Abstract
Background
Long‐term treatment of cats with ionized hypercalcemia using alendronate has not been evaluated.
Hypothesis/Objectives
Alendronate is well tolerated in treatment of ionized hypercalcemia in cats.
Animals
A total of 12 cats with ionized hypercalcemia.
Methods
Prospective study of 12 cats with ionized hypercalcemia of idiopathic origin was identified by telephone and email communication with a convenience sample of consulting veterinarians. Cats were treated with alendronate at a dose of 5–20 mg per feline PO q7d. Serum ionized calcium concentration (iCa) was measured before beginning treatment with alendronate, and after 1, 3, and 6 months of treatment. Alendronate dosage was adjusted according to iCa. Evaluation included physical examination, CBC, biochemistry profile, and diagnostic imaging. The owners and referring veterinarians were questioned about any observed adverse effects. The Wilcoxon matched‐pairs signed rank test was used to compare baseline iCa to iCa at different time periods.
Results
Alendronate treatment resulted in a decrease in iCa in all 12 cats. The median percentage change in iCa was −13.2%, −15.9%, and −18.1% (range, −29.6 to +7.6; −30.5 to −1.9; −45.8 to +1.5%) at the 1, 3, and 6 month time points, respectively. Baseline iCa was significantly different from 1 month (P = .0042), 3 months (P = .0005), and 6 months (P = .0015). No adverse effects were reported for any of the cats.
Conclusions and Clinical Importance
Alendronate was well tolerated and decreased iCa in most cats for the 6‐month period of observation.
Keywords: Bisphosphonate, Feline, Idiopathic hypercalcemia, Ionized calcium, Safety
Abbreviations
- BUN
blood urea nitrogen
- CKD
chronic kidney disease
- FORL
feline odontoclastic resorptive lesion
- FPPS
farnesyl diphosphate synthase
- GI
gastrointestinal
- iCa
ionized calcium
- IHC
idiopathic hypercalcemia
- N‐BPs
nitrogen‐containing bisphosphonates
- PTH
parathyroid hormone
- PTHrp
parathyroid hormone‐related protein
Unexplained disturbances in calcium homeostasis resulting in ionized hypercalcemia are attributed to parathyroid‐independent hypercalcemia in over 50% of cats.1 Most cats with parathyroid‐independent ionized hypercalcemia have idiopathic hypercalcemia (IHC), with malignancy and hypervitaminosis D thought to be less common. Based on measurement of total serum calcium concentration, malignancy‐associated hypercalcemia and chronic kidney disease (CKD) are the most common causes in a case series of cats with hypercalcemia.1 A diagnosis of IHC requires eliminating other known causes of hypercalcemia including neoplasia, CKD, primary hyperparathyroidism, hypoadrenocorticism, toxin ingestion (eg, cholecalciferol, calcipotriene), granulomatous disease, and osteolytic disease.2 Cats with IHC typically are middle‐aged (mean, 5.8–9.8 years),2,2 with mild‐to‐moderate increases in total and ionized serum calcium concentrations. Most cats with IHC have few or no clinical signs and ionized hypercalcemia often is detected on routine laboratory evaluation for seemingly unrelated conditions, wellness exams, and pre‐anesthesia screening.2 Vomiting, weight loss, dysuria, anorexia, constipation, calcium oxalate urolithiasis, and inappropriate urination are clinical signs most commonly noted by owners of cats with IHC. Concentrations of the calcium regulatory hormones parathyroid hormone (PTH), parathyroid hormone‐related protein (PTHrP), 25‐hydroxycholecalciferol (25(OH)‐vitamin D, calcidiol), and 1,25‐dihydroxycholecalciferol (1,25(OH)2‐vitamin D, calcitriol) usually are either low or within the normal reference range in cats with IHC.2,2, 3
Calcium homeostasis is maintained by precise regulation of dietary calcium absorption from the gastrointestinal tract, calcium excretion in the urine after glomerular filtration and tubular reabsorption, and the balance of bone production (by osteoblasts) and resorption (by osteoclasts). Current recommendations for management of IHC target 2 of these areas of control: diet change to a nonacidifying, high fiber diet to decrease calcium absorption from the gastrointestinal (GI) tract,4 and prednisolone5 to promote lowering of serum calcium concentration by several mechanisms of action.2 Response to 1 or a combination of these treatments has been successful in some cases, but response is unpredictable and often transient.
