Abstract
Background
Acid suppressant drugs are a mainstay of treatment for cats with gastrointestinal erosion and ulceration. However, clinical studies have not been performed to compare the efficacy of commonly PO administered acid suppressants in cats.
Hypothesis/Objectives
To compare the effect of PO administered famotidine, fractionated omeprazole tablet (fOT), and omeprazole reformulated paste (ORP) on intragastric pH in cats. We hypothesized that both omeprazole formulations would be superior to famotidine and placebo.
Animals
Six healthy adult DSH colony cats.
Methods
Utilizing a randomized, 4‐way crossover design, cats received 0.88–1.26 mg/kg PO q12h fOT, ORP, famotidine, and placebo (lactose capsules). Intragastric pH monitoring was used to continuously record intragastric pH for 96 hours beginning on day 4 of treatment. Plasma omeprazole concentrations at steady state (day 7) were determined by high performance liquid chromatography (HPLC) with ultraviolet detection. Mean percentage time that intragastric pH was ≥3 and ≥4 were compared among groups using ANOVA with a posthoc Tukey‐Kramer test (α = 0.05).
Results
The mean percentage time ± SD that intragastric pH was ≥3 was 68.4 ± 35.0% for fOT, 73.9 ± 23.2% for ORP, 42.8 ± 18.6% for famotidine, and 16.0 ± 14.2% for placebo. Mean ± SD plasma omeprazole concentrations were similar in cats receiving fOT compared to those receiving ORP and in a range associated with acid suppression reported in other studies.
Conclusions and Clinical Importance
These results suggest that both omeprazole formulations provide superior acid suppression in cats compared to famotidine or placebo. Fractionated enteric‐coated OT is an effective acid suppressant despite disruption of the enteric coating.
Keywords: Bravo monitoring, Famotidine, Feline, Omeprazole
Abbreviations
- AUC
area under the concentration‐time curve
- fOT
fractionated omeprazole tablet
- H2RA
histamine 2 receptor antagonists
- HPLC
high performance liquid chromatography
- ORP
omeprazole reformulated paste
- OT
omeprazole tablet
- PK
pharmacokinetics
- PPIs
proton pump inhibitors
When the protective mechanisms of the stomach are compromised, the acidic and proteolytic environment can contribute to the development of gastroduodenal ulceration.1, 2, 3 Disorders that may disrupt the gastroduodenal mucosal barrier include both gastric and nongastric diseases including gastrointestinal neoplasia, liver failure, critical illness, drug toxicity, gastrointestinal infections (eg, Helicobacter) and inflammatory bowel disorders among others.2, 4, 5, 6, 7 Gastric ulceration and its sequelae can be severe.2, 6, 7, 8, 9 Thus, gastric acid suppressants including histamine‐2 receptor antagonists (H2RA) and proton pump inhibitors (PPIs) are among the most widely prescribed medications for adjunctive treatment of diseases that disrupt the mucosal barrier and predispose cats to development of gastrointestinal erosion and ulceration. Proton pump inhibitors including omeprazole increase intragastric pH by covalently binding to parietal cell proton pumps (H+, K+‐ATPase), which represent the final common target for acid secretion.10 This inhibition is potent, acting against basal and stimulated gastric acid production. Omeprazole is more effective at increasing intragastric pH than H2RAs in both humans11 and dogs12, 13, 14 and is widely used for the treatment of acid‐related disorders in these species.
Several barriers prevent optimal dosing of commercially available omeprazole formulations in cats. These include proposed dosing requirements that are not practically obtained by manipulation of dosage forms intended for humans and the inclusion of substances in some omeprazole formulations that are potentially toxic to cats. For example, the omeprazole suspension approved for humans1 is not a good option because it has a low concentration of omeprazole (2 mg/mL), is potentially toxic (contains xylitol) and is inappropriately flavored (pineapple) for cats. Omeprazole capsules are only widely available in sizes that make dosing for cats difficult. Although omeprazole tablets are easier to manipulate, they contain a protective coating on their surface to prevent premature gastric degradation.15 Lacking other practical alternatives, veterinary practitioners often divide or crush these tablets for optimal dosing in cats; however, the efficacy of the fractionated tablets to increase intragastric pH after damage to their enteric coating is unknown. Breaking the tablets potentially could result in a lower systemic bioavailability because of drug degradation in the acid environment of the stomach, but this effect could be temporary because continued administration may result in suppression of gastric acid secretion, and therefore inhibition of premature degradation.16 Thus, fractionated omeprazole tablets may be a good option in cats. Finally, an omeprazole paste,2 widely used in equine medicine, recently was found to be effective in dogs.12 Omeprazole reformulated paste can be diluted in a fixed oil (eg, cod liver oil, corn oil), thus providing a more palatable omeprazole formulation that also is easily dosed for a range of cat sizes. To the authors' knowledge, comparative studies investigating the clinical efficacy of PO administered omeprazole formulations and famotidine in cats are not available. Therefore, the purpose of this study was to compare the effect of PO administered fractionated omeprazole tablet, omeprazole reformulated paste and famotidine on intragastric pH in cats. On the basis of previous evidence in human and canine medicine, we hypothesized that both omeprazole formulations would be more efficacious in increasing feline intragastric pH than famotidine or placebo. A continuous analysis of the pharmacodynamics of omeprazole and famotidine on gastric pH in cats was obtained using a minimally invasive continuous intragastric pH monitoring device.3 In addition, we sought to compare the pharmacokinetics of the 2 omeprazole formulations at steady state.
