with Doppler‐derived evidence of pulmonary hypertension: 1992–1996. J Vet Intern Med 1999;13:440–447. [DOI] [PubMed] [Google Scholar]
- 26. Schober KE, Baade H. Doppler echocardiographic prediction of pulmonary hypertension in west highland white terriers with chronic pulmonary disease. J Vet Intern Med 2006;20:912–920. [DOI] [PubMed] [Google Scholar]
- 27. Chetboul V, Serres F, Tissier R, et al. Association of plasma N‐terminal pro‐B‐type natriuretic peptide concentration with mitral regurgitation severity and outcome in dogs with asymptomatic degenerative mitral valve disease. J Vet Intern Med 2009;23:984–994. [DOI] [PubMed] [Google Scholar]
- 28. Boswood A, Dukes‐McEwan J, Loureiro J, et al. The diagnostic accuracy of different natriuretic peptides in the investigation of canine cardiac disease. J Small Anim Pract 2008;49:26–32. [DOI] [PubMed] [Google Scholar]
- 29. Guglin M, Hourani R, Pitta S. Factors determining extreme brain natriuretic peptide elevation. Congest Heart Fail 2007;13:136–141. [DOI] [PubMed] [Google Scholar]
- 30. Popat J, Rivero A, Pratap P, et al. What is causing extremely elevated amino terminal brain natriuretic peptide in cancer patients? Congest Heart Fail 2013;19:143–148. [DOI] [PubMed] [Google Scholar]
- 31. Raffan E, Loureiro J, Dukes‐McEwan J, et al. The cardiac biomarker NT‐proBNP is increased in dogs with azotemia. J Vet Intern Med 2009;23:1184–1189. [DOI] [PubMed] [Google Scholar]
- 32. Miyagawa Y, Tominaga Y, Toda N, et al. Relationship between glomerular filtration rate and plasma N‐terminal pro B‐type natriuretic peptide concentrations in dogs with chronic kidney disease. Vet J 2013;197:445–450. [DOI] [PubMed] [Google Scholar]
- 33. Kellihan HB, Mackie BA, Stepien RL. NT‐proBNP, NT‐proANP and cTnI concentrations in dogs with pre‐capillary pulmonary hypertension. J Vet Cardiol 2011;13:171–182. [DOI] [PubMed] [Google Scholar]
- 34. Kellihan HB, Oyama MA, Reynolds CA, et al. Weekly variability of plasma and serum NT‐proBNP measurements in normal dogs. J Vet Cardiol 2009;11:S93–S97. [DOI] [PubMed] [Google Scholar]
- 35. Bruins S, Fokkema MR, Römer JW, et al. High intraindividual variation of B‐type natriuretic peptide (BNP) and amino‐terminal proBNP in patients with stable chronic heart failure. Clin Chem 2004;50:2052–2058. [DOI] [PubMed] [Google Scholar]
- 36. Sjöstrand K, Wess G, Ljungvall I, et al. Breed differences in natriuretic peptides in healthy dogs. J Vet Intern Med 2014;28:451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]