Treatment with bisphosphonates to decrease bone resorption is another strategy that can decrease serum calcium concentration in some diseases. The nitrogen‐containing bisphosphonates (N‐BPs; alendronate,3 pamidronate,4 ibandronate,5 risedronate,6 zoledronate7) inhibit osteoclast function by inhibition of the enzyme farnesyl diphosphate synthase (FPPS) in the HMG‐CoA reductase (ie, mevalonate) pathway. The end products of this pathway are required for normal intracellular signaling, as well as cytoskeletal functions required for hydroxyapatite crystal dissolution. Metabolism of N‐BPs also results in the production of an intracellular ATP analog Apppi (triphosphoric acid 1‐adenosin‐5'‐yl ester 3‐[3‐methylbut‐3‐enyl] ester), which is believed to directly induce apoptosis of osteoclasts.6
We hypothesized that oral administration of an N‐BP would decrease the magnitude of serum ionized hypercalcemia in cats as a consequence of its potent inhibition of bone resorption. The purpose of this study is to report tolerance of PO‐administered alendronate and change in ionized calcium concentration in the treatment of 12 cats with ionized hypercalcemia for a period of 6 months.
Materials and Methods
Twelve cats with ionized hypercalcemia were identified after telephone and email communications with a convenience sample of consulting veterinarians. Criteria for inclusion in the study were an initial finding of increased serum total calcium concentration (reference range, 8.2–10.8 mg/dL) followed by finding of an increased serum iCa concentration (>1.4 mmol/L; >5.6 mg/dL). Cats were excluded if they had an identifiable cause for hypercalcemia, if PTH exceeded the upper half of the reference range if measured, if they did not have complete data (ie, serum iCa concentration before, and 1, 3, and 6 months after beginning treatment with alendronate), or if they had received corticosteroids during the study period. Cats were not excluded if they had previously failed to respond to treatment with corticosteroids or dietary management.
Serum PTH, PTHrP, 25‐hydroxycholecalciferol, and serum iCa concentrations were measured by the Endocrine Section of the Diagnostic Center for Population Animal Health (DCPAH) at Michigan State University, East Lansing, Michigan. Serum PTH concentration was measured by an immunoradiometric assay for intact PTH that previously has been validated for use in cats.7 Serum PTHrP concentration was measured by an immunoradiometric assay for PTHrP (1–84) that previously has been validated in cats.7 Serum 25‐hydroxycholecalciferol concentration was measured by a commercially available radioimmunoassay. Serum iCa was measured by ion‐specific electrode methodology.8 Serum biochemistry was performed by routine automated techniques.
Corticosteroid treatment was discontinued for at least 4 weeks before beginning alendronate treatment, and no other medications known to alter serum iCa concentration were administered during the study period. Diet was not changed during the 6 months of the study in any of the cats. Detailed treatment history was collected from the feline owners and the attending veterinarians with emphasis on any possible adverse effects since starting alendronate treatment.
Eight cats were started at a dose of 10 mg alendronate3 in tablet form PO q7d. Four cats were started at 5 mg PO q7d as a consequence of their lower body weight. Owners were instructed to follow pill administration with 6 mL of tap water8 by syringe and a small amount of butter9 placed on the cat's nasal planum to increase salivation and shorten esophageal transit time.
Serum iCa concentrations were measured before starting treatment, and 1, 3, and 6 months after once weekly drug administration. Serum iCa concentrations are presented as median (range). The median percentage change from the baseline iCa is reported at the 1, 3, and 6 month time intervals. The Wilcoxon matched‐pairs signed rank test was used to compare baseline serum iCa concentrations to serum iCa concentrations at each time period.