Materials and Methods
Cats
The Institutional Animal Care and Use Committee (IACUC) at the University of Tennessee approved the protocol for this study (Approval #2193‐0693). The subjects of this study were 7 healthy adult domestic shorthair cats from a research colony at the University of Tennessee (3 neutered females, 4 neutered males), aged 3–10 years (median, 5 years) and weighing 3.97–5.68 kg (median, 4.55 kg). Cats included in the study were deemed healthy on the basis of normal physical examination, normal CBC and biochemistry profile performed within 6 months of study entry. In addition, cats had no evidence of gastrointestinal disease and had normal PCV, as well as normal total serum protein, blood urea nitrogen and blood glucose concentrations and urinalysis results at study entry. To ensure inclusion of healthy cats and to comply with IACUC guidelines, cats were excluded from the study if they developed inappetence for >24 hours, lost >10% of their body weight, had gross evidence of disease on gastroesophagoscopy during the study period or some combination of these. Four times per day, cats were allowed to roam in an enclosed room and were engaged in playful activities. The receivers remained in the middle of the room within 6 feet of the cats at all times to allow for continued data collection during free‐roaming periods.
Study Design
In a randomized, open‐label, 4‐way crossover design, cats were administered placebo4 (250 mg lactose capsule PO q12h), famotidine5 (0.88–1.26 mg/kg PO q12h), fractionated omeprazole tablets (fOT6 ; 0.88–1.26 mg/kg PO q12h) or omeprazole reformulated paste (ORP2; 0.88–1.26 mg/kg PO q12h) for 7 consecutive days followed by a minimum 10‐day washout period. Cats were randomized to a treatment group using a random number generator. The goal of treatment was to achieve a dosage that approximated 1 mg/kg PO q12h in all treatment arms. The dosage of each drug was kept consistent among treatment arms for each cat (eg, cat 1 received 1.1 mg/kg PO q12h for all study drugs). Omeprazole paste was reformulated to a suspension at a concentration of 10 mg/ml by mixing an approved oral paste for horses (Gastrogard) in cod liver oil at a ratio of 1:39 and stored at a controlled cold temperature (7°C) protected from light. To be conservative, a beyond use date of 90 days was assigned to the refrigerated reformulated paste, which is within the US Pharmacopeial standard of 180 days for nonaqueous compounded oral liquids. Cats were medicated at 7:30 am and 5:30 pm daily. Each medication was followed by oral syringe administration of 3 ml of water and swallowing of the medication was witnessed. Cats were fed a maintenance diet7 30 minutes after medicating at 8:00 am and 6:00 pm daily. This time interval was selected to best mimic the feeding schedule of many client‐owned cats. Cats had unlimited access to water during the pH monitoring period. Clinical signs, including changes in attitude, inappetence, vomiting, number of defecations, and fecal consistency were recorded twice daily. An episode of inappetence was defined as <50% of the meal ingested. Vomitus was evaluated for the presence of medication when it occurred. Feces were graded from 1 to 7 by a standardized fecal scoring system.8 Diarrhea was defined as a fecal score of ≥4.
Placement of Intragastric pH Monitor
On the morning of day 4 of each treatment period, the morning meal was withheld and cats were anesthetized for gastroscopy‐assisted placement of a Bravo pH capsule. Cats were premedicated with dexmedetomidine9 (0.005 mg/kg), ketamine10 (5 mg/kg) and butorphanol11 (0.4 mg/kg) IM. An IV catheter was placed and general anesthesia was induced with propofol12 to effect. Cats were maintained with sevoflurane13 in 100% oxygen after endotracheal tube placement. Peripheral catheters were placed under premedication to allow for induction drug administration and blood sampling. Gastroscopy was performed with cats in left lateral recumbency to aid in position and attachment of the pH capsule to the fundic mucosa, 2–5 cm distal to the lower esophageal sphincter. This location was kept consistent among treatment groups by utilizing the measurements on the insertion tube of the scope to measure the distance from the canine teeth to the lower esophageal sphincter (LES) and from the LES to the area of desired capsule placement. At initial endoscopy for each cat, the entire stomach and esophagus were evaluated for evidence of gross disease. Immediately before capsule placement, the capsule and 2 receivers for each cat (1 for the first 48 hours and 1 for the second 48 hours) were calibrated with commercial buffer solutions (pH 1.07 and 7.01) according to manufacturer's instructions. The capsule, preassembled with a catheter delivery system, was introduced into the stomach transorally. Once the desired position of the capsule was verified, mucosal attachment of the pH capsule was achieved as previously reported12 according to the manufacturer's instructions with some modifications. External vacuum suction was applied to the capsule delivery system to achieve a minimum of 510 mmHg for 15 seconds. This protocol was altered from the manufacturer recommendations of 30 seconds to allow for natural passage of the capsule in cats. The protocol was altered because of 1 of the author's (MKT) experience that cats retain capsules for a longer period of time compared to dogs. After application of vacuum suction (Fig. 1A), a spring‐loaded pin mechanism was initiated to engage suctioned mucosa within the capsule well. The vacuum source was turned off and the delivery system was withdrawn from the cat. Mucosal attachment of the capsule was confirmed by endoscopic visualization of secured mucosa within the well of the capsule (Fig. 1B). Capsules that remained adhered to the gastric mucosa from the previous treatment upon placement of a new capsule were either removed by a polypectomy snare or were allowed to slough off the gastric mucosa. Cats were reversed with atipamezole14 (0.05 mg/kg IM) after the procedure.
Figure 1.
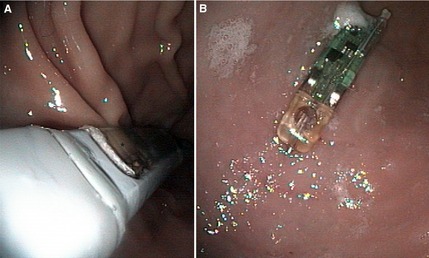
(A) Placement of Bravo pH capsule in the gastric fundus of a cat under endoscopic guidance using manufacturer supplied delivery device. Mucosa can be seen within the well of the capsule (black asterisk). (B) Bravo pH capsule adhered to fundic mucosa as visualized by endoscope (resting immediately beyond lower esophageal sphincter).
pH Recordings
Intragastic pH recordings were obtained telemetrically at 6‐second sampling intervals for 96 hours (days 4–7 of treatment) after capsule placement. Data receivers were kept near the cage or on the cage door of all cats during the data acquisition phase. After 48 hours of pH data acquisition (the maximal amount of data held by the receiver), pH data was uploaded from the first receiver to the computer using manufacturer software (Polygram Net15 ). The percentage of time that intragastric pH was ≥3 and ≥4 and in 1 of each of 8 categories (pH 0–1, 1–2, 2–3, 3–4, 4–5, 5–6, 6–7, 7–8) was calculated by the computer software. The second receiver previously calibrated to the existing capsule was used to collect the final 48 hours of data.
Sample Collection and Pharmacokinetic Analysis
Blood samples were obtained from cats receiving fOT or ORP on day 7 of treatment (steady state). Blood (1 ml) was collected from a peripheral catheter into heparinized tubes 0.25, 0.5, 1, 2, 4, 6, and 8 hours after administration of the morning medication. Blood samples were centrifuged at 250 × g for 10 minutes. Plasma was transferred to a cryovial and stored at −80°C until analyzed. All plasma samples were analyzed for omeprazole concentration within 3 months of collection using HPLC with ultraviolet detection using a previously published method with partial validation to account for feline plasma.16, 17 The limit of quantification was 0.01 μg/ml. A zero value was assigned to all measurements determined to be below the limit of quantification. Pharmacokinetic parameter estimates for each omeprazole formulation were estimated using computer software.16 A 1‐compartment model with first order input and elimination and no lag time, according to the following equation was used:
where C is plasma concentration at time t, D po is oral dose, F is bioavailability (unknown in this study), V is volume of distribution divided by bioavailability, K 01 is the absorption rate constant, and K 10 is the elimination rate constant. Plasma concentrations were weighted by the reciprocal of the predicted value squared to obtain the best fit. The best fit model was chosen based on visual inspection of the data, residual plots, and Aikake's Information Criterion (AIC). Values for C MAX and T MAX were taken directly from the data.
Statistical Analysis
Intragastric pH measurements, fecal scores, and percentage food consumption were compared by treatment. For pH, the effects of day of treatment and time of treatment and feeding also were assessed. Data were analyzed using mixed effects analysis of variance (ANOVA) to model the 4‐way crossover design and account for the random effect of subjects nested within treatment sequence. When repeated measurements occurred, a first order autoregressive covariance structure was specified for the residual matrix. Covariance parameters were estimated using a restricted maximum likelihood estimation method, and denominator degrees of freedom for the tests of fixed effects were computed using the Kenward‐Roger method. F‐tests were obtained for all main effects and for interactions when multifactor models were used. The Shapiro‐Wilk test was used to test the ANOVA assumption of normally distributed errors, and the Levene's F test was used to test the assumption of homogeniety of variance. When necessary, data were logarithmically (normal) transformed. Both assumptions were met with transformed data. To minimize the probability of type I error, a Bonferroni adjusted alpha of 0.003 was used to evaluate the ANOVA results. When a significant treatment effect was observed, a posthoc Tukey‐Kramer test was performed to determine which groups were significantly different from each other (protected α = 0.05). No significant period or sequence effects were found for any dependent variable. A t‐test and Mann‐Whitney Rank Sum test were used to analyze parametric and nonparametric pharmacokinetic data, respectively. For pharmacokinetic analyses, a P value of <.05 was considered significant. Commercially available statistical software was used to perform all data analysis and to produce all descriptive statistics.17
Results
Use of the Bravo pH Monitoring System in Cats