Results
No identifiable cause of hypercalcemia was found after physical examination, which was normal in all cats. Two cats had a history of mild weight loss. No cause for ionized hypercalcemia, such as mediastinal mass, abdominal organomegaly, lymphadenopathy, infiltrative pulmonary disease, was identified by diagnostic imaging. Abdominal ultrasound examination was performed in 8 cats, 4 cats had abdominal radiography, and all 12 cats underwent thoracic radiography. Evaluation of results from CBC and serum biochemistry profile failed to identify any known causes of hypercalcemia in cats. Two cats had mild azotemia (serum creatinine concentrations of 2.1 and 3.1 mg/dL in association with urine specific gravity <1.030).
Calcium Regulatory Hormones
Concentrations of PTH, PTHrP, and 25‐hydroxycholecalciferol were low or within the normal reference range for all cats in the study. Serum PTH concentrations ranged from 0 to 1.9 pmol/L (n = 10; median, 0 pmol/L; reference range, 0–4 pmol/L). The range of serum PTHrP concentrations was 0–0.7 pmol/L (n = 6; median 0 pmol/L; reference range, 0–1 pmol/L) and the range of serum 25‐hydroxycholecalciferol concentrations was 60–145 nmol/L (n = 5; median 90.5 nmol/L; reference range, 65–170 nmol/L).
Dose and Effect of Alendronate
The alendronate dose was increased during the study period in 8 cats. Only 1 of the 4 cats that initially were given 5 mg PO q7d remained on this dose by the end of the 6‐month period. At the end of the study, the other 3 cats were receiving 10 mg PO q7d and 3 other cats remained on their initial dose of 10 mg PO q7d. The dose was increased to 15 mg PO q7d in 1 cat and to 20 mg PO q7d in 4 cats. The median dose of alendronate remained at 10 mg PO q7d throughout the 6 months of the study. In total, 8 of the 12 cats required dose escalation.
The median serum iCa concentration before alendronate treatment was 1.82 mmol/L (range, 1.48–2.25 mmol/L). After 1, 3, and 6 months of treatment, respectively, median serum iCa concentrations were 1.51, 1.59, and 1.42 mmol/L (range, 1.34–1.9, 1.18–2.13, and 1.03–2.02 mmol/L, respectively). Figures 1 and 2 detail the changes in serum iCa concentration for all 12 cats. This corresponds to a median change of −0.22, −0.29, and −0.34 mmol/L (range, −0.59 to +0.13, −0.58 to −0.03, −0.87 to +0.03 mmol/L) and a median percentage change of −13.2%, −15.9%, and −18.1% (range, −29.6 to +7.6; −30.5 to −1.9; −45.8 to +1.5%), respectively, over the same time period. Based on the Wilcoxon matched pairs signed rank test, baseline serum iCa concentration was significantly different from that measured at 1 month (P = .0042), 3 months (P = .0005), and 6 months (P = .0015) after beginning treatment. When comparing 1–3 months (P = 1.000), 1–6 months (P = .1165), and 3–6 months (P = .2552), serum iCa concentrations were not significantly different. In 8 of 12 cats, the serum iCa concentrations entered the normal reference range at some point during the 6‐month study period (Table 1). The median dose at the beginning of the study was 10 mg/cat (range, 5–10 mg) and was 10 mg/cat (range, 5–20 mg) at the end of the study.
Figure 1.
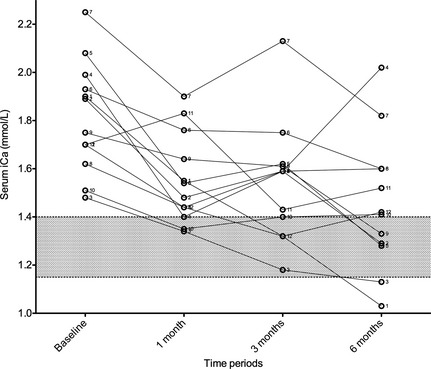
Serum iCa concentration at baseline, and following 1, 3, and 6 months of alendronate treatment for all 12 cats.
Figure 2.
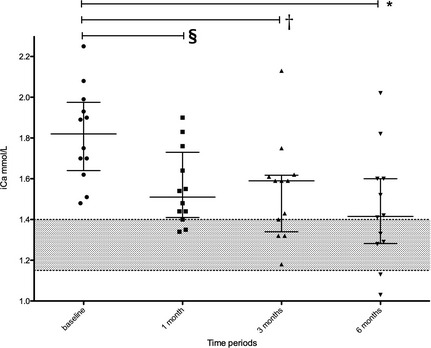
Serum iCa concentration at baseline, and following 1, 3, and 6 months of alendronate therapy for all 12 cats. Scatter dot plot showing the median with the interquartile range. Wilcoxon matched pairs signed rank test was used to compare baseline iCa to different time periods. Baseline iCa was significantly different from 1 month (P = .0042)§, 3 months (P = .0005)†, and 6 months (P = .015)*.