All 24 Bravo pH capsules were successfully attached to the fundic gastric mucosa. Total procedure times for gastroscopy‐assisted capsule attachment ranged from 5 to 10 minutes. On 12 of 24 occasions, the previously placed Bravo pH capsule remained in place and an additional 5–15 minutes of procedure time was required to remove the attached capsule. Because of difficulty removing the capsule and concern for perforation associated with removal of firmly adhered capsules, 5 capsules were left adhered followed by placement of a new capsule in the area immediately adjacent to the previous capsule. On 3 occasions, the capsule dislodged during the washout period and passed uneventfully in the feces. In contrast to experience with dogs, no capsules detached prematurely during the study period. Because of receiver malfunction, data was not captured for 86 hours of a total of 2304 hours, although the Bravo pH capsules remained appropriately adhered. This may have been the result of lost signal because the receivers could not be placed directly on the cats because of the size of the receiver and patient. The majority of the lost data occurred in the evening when the receivers were not monitored as frequently. On 4 occasions, the receiver continued to read from the previously placed capsule (at least 14 days earlier). Data from 1 cat were excluded from study results after the onset of progressive inappetence, weight loss, and suspicion of eosinophilic gastroenteritis. Additional information on this cat is published elsewhere.18
Intragastric pH Recordings
The mean percentage time gastric pH is ≥3 and ≥4 is considered the ideal baseline for encouraging healing of gastrointestinal disease as determined by meta‐analysis studies in humans.11 Thus, mean percentage time intragastric pH was ≥3 and ≥4 was used for comparative analyses of treatments. The mean percentage time ± SD the intragastric pH was ≥3 and ≥4 were 68.4 ± 35.0% and 57.8 ± 37.1% for fOT, 73.9 ± 23.2% and 55.7 ± 25.3% for ORP, 42.8 ± 18.6% and 22.4 ± 14.7% for famotidine, and 16.0 ± 14.2% and 9.6 ± 10.1% for placebo, respectively. For the mean percentage time intragastric pH was ≥3 and ≥4 over the 4‐day study period, both omeprazole formulations significantly increased intragastric pH compared to famotidine or placebo (P < .0001; Fig. 2). Differences also were observed in the distribution of intragastric pH over pH categories 1–8 when comparing omeprazole formulations to famotidine and placebo (Fig. 3). No differences were observed when comparing omeprazole formulations to each other in any category. As in dogs, intragastric pH fluctuated widely across all pH categories in cats receiving all treatments, but both omeprazole formulations resulted in more time intragastric pH fell between pH categories 3–4 and 7–8. The mean percentage time intragastric pH was ≥3 and ≥4 was significantly higher in cats receiving famotidine compared to placebo (P < .0001).
Figure 2.
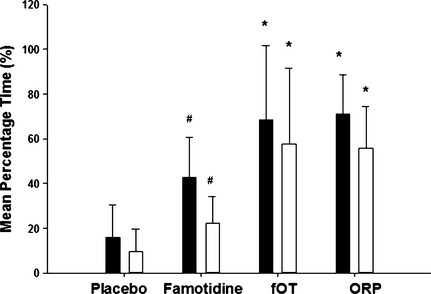
Comparison of the effect of oral placebo, famotidine, fractionated omeprazole tablet (fOT) and omeprazole reformulated paste (ORP) on intragastric pH in six cats on days 4–7 of treatment. Bars represent the mean ± SD percentage of time that intragastric pH was ≥3 (black bars) and 4 (white bars). *Value significantly increased compared to famotidine and placebo. (P < .001) #Value significantly increased compared to placebo (P < .001).
Figure 3.
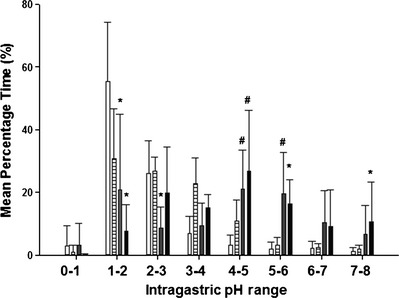
Comparison of the effect of oral placebo (white bars), famotidine (horizontal striped bars), fractionated omeprazole tablet (gray bars) and omeprazole reformulated paste (black bars) on intragastric pH distribution in six cats on days 4–7 of treatment. *Value significantly different from famotidine and placebo (P < 0.001). #Value significantly different from placebo (P < 0.001).
The mean percentage time intragastric pH was ≥3 and ≥4 also was used to determine if there was an effect on the order of treatment, day of treatment, and time of day (morning or evening treatment) on intragastric pH between or within certain groups. For all cats, on the order of treatment did not significantly affect the percentage time intragastric pH was ≥3 and ≥4 over the 96‐hour recording period (P = .8807). Similarly, no significant differences were identified for the mean percentage time intragastric pH was ≥3 and ≥4 when comparing days 4 through 7 of treatment within each group (P = .3043). There were no significant differences in the percentage time intragastric pH was ≥3 and ≥4 between the 1st and 2nd 12 hours treatment periods within each treatment group (P = .1388). In addition, a buffering effect of food on intragastric pH was not identified in the placebo control group (P = .7851). The mean pH ± SD for cats in the placebo group over the 96‐hour period was 2.3 ± 0.4.
Adverse Effects of Treatment
Vomiting was noted in 2 cats with a total occurrence of 6 episodes of emesis. None of these episodes occurred immediately after medicating, and no medications were observed in the vomitus. There were 3 episodes of vomiting each in the placebo and ORP groups. There was no significant association with treatment received and inappetence, consistency of stool, number of defecations with a fecal score of ≥4, or changes in attitude. There were 8 episodes of inappetence in the famotidine group, 16 episodes in the fOT group, 11 episodes in the ORP, and 7 episodes for the placebo group. The mean ± SD fecal score was 3.1 ± 0.8 for the famotidine group, 2.6 ± 0.5 for the fOT group, 2.7 ± 0.9 for the ORP group, and 2.9 ± 0.1 for the placebo group. There were 9 episodes of fecal scores ≥4 in the famotidine group and 6 episodes each in the fOT and ORP groups. There were 6 days of defecations with a fecal score ≥4 in the famotidine group, 3 days in the fOT group and 5 days in the ORP group. No defecations with a fecal score ≥4 occurred in the placebo group.
Pharmacokinetic Analysis
Plasma concentration (mean ± SD) data were available for 6 cats receiving ORP and 5 cats receiving fOT (Fig. 4). In 2 cats that received fOT, a full set of data was not obtained and thus values from these cats were omitted from analysis of pharmacokinetic parameters. In 1 of these 2 cats, concentration data were only detectable for 3 time points which precluded fitting to the compartmental model; however, these 3 time points were included in graphical representation of the data (Fig. 4). Plasma concentrations were detectable in all 6 cats receiving ORP and in 2 of 5 cats receiving fOT at 15 minutes postmedication. Plasma concentrations were undetectable in 5 of 6 cats receiving ORP and in 3 of 5 cats receiving fOT by 6 hours postmedication. Pharmacokinetic parameter estimates are listed in Table 1. A significant difference was found when comparing T MAX in cats receiving ORP compared to fOT (P = .03), where the median TMAX for ORP was 0.5 hours versus 2 hours for fOT. No other parameters were found to be significantly different.
Figure 4.
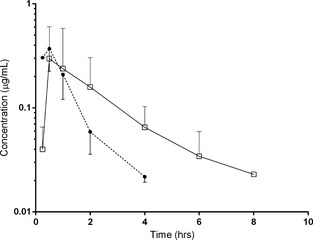
Mean ± SD of omeprazole concentrations in cats receiving omeprazole reformulated paste (closed circle, n = 6) or fractionated omeprazole tablet (open square) on day 7 of treatment.
Table 1.
Pharmacokinetic variable estimates (median and range) for cats receiving omeprazole reformulated paste (ORP) or fractionated omeprazole tablet (fOT) on day 7 of treatment
| Parameter | ORP (n = 6) | fOT (n = 4) | P value | ||
|---|---|---|---|---|---|
| Median | Range | Median | Range | ||
| AUC (μg/hr/mL) | 0.46 | 0.21–0.94 | 0.57 | 0.33–1.4 | .394 |
| Cmax (μg/mL) | 0.35 | 0.05–0.78 | 0.11 | 0.09–0.85 | .705 |
| Tmax (hr) | 0.5 | 0.25–1.0 | 2.0 | 0.5–4.0 | .03 |
| Elim T1/2 (hr) | 0.9 | 0.07–0.25 | 0.83 | 0.66–1.75 | .806 |
Discussion
Gastric erosion and ulceration are common sequelae to a wide range of gastric and nongastric acid‐related disorders in cats. For this reason, acid suppressants are among the most widely prescribed medications for cats. Generic tablets containing omeprazole (OT) or famotidine are commonly prescribed acid suppressants. However, to the authors' knowledge, no clinical studies have been performed in cats to determine the efficacy of these different acid suppressants. This study was undertaken to compare the efficacy of PO administered famotidine to omeprazole formulations in cats using the Bravo pH monitoring system. The mean percentage time gastric pH is ≥3 and ≥4 is considered the ideal baseline for encouraging healing of gastrointestinal disease as determined by meta‐analysis studies of humans.11 Therefore, the mean percentage of time that intragastric pH was ≥3 and ≥4 was used for purposes of comparative analyses among treatment arms. Our results demonstrate that omeprazole formulations provide superior acid suppression compared to famotidine and placebo. Moreover, only omeprazole formulations administered to cats approached goals established for treatment of people with gastroduodenal ulcers and gastroesophageal reflux disease, demonstrated to be optimal at an intragastric pH of ≥3.0 and ≥4.0 for approximately 75% and 67% of the day, respectively.11
Delayed‐release OT contains a protective enteric coating on its surface to prevent premature gastric degradation. Omeprazole tablets are only widely available in sizes of 20 mg and larger. A major limitation in appropriately dosing cats is their small body size, resulting in a much smaller total dose of medication needed than provided as a nondivided tablet. Thus, lacking other practical alternatives, veterinary practitioners often divide these tablets for optimal dosing in cats. The efficacy of the tablets after damage to their enteric coating is unknown. To evaluate the efficacy of omeprazole tablets after disruption to their protective coating, we directly compared their pharmacokinetics at steady state (day 7 of treatment) and their pharmacodynamics (days 4–7 of treatment) using mean percentage time intragastric pH was ≥3 and ≥4 over the 4‐day study period to an omeprazole reformulated paste (ORP) recently shown to be highly efficacious in dogs.12 No significant differences were found between fOT and ORP when comparing mean percentage time intragastric pH was ≥3 and ≥4 over the 4‐day study period. Moreover, there were no differences in any of 8 pH categories when comparing the 2 omeprazole formulations. Mean ± SD plasma omeprazole concentrations of both formulations were similar. Evaluation of the pharmacokinetic parameter estimates indicated that time to peak omeprazole concentration (T MAX) occurred earlier in cats receiving ORP, perhaps because fOT required more time for tablet dissolution. Many factors affect systemic absorption after oral drug administration, including rate of tablet dissolution in gastrointestinal (GI) fluid.18 However, the area under the concentration‐time curve (AUC) median and range, which have been shown to best reflect omeprazole's inhibitory effect on gastric acid secretion19, 20, were similar between formulations. The inability to detect a significant difference in AUC may have been a result of small sample size and therefore underpowered analysis. However, there were no significant differences between the 2 formulations when comparing peak omeprazole concentration or other pharmacokinetic variables at steady state. All medications were dosed on an empty stomach when intragastric pH is reportedly higher because of less food stimulation of acid secretion,21 which may have contributed to less degradation of the fractionated tablet.