Table 1.
iCa concentration, change in iCa concentration and percent change in iCa concentration (range) at baseline and following 1, 3, and 6 months of therapy with alendronate
| Baseline | 1 Month | 3 Months | 6 Months | |
|---|---|---|---|---|
| [iCa] mmol/L (median, range) | 1.82 (1.48–2.25) | 1.51 (1.34–1.90) a | 1.59 (1.18–2.13) b | 1.42 (1.03–2.02) c |
| Change in [iCa] mmol/L (median, range) | n/a | −0.22 (−0.59 to 0.13) | −0.29 (−0.58 to −0.03) | −0.34 (−0.87 to 0.03) |
| % change in [iCa] (median, range) | n/a | −13.2 (−29.6 to 7.6) | −15.9 (−30.5 to −1.9) | −18.1 (−45.8 to 1.5) |
| Number of cats above reference range (>5.5 mg/dL, >1.4 mmol/L) | 12/12 | 9/12 | 8/12 | 7/12 d |
Baseline iCa was significantly different from 1 month (P = .0042)a, 3 months (P = .0005)b, and 6 months (P = .0015)c.
dTwo cats were below the reference range.
Adverse Effects of Alendronate
No reports of adverse effects were found in any of the cats treated with alendronate in this study.
Discussion
Once weekly alendronate treatment was successful in decreasing serum ionized calcium concentrations in most cats of this study without clinically apparent adverse effects during 6 months of treatment. Dose escalation above the average weekly dose of 10 mg weekly was required to achieve adequate control of serum ionized calcium concentration in 8 cats and dose reduction was necessary because of development of hypocalcemia in 2 cats. Some of the variability in the degree of serum ionized calcium concentration achieved in this study likely reflects lack of standardization of fasting time before and after oral administration of alendronate. Ours is the first report describing use of PO‐administered alendronate to treat a series of cats with ionized hypercaclemia. Bone accumulation of alendronate and decreased activity or number of osteoclasts is thought to be responsible for decreased serum ionized calcium concentration during treatment. The use of bisphosphonates in veterinary medicine has been limited primarily to IV administration of pamidronate and zoledronate as palliative treatment in dogs with osteolytic neoplasia. Bisphosphonate treatment for idiopathic ionized hypercalcemia previously has been reported in 2 cats. In 1 cat, normocalcemia was achieved after IV administration of pamidronate9 and in the other cat combined treatment with pamidronate IV and alendronate PO was used to treat ionized hypercalcemia.3
Alendronate is an amino‐bisphosphonate that inhibits the cellular processes of osteoclasts required for hydroxyapatite crystal dissolution as well as directly inducing osteoclast apoptosis, thereby decreasing bone resorption.10 Alendronate is poorly absorbed from the Gl tract, with oral bioavailability of 1.54 ± 0.30% when administered in water and 0.20 ± 0.03% when administered in tuna juice‐flavored water to cats.11 Alendronate that is absorbed from the GI tract is rapidly and preferentially sequestered in the skeleton or excreted unchanged by the kidneys.10 Although not known specifically for the cat, the half‐life for alendronate in skeletal tissue is years in the dog and humans.10
The underlying mechanisms of IHC are unknown, but increased bone resorption has been hypothesized as a cause or important component of IHC in cats.2 Therefore, inhibiting osteoclast activity should decrease serum iCa concentrations and improve clinical signs if this hypothesis is true. Possible explanations for increased bone resorption include feeding an acidifying,12 magnesium‐restricted diet13; increased vitamin D14 intake with or without increased vitamin A15 intake; decreased copper intake16; increased aluminum intake17; and, acquired or genetic abnormalities in the calcium‐sensing receptor.18
The starting dose of 5–10 mg of alendronate PO q7d was extrapolated from the standard human dose of 70 mg PO q7d for the treatment of osteoporosis. Most cats started treatment at 10 mg weekly. By the end of the 6‐month period, many cats were receiving 15 mg (1 cat) or 20 mg per week (4 cats), but 1 cat had normal serum iCa concentration whereas being maintained on as little as 5 mg PO q7d. Based on serum iCa concentration, doses were increased or decreased as needed, typically in 5‐mg increments. The highest dose administered to any of the study cats was 20 mg PO q7d, with 8 of 12 cats requiring dose escalation. In contrast, 2 cats required dose reduction because of development of borderline hypocalcemia. In a study investigating the use of alendronate in the treatment of 9 cats with FORL, dosages of 3 mg/kg PO q7d for 22 weeks followed by 9 mg/kg PO q3–4d for 27 weeks were administered without recognition of adverse effects.11 These dosages are much higher than zolendrate dosages used in dogs (0.25 mg/kg IV)6 or a pamidronate dosage (1.05–2.0 mg/kg IV) reported in cats.9 The marked dosage differences reported among alendronate, pamidronate, and zolendronate are a consequence of the low gastrointestinal absorption of bisphosphonates. The alendronate dosage used in our study was similar to those used in other studies of alendronate.3, 11
Alendronate is available in different sizes and formulations, with a smallest available tablet size of 5 mg. Most cats can be readily treated with combinations of 10‐ and 5‐mg tablets. A liquid suspension also is available, but this formulation was not used in any of the cats in this study and it is not likely to be useful in cats as a consequence of the flavoring added. Oral absorption in humans is comparable between the liquid and tablet forms.3 In humans, tablets that are crushed or chewed can be highly irritating to the oral cavity and esophagus, and thus splitting tablets is not recommended.19, 20
Dosing recommendations given to owners were to follow administration of alendronate with administration of 6‐mL tap water8 PO by syringe and then application of a small amount of butter9 on the nasal planum to facilitate passage of the pill down the esophagus and into the stomach in as short a time as possible. These precautions are taken to minimize the risk of esophageal erosion or ulceration and subsequent strictures that have been described in humans taking alendronate for osteoporosis.21 The effect, if any, of butter on the GI intestinal absorption of alendronate has not been evaluated.
None of the cats in the study experienced any adverse effects from alendronate administration. We are aware of 1 cat (not included in this study because of limited laboratory evaluation) that experienced acute, flu‐like clinical signs, characterized by fever (104°F) and lethargy after 1 dose of alendronate. Similar signs have been reported in humans taking bisphosphonates and are believed to be the result of a systemic inflammatory response, an effect that is more common after first dose IV administration.22, 23 Other adverse effects reported in humans are esophageal irritation and ulceration because of prolonged esophageal mucosa‐drug contact time and potential subsequent stricture24, 25; gastrointestinal signs (inappetence, vomiting, diarrhea, abdominal pain)26; osteonecrosis of the mandible and maxilla27; acute, and chronic kidney failure (especially with higher doses and rapid parenteral administration)28; nephrotic syndrome29; and, electrolyte abnormalities (hypophosphatemia, hypocalcemia, hypomagnesemia).30 Osteonecrosis of the jaw has been reported in 2 Beagle dogs that received long‐term PO alendronate treatment. In both instances, the lesion was microscopic and not grossly observable or associated with detectable oral pain as occurs in humans.27, 31 The number of cats included in our study population was small and treatment time was only 6 months, thus some of these complications potentially could have developed if a larger number of cats had been treated for a longer period of time. In addition, the dosing precautions described above were recommended to all clients to minimize the risk of esophagitis and esophageal ulceration. Although we have not identified esophagitis or esophageal ulceration in cats treated with alendronate, we believe caution should be taken to minimize the potential risk of such complications. Two cats in this study had stable CKD that did not progress during the study period. These cats had pretreatment BUN and serum creatinine concentrations of 30 and 2.1 mg/dL; and 48 and 3.1 mg/dL, respectively and posttreatment BUN and serum creatinine concentrations of 33 and 2.0 mg/dL and 44 and 3.2 mg/dL, respectively. Although possible, we have not detected deterioration in renal function in cats with CKD and ionized hypercalcemia that we could attribute to the use of long‐term administration of alendronate (D. J. Chew and J. F. de Brito Galvao, unpublished observations).