Similar to previous studies in dogs,9, 13, 14 famotidine was found to be more efficacious than placebo in cats but was inferior to omeprazole in suppression of acid production. Thus, the authors recommend that omeprazole be administered to cats with documented acid‐related disorders.
The optimal omeprazole dosage in small animal patients has not been established.5, 14, 22 However, studies in dogs in which lower dosages of omeprazole (0.7–1.0 mg/kg PO q24h) were used resulted in suboptimal performance, did not meet goals established for people with acid‐related disorders or both.9, 13, 14, 23 The chosen dosage and frequency for omeprazole in this study (approximately, 1 mg/kg PO q12h) were based on those recently demonstrated to provide superior acid suppression in dogs.12, 14 Future work will be necessary to determine if this is the appropriate dosage for cats at risk for or with documented acid‐related disorders.
Previous studies performed on cats evaluated the efficacy of acid suppressants by measurement of the pH of gastric secretions obtained from a surgically placed gastric fistula.3, 24, 25 Artificial disruption of normal gastric anatomy and physiology by placement of a gastric fistula and pharmacologic stimulation of gastric acid secretion may not accurately reflect intragastric pH in the clinical patient. The Bravo pH monitoring system was selected in this study, because wireless continuous pH monitoring systems are being used with increasing frequency, are better tolerated by patients than nasally placed pH probes (ie, less impairment of normal activities), and provide reliable results.26, 27 These monitoring systems have been shown to be more physiologic and more accurate than aspiration of gastric secretions, because acid is not removed from the stomach and readings are obtained every 6 seconds.26, 28, 29 Moreover, capsules could be reproducibly adhered to the gastric fundus, which has been shown to have the most accurate results and is considered the optimal location for evaluation of gastric pH.30 The Bravo monitoring system has been demonstrated to be successful and safe in several studies in humans28, 29, 31, 32, 33 and dogs12, 34, but, before this study, the methodology had not been explored in cats. In contrast to previous studies,12, 32 premature detachment of the Bravo pH capsule was not detected in our study population. Although this may be because of small sample size, it also may be because of differences in thickness of human and canine gastric mucosa in comparison to the feline gastric mucosa. Future work is needed to adjust the protocol (eg, lower vacuum pressure or time) with which the Bravo pH capsule is adhered to the stomach to promote natural passing of the capsule in cats.
In accordance with studies performed in dogs,12, 34, 35 this study did not identify a buffering effect of food on intragastric pH in cats receiving placebo. This may be a result of the pH capsule methodology, which unlike digital probes, allows direct adherence to the gastric mucosa and provides a direct measurement of intragastric pH. In addition, gastroduodenal reflux (defined as a rapid increase in pH > 4) occurred infrequently, with an average of 1.2 ± 1.2 episodes/day.
Adverse GI events including vomiting and diarrhea are the most commonly reported effects in dogs receiving omeprazole.8, 12, 13, 14 No statistically significant differences in occurrence of adverse events (inappetence, vomiting, diarrhea) were identified in cats receiving any of the acid suppressants tested. These results suggest that omeprazole paste, fractionated omeprazole tablet as well as famotidine generally are well tolerated by cats even at higher doses. However, we found it notable that episodes of fecal scores ≥4 were identified in all treatment groups with the exception of placebo, even though cats receiving placebo continued to be medicated PO and were exposed to the same environmental conditions as cats receiving acid suppressants. Thus, it is possible that acid suppressants are associated with adverse GI events as reported in dogs and that small sample size prevented the ability to detect such a difference. Proton pump inhibitor (PPI) administration leads to quantitative changes in the intestinal microbiota of healthy dogs although the role of dysbiosis in PPI‐induced diarrhea is unknown.36 Future work will be necessary to determine if acid suppressant drugs alter the composition of fecal microbiota in cats and play a causal role in dysbiosis and PPI‐induced diarrhea.
This study included a small group of cats with no known history of GI disease. Histopathologic examination of gastric biopsies was not performed to evaluate for subclinical gastritis, but physical examination, laboratory results and gastroscopic evaluation identified no clinically relevant abnormalities. Moreover, in the unlikely event that a cat did have subclinical gastritis, the crossover design of the study ensured that each cat served as its own control. Larger studies evaluating the efficacy of these drugs in cats with overt gastritis are needed.
In conclusion, these results suggest that both omeprazole formulations provide superior acid suppression in cats compared to famotidine or placebo. The fractionated enteric‐coated omeprazole tablet remains efficacious despite disruption of its enteric coating, allowing for more convenient, titratable dosing in cats. In addition, the Bravo pH monitoring system can be safely used in healthy cats for continuous intragastric pH monitoring, but, the safety of its use in cats with GI disease needs to be evaluated.
Acknowledgments
The authors thank Randy Buddington, Gina Galyon and Shanna Hillsman for their technical support. This study was made possible through financial support from the Comparative Gastroenterology Society/Waltham grant and the University of Tennessee fund for Companion Animal Research.
Conflict of Interest: Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Work for this manuscript was performed at the University of Tennessee, College of Veterinary Medicine in Knoxville, Tennessee (pH study) and North Carolina State University in Raleigh, North Carolina (PK analysis).
This manuscript was presented in part as a research report at the American College of Veterinary Internal Medicine Forum in Nashville, TN in June of 2014.
Footnotes
Zegerid, Salix Pharmaceuticals, Inc./Santarus Inc., Raleigh, NC
Reformulated paste was diluted to 10 mg/mL in cod liver oil; Gastrogard, Merial, Duluth, GA
Bravo pH monitoring system, Given Imaging, Yoqneam, Israel
Lactose 250 mg encapsulated in size #3 gelatin capsule, Spectrum Chemical Mfg Corp, Gardena, CA
10 mg from Teva Pharmaceuticals from Sellersville, PA
20 mg tablets from Dexcel Pharma Technologies, Yonkneam, Isreal
Hill's Science Diet Adult Light, Hill's Pet Nutrition, Inc, Topeka, KS
Fecal Scoring System, Nestlè Purina PetCare Company, St Louis, MO
Dexdomitor 0.5 mg/mL injection, Orion Pharma, Espoo, Finland
Ketacine 100 mg/mL injection, VetOne, Boise, ID
Torbugesic 10 mg/mL injection, Fort Dodge Animal Health, Fort Dodge, IA
PropoFlo 10 mg/mL injection, Abbott Laboratories, North Chicago, IL
SevoFlo, Abbott Laboratories, North Chicago, IL
Antisedan 5 mg/mL injection, Orion Pharma, Espoo Finland
Polygram Net Software, Given Imaging, Yoqneam, Israel
Phoenix WinNonLin, Certara, LP., St. Louis, MO
SAS 9.2, SAS Institute Inc., Cary, NC
Tolbert K, Odunayo A, Craig L. Gastric perforation following endoscopic removal of a Bravo pH capsule in a cat. Manuscript in press. JFMS Open Reports
References
- 1. Lee A. Animal models of gastroduodenal ulcer disease. Baillieres Best Pract Res Clin Gastroenterol 2000;14:75–96. [DOI] [PubMed] [Google Scholar]
- 2. Sullivan M, Yool DA. Gastric disease in the dog and cat. Vet J 1998;156:91–106. [DOI] [PubMed] [Google Scholar]
- 3. Chiavarini M, Barocelli E, Ballabeni V, et al. Omeprazole‐like compounds on histamine‐stimulated acid and peptic secretions in conscious dog and cat. Boll Soc Ital Biol Sper 1992;68:429–436. [PubMed] [Google Scholar]
- 4. Khoshnegah J, Jamshidi S, Mohammadi M, et al. The efficacy and safety of long‐term Helicobacter species quadruple therapy in asymptomatic cats with naturally acquired infection. J Feline Med Surg 2011;13:88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Papich MG. Antiulcer therapy. Vet Clin North Am Small Anim Pract 1993;23:497–512. [DOI] [PubMed] [Google Scholar]
- 6. Jergens AE, Moore FM, March P, et al. Idiopathic inflammatory bowel disease associated with gastroduodenal ulceration‐erosion: A report of nine cases in the dog and cat. J Am Anim Hosp Assoc 1992;28:21–26. [Google Scholar]
- 7. Liptak JM, Hunt GB, Barrs VR, et al. Gastroduodenal ulceration in cats: Eight cases and a review of the literature. J Feline Med Surg 2002;4:27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davis MS, Willard MD, Nelson SL, et al. Efficacy of omeprazole for the prevention of exercise‐induced gastritis in racing Alaskan sled dogs. J Vet Intern Med 2003;17:163–166. [DOI] [PubMed] [Google Scholar]
- 9. Williamson KK, Willard MD, McKenzie EC, et al. Efficacy of famotidine for the prevention of exercise‐induced gastritis in racing Alaskan sled dogs. J Vet Intern Med 2007;21:924–927. [DOI] [PubMed] [Google Scholar]
- 10. Wallmark B, Larsson H, Humble L. The relationship between gastric acid secretion and gastric H+, K + ‐ATPase activity. J Biol Chem 1985;260:13681–13684. [PubMed] [Google Scholar]
- 11. Bell NJ, Burget D, Howden CW, et al. Appropriate acid suppression for the management of gastro‐oesophageal reflux disease. Digestion 1992;51(Suppl 1):59–67. [DOI] [PubMed] [Google Scholar]
- 12. Tolbert K, Bissett S, King A, et al. Efficacy of oral famotidine and 2 omeprazole formulations for the control of intragastric pH in dogs. J Vet Intern Med 2011;25:47–54. [DOI] [PubMed] [Google Scholar]
- 13. Williamson KK, Willard MD, Payton ME, et al. Efficacy of omeprazole versus high‐dose famotidine for prevention of exercise‐induced gastritis in racing Alaskan sled dogs. J Vet Intern Med 2010;24:285–288. [DOI] [PubMed] [Google Scholar]
- 14. Bersenas AM, Mathews KA, Allen DG, et al. Effects of ranitidine, famotidine, pantoprazole, and omeprazole on intragastric pH in dogs. Am J Vet Res 2005;66:425–431. [DOI] [PubMed] [Google Scholar]