Limitations of this study include lack of a specific protocol for fasting before PO administration of alendronate at the time this study was undertaken. Whether or not the cat was fasted before collection of samples to measure serum iCa concentrations also was not determined. At the time of this study, we were not aware that gastric contents other than water could impair gastrointestinal absorption of alendronate. Consequently, owners were not instructed to fast their cats before and after administration of alendronate. Residual gastric contents at the time of dosing or feeding soon after dosing could explain some of the observed variability in ionized calcium concentrations after treatment with alendronate. Our study did not provide a control placebo treatment population for comparison; and therefore we were unable to determine daily variation in serum iCa concentration independent of treatment with alendronate. Although we have observed apparent resolution of ionized hypercalcemia in some IHC cats without treatment, this appears to have occurred in cats with marginal increases in serum iCa concentration. Overall, we consider spontaneous resolution of ionized hypercalcemia unlikely in the cats of this study, especially because many cats were still hypercalcemic after initial treatment until dose escalation resulted in additional reduction in ionized calcium concentrations. The dose of alendronate was not standardized, and in most of the cats the dose was changed based on the individual need of the cat as guided by follow‐up serum iCa concentrations that had not decreased into the desired target range of normal or near‐normal serum iCa concentration. Calcium regulatory hormones were not measured during treatment.
The decrease in serum iCa concentrations after PO administration of alendronate seen in the cats of this study suggests that increased bone turnover might be an important component of IHC. The decreases in serum iCa concentration, however, simply might have been the result of inhibition of normal bone turnover overcoming increased GI absorption, decreased renal excretion of calcium, or both that ultimately could be responsible for generation and maintenance of ionized hypercalcemia. Other causes of hypercalcemia, such as hypervitaminosis D32, 33 and acidifying diets also would be expected to respond well to bisphosphonate treatment.
Based on results of this preliminary study, alendronate appears to be a well tolerated treatment that decreased serum iCa concentration in most cats with IHC by a median of −0.34 mmol/L after 6 months of treatment. Most cats (n = 8) had serum iCa concentrations that decreased to within the reference range (1.15–1.4 mmol/L) at some point during treatment. These results suggest that increased bone resorption is an important component of IHC in many cats. The lack of response in some cats suggests that IHC in cats likely is the manifestation of a number of disease processes, rather than a single disease in all cases. Three cats had serum iCa concentrations that did not decrease by more than 20%, or experienced only transient decreases in serum iCa concentration despite dose escalation. The beneficial effects seen in this study warrant a prospective, placebo‐controlled study of alendronate treatment of cats with IHC to determine the drug's effect on serum iCa concentrations, calciotropic hormones, and bone turnover biomarkers. As determined in this study, alendronate treatment of cats with ionized hypercalcemia of unknown cause (ie, IHC) appears to be well tolerated for at least 6 months, but we caution that long‐term observations in a larger number of cats are necessary to further determine its tolerance safety when used for extended periods. It would be preferable to treat the underlying causes of ionized hypercalcemia in cats (when they can be identified) rather than correcting hypercalcemia by slowing the rate of bone resorption with a bisphosphonate.
Acknowledgments
The authors thank the veterinarians who provided history, diagnostic, treatment, and follow‐up information for this study: Dr Amy Bell Shirley, Murfreesboro, TN; Dr Nancy DiMarco, Jupiter, FL; Dr Leah Gibbs, West Orange, NJ; Dr Mike Herko, Baltimore, MD; Dr Kris Hoyt, Scarborough, ME; Dr Dana Kuehn, Washington, DC; Dr Sofia Morales, Coral Springs, FL; Dr Leslie Nixon, Ormond Beach, FL; and Dr Heidi Ward, Sarasota, FL.
Conflict of Interest Declaration: Dr. Stephen P. DiBartola is a Co‐Editor‐in‐Chief of the Journal of Veterinary Internal Medicine.
Off‐label Antimicrobial Declaration: The authors declare no off‐label use of antimicrobials.