- 15. Horn JR, Howden CW. Review article: Similarities and differences among delayed‐release proton‐pump inhibitor formulations. Aliment Pharmacol Ther 2005;22(Suppl 3):20–24. [DOI] [PubMed] [Google Scholar]
- 16. Andersson T, Andren K, Cederberg C, et al. Pharmacokinetics and bioavailability of omeprazole after single and repeated oral administration in healthy subjects. Br J Clin Pharmacol 1990;29:557–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Poulsen KP, Smith GW, Davis JL, et al. Pharmacokinetics of oral omeprazole in llamas. J Vet Pharmacol Ther 2005;28:539–543. [DOI] [PubMed] [Google Scholar]
- 18. Buxton ILO, Benet LZ. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 12th ed. Chapter 2, Pharmacokinetics: The Dynamics of Drug Absorption, Distribution, Metabolism, and Elimination. New York: McGraw‐Hill, 2011: 17–40. [Google Scholar]
- 19. Andersson T. Pharmacokinetics, metabolism and interactions of acid pump inhibitors. Focus on omeprazole, lansoprazole and pantoprazole. Clin Pharmacokinet 1996;31:9–28. [DOI] [PubMed] [Google Scholar]
- 20. Andersson T, Rohss K, Bredberg E, et al. Pharmacokinetics and pharmacodynamics of esomeprazole, the S‐isomer of omeprazole. Aliment Pharmacol Ther 2001;15:1563–1569. [DOI] [PubMed] [Google Scholar]
- 21. Huang JQ, Hunt RH. Pharmacological and pharmacodynamic essentials of H(2)‐receptor antagonists and proton pump inhibitors for the practising physician. Best Pract Res Clin Gastroenterol 2001;15:355–370. [DOI] [PubMed] [Google Scholar]
- 22. Trepanier L. Acute vomiting in cats: Rational treatment selection. J Feline Med Surg 2010;12:225–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jenkins CC, DeNovo RC, Patton CS, et al. Comparison of effects of cimetidine and omeprazole on mechanically created gastric ulceration and on aspirin‐induced gastritis in dogs. Am J Vet Res 1991;52:658–661. [PubMed] [Google Scholar]
- 24. Coruzzi G, Bertaccini G. Antisecretory activity of omeprazole in the conscious gastric fistula cat: Comparison with famotidine. Pharmacol Res 1989;21:499–506. [DOI] [PubMed] [Google Scholar]
- 25. Coruzzi G, Bertaccini G, Noci MT, et al. Inhibitory effect of famotidine on cat gastric secretion. Agents Actions 1986;19:188–193. [DOI] [PubMed] [Google Scholar]
- 26. Savarino V, Mela GS, Zentilin P, et al. Gastric aspiration versus antimony and glass pH electrodes. A simultaneous comparative in vivo study. Scand J Gastroenterol 1989;24:434–439. [DOI] [PubMed] [Google Scholar]
- 27. Pandolfino JE, Richter JE, Ours T, et al. Ambulatory esophageal pH monitoring using a wireless system. Am J Gastroenterol 2003;98:740–749. [DOI] [PubMed] [Google Scholar]
- 28. Chiverton SG, Burget DW, Hunt RH. Validation of pH dataloggers for pharmacologic studies. Scand J Gastroenterol 1989;24:975–981. [DOI] [PubMed] [Google Scholar]
- 29. Mela GS, Savarino V, Vigneri S. Optimizing the information obtained from continuous 24‐hour gastric pH monitoring. Am J Gastroenterol 1992;87:961–966. [PubMed] [Google Scholar]
- 30. Fackler WK, Vaezi MF, Richter JE. Ambulatory gastric pH monitoring: Proper probe placement and normal values. Aliment Pharmacol Ther 2001;15:1155–1162. [DOI] [PubMed] [Google Scholar]
- 31. Chang JH, Choi MG, Yim DS, et al. A novel placement method of the Bravo wireless pH monitoring capsule for measuring intragastric pH. Dig Dis Sci 2009;54:578–585. [DOI] [PubMed] [Google Scholar]
- 32. Pandolfino JE, Schreiner MA, Lee TJ, et al. Bravo capsule placement in the gastric cardia: A novel method for analysis of proximal stomach acid environment. Am J Gastroenterol 2005;100:1721–1727. [DOI] [PubMed] [Google Scholar]
- 33. Yamaguchi T, Seza A, Odaka T, et al. Placement of the Bravo wireless pH monitoring capsule onto the gastric wall under endoscopic guidance. Gastrointest Endosc 2006;63:1046–1050. [DOI] [PubMed] [Google Scholar]
- 34. Mahar KM, Portelli S, Coatney R, et al. Gastric pH and gastric residence time in fasted and fed conscious beagle dogs using the Bravo pH system. J Pharm Sci 2012;101:2439–2448. [DOI] [PubMed] [Google Scholar]
- 35. Sagawa K, Li F, Liese R, et al. Fed and fasted gastric pH and gastric residence time in conscious beagle dogs. J Pharm Sci 2009;98:2494–2500. [DOI] [PubMed] [Google Scholar]
- 36. Garcia‐Mazcorro JF, Suchodolski JS, Jones KR, et al. Effect of the proton pump inhibitor omeprazole on the gastrointestinal bacterial microbiota of healthy dogs. FEMS Microbiol Ecol 2012;80:624–636. [DOI] [PubMed] [Google Scholar]