Footnotes
Schenck PA. Serum magnesium concentrations in association with feline calcium metabolic disorders. J Vet Intern Med 2008;22:796–797
Schenck PA, Chew DJ, Refsal K, Nachreiner R, Rick M. Calcium metabolic hormones in feline idiopathic hypercalcemia. J Vet Intern Med 2004;18:442
Fosamax; Merck & Co. Inc, 1 Merck Access Drive, Whitehouse Station, NJ
pamidronate disodium 3 mg/mL injection; Mylan Institutional LLC, Rockford, IL
Boniva; Roche Laboratories Inc, Nutley, NJ
Actonel; Warner Chilcott Plc, 100 Enterprise Drive, Rockaway, NJ
Zometa; Novartis Pharma, East Hanover, NJ
Nova 8 + ion‐selective analyzer; Nova Biomedical, Waltham, MA
Griffin B, Beard DM, Klopfenstein KA. Use of butter to facilitate the passage of tablets through the esophagus in cats. J Vet Intern Med 2003;17:445
References
- 1. Savary KC, Price GS, Vaden SL. Hypercalcemia in cats: A retrospective study of 71 cases (1991–1997). J Vet Intern Med 2000;14:184–189. [DOI] [PubMed] [Google Scholar]
- 2. Midkiff AM, Chew DJ, Randolph JF, et al. Idiopathic hypercalcemia in cats. J Vet Intern Med 2000;14:619–626. [DOI] [PubMed] [Google Scholar]
- 3. Whitney JL, Barrs VR, Wilkinson MR, et al. Use of bisphosphonates to treat severe idiopathic hypercalcaemia in a young Ragdoll cat. J Feline Med Surg 2011;13:129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McClain HM, Barsanti JA, Bartges JW. Hypercalcemia and calcium oxalate urolithiasis in cats: A report of five cases. J Am Anim Hosp Assoc 1999;35:297–301. [DOI] [PubMed] [Google Scholar]
- 5. Graham‐Mize CA, Rosser EJ. Bioavailability and activity of prednisone and prednisolone in the feline patient. Vet Dermatol 2004;15:7–10. [Google Scholar]
- 6. Fan TM, de Lorimier LP, Garrett LD, et al. The bone biologic effects of zoledronate in healthy dogs and dogs with malignant osteolysis. J Vet Intern Med 2008;22:380–387. [DOI] [PubMed] [Google Scholar]
- 7. Bolliger AP, Graham PA, Richard V, et al. Detection of parathyroid hormone‐related protein in cats with humoral hypercalcemia of malignancy. Vet Clin Pathol 2002;31:3–8. [DOI] [PubMed] [Google Scholar]
- 8. Westfall DS, Twedt DC, Steyn PF, et al. Evaluation of esophageal transit of tablets and capsules in 30 cats. J Vet Intern Med 2001;15:467–470. [DOI] [PubMed] [Google Scholar]
- 9. Hostutler RA, Chew DJ, Jaeger JQ, et al. Uses and effectiveness of pamidronate disodium for treatment of dogs and cats with hypercalcemia. J Vet Intern Med 2005;19:29–33. [DOI] [PubMed] [Google Scholar]
- 10. Lin JH, Duggan DE, Chen IW, et al. Physiological disposition of alendronate, a potent anti‐osteolytic bisphosphonate, in laboratory animals. Drug Metab Dispos 1991;19:926–932. [PubMed] [Google Scholar]
- 11. Mohn KL, Jacks TM, Schleim KD, et al. Alendronate binds to tooth root surfaces and inhibits progression of feline tooth resorption: A pilot proof‐of‐concept study. J Vet Dent 2009;26:74–81. [DOI] [PubMed] [Google Scholar]
- 12. Ching SV, Fettman MJ, Hamar DW, et al. The effect of chronic dietary acidification using ammonium chloride on acid‐base and mineral metabolism in the adult cat. J Nutr 1989;119:902–915. [DOI] [PubMed] [Google Scholar]
- 13. Macintyre I, Davidsson D. The production of secondary potassium depletion, sodium retention, nephrocalcinosis and hypercalcaemia by magnesium deficiency. Biochem J 1958;70:456–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wehner A, Katzenberger J, Groth A, et al. Vitamin D intoxication caused by ingestion of commercial cat food in three kittens. J Feline Med Surg 2013;15:730–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wieland RG, Hendricks FH, Amat y Leon F, et al. Hypervitaminosis A with hypercalcaemia. Lancet 1971;1:698. [DOI] [PubMed] [Google Scholar]
- 16. Roughead ZK, Lukaski HC. Inadequate copper intake reduces serum insulin‐like growth factor‐I and bone strength in growing rats fed graded amounts of copper and zinc. J Nutr 2003;133:442–448. [DOI] [PubMed] [Google Scholar]
- 17. Henry DA, Goodman WG, Nudelman RK, et al. Parenteral aluminum administration in the dog: I. Plasma kinetics, tissue levels, calcium metabolism, and parathyroid hormone. Kidney Int 1984;25:362–369. [DOI] [PubMed] [Google Scholar]
- 18. Thakker RV. Diseases associated with the extracellular calcium‐sensing receptor. Cell Calcium 2004;35:275–282. [DOI] [PubMed] [Google Scholar]
- 19. Yue QY, Mortimer O. Alendronate—Risk for esophageal stricture. J Am Geriatr Soc 1998;46:1581–1582. [DOI] [PubMed] [Google Scholar]
- 20. Gonzalez‐Moles MA, Bagan‐Sebastian JV. Alendronate‐related oral mucosa ulcerations. J Oral Pathol Med 2000;29:514–518. [DOI] [PubMed] [Google Scholar]
- 21. Abraham SC, Cruz‐Correa M, Lee LA, et al. Alendronate‐associated esophageal injury: Pathologic and endoscopic features. Mod Pathol 1999;12:1152–1157. [PubMed] [Google Scholar]
- 22. Hewitt RE, Lissina A, Green AE, et al. The bisphosphonate acute phase response: Rapid and copious production of proinflammatory cytokines by peripheral blood gd T cells in response to aminobisphosphonates is inhibited by statins. Clin Exp Immunol 2005;139:101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Recker RR, Lewiecki EM, Miller PD, et al. Safety of bisphosphonates in the treatment of osteoporosis. Am J Med 2009;122:S22–S32. [DOI] [PubMed] [Google Scholar]
- 24. Paul AK, Seetharaman M. Esophageal stricture associated with alendronate use. CMAJ 2011;183:E429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Levine J, Nelson D. Esophageal stricture associated with alendronate therapy. Am J Med 1997;102:489–491. [DOI] [PubMed] [Google Scholar]
- 26. Kelly R, Taggart H. Incidence of gastrointestinal side effects due to alendronate is high in clinical practice. BMJ 1997;315:1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Allen MR, Burr DB. Mandible matrix necrosis in beagle dogs after 3 years of daily oral bisphosphonate treatment. J Oral Maxillofac Surg 2008;66:987–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Body JJ, Pfister T, Bauss F. Preclinical perspectives on bisphosphonate renal safety. Oncologist 2005;10(Suppl 1):3–7. [DOI] [PubMed] [Google Scholar]
- 29. Markowitz GS, Appel GB, Fine PL, et al. Collapsing focal segmental glomerulosclerosis following treatment with high‐dose pamidronate. J Am Soc Nephrol 2001;12:1164–1172. [DOI] [PubMed] [Google Scholar]
- 30. Fan TM. The role of bisphosphonates in the management of patients that have cancer. Vet Clin North Am Small Anim Pract 2007;37:1091–1110; vi. [DOI] [PubMed] [Google Scholar]
- 31. Burr DB, Allen MR. Mandibular necrosis in beagle dogs treated with bisphosphonates. Orthod Craniofac Res 2009;12:221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rumbeiha WK, Fitzgerald SD, Kruger JM, et al. Use of pamidronate disodium to reduce cholecalciferol‐induced toxicosis in dogs. Am J Vet Res 2000;61:9–13. [DOI] [PubMed] [Google Scholar]
- 33. Rumbeiha WK, Kruger JM, Fitzgerald SF, et al. Use of pamidronate to reverse vitamin D3‐induced toxicosis in dogs. Am J Vet Res 1999;60:1092–1097. [PubMed] [Google Scholar]


