Abstract
Background
Computed tomography (CT) is highly accurate for diagnosing pancreatitis in humans. The diagnosis of pancreatitis in dogs is based on clinical signs, laboratory findings, and ultrasonographic (US) changes. There are, however, inherent limitations in relying on laboratory and ultrasound findings for the clinical diagnosis of pancreatitis in dogs.
Hypothesis/Objectives
We hypothesized that CT angiography would be a rapid and reliable method to confirm pancreatitis in dogs compared to ultrasonography. The aim was to describe the CT characteristics and compare them to ultrasound findings and correlate the CT appearance to the severity of the patients' clinical course.
Animals
A prospective pilot case series; 10 dogs with pancreatitis were enrolled if the history, clinical signs, laboratory, and ultrasonographic findings were indicative of pancreatitis.
Methods
A 3‐phase angiographic CT was performed under sedation. Afterward, each dog had US‐guided aspirates of the pancreas collected and blood drawn for cPLi assay. Images were evaluated for portion of visible pancreas, pancreatic size and margin, pancreatic parenchyma, presence of peripancreatic changes and contrast enhancement pattern. The results were compared with outcome.
Results
An enlarged, homogeneously to heterogeneously attenuating and contrast‐enhancing pancreas with ill‐defined borders was identified in all dogs. CT identified more features characterizing pancreatic abnormalities compared to US. Thrombi were found in 3/10 dogs. Three dogs with heterogeneous contrast enhancement had an overall poorer outcome than those with homogenous enhancement.
Conclusions and Clinical Importance
CT angiography under sedation was used in dogs to confirm clinically suspected pancreatitis and identified clinically relevant and potentially prognostic features of pancreatitis in dogs.
Keywords: CT, Dog, Pancreas, Ultrasound
Abbreviations
- CT
computed tomography
- US
ultrasound
Pancreatitis is the most common exocrine pancreatic disease in dogs, with an overall incidence of exocrine pancreatic diseases reported to be 1.7%.1 However, grossly normal pancreata of 93/101 dogs were found to have histological evidence of acute or chronic pancreatitis when histologic sections were taken every 2 cm throughout the organ, suggesting that histological lesions occur much more frequently than gross lesions.2 An additional study of 200 canine pancreata evaluated histologically found that the prevalence of acute or chronic pancreatitis in 37% of those animals, prompting the authors to suggest that pancreatitis may be a common and underestimated disease.3 The true overall incidence of clinical or subclinical pancreatitis in dogs remains unknown.
In veterinary medicine, a combination of clinical signs, laboratory findings including pancreatic lipase (cPLI) and B‐mode ultrasonography (US) commonly are used to diagnose pancreatitis clinically in dogs. Clinical signs include anorexia, vomiting, weakness, diarrhea, and abdominal pain, but these signs are not specific for pancreatitis. Hematological and biochemical laboratory testing provides further supportive evidence of the diagnosis but still lacks specificty.4 The cPLI test is considered the most sensitive assay for diagnosing clinical pancreatitis with a reported sensitivity of approximately 91% and a specificity of 72%.1 , 1, 2, 5, 6, 7 Imaging plays an important adjunctive role not only to aid in the diagnosis of pancreatitis but also to assess the severity of pancreatic changes.
B‐mode US is the most common imaging modality used to support the diagnosis of pancreatitis in dogs but also has limitations. In a retrospective study evaluating acute fatal pancreatitis, only 68% of dogs with histologically confirmed pancreatitis had US evidence of pancreatitis.8 Based on the anatomic location of the pancreas, gas within the gastrointestinal tract may hinder evaluation. In addition, abdominal pain from pancreatitis often prevents application of the transducer with sufficient pressure to properly image the right cranial abdomen. Ultrasonography also is very operator dependent, and results are influenced in part by operator experience.
In humans, computed tomography (CT) with IV contrast administration frequently is used for diagnosing and staging pancreatitis, and is considered to be highly sensitive and specific (for acute pancreatitis, 87–90% and 90–92%, respectively; for chronic pancreatitis, 60–95% sensitive and 90% sensitivity to detect necrosis).9, 10, 11, 12, 13, 14, 15, 16, 17, 18 The inflammatory changes associated with pancreatitis can lead to visible attenuation differences and altered contrast enhancement. The diagnosis of pancreatitis in humans is based on the combination of clinical signs, hematologic and biochemical findings, and characteristic CT changes. Pancreatic biopsies are no longer routinely performed in people to diagnose acute or chronic pancreatitis, because CT results generally are considered accurate because of strong correlation with histology.13 Computed tomography is more sensitive to soft tissue pathology than is ultrasonography caused by lack of superimposition of gastrointestinal tract gas and better soft tissue contrast, which allows for improved identification of subtle attenuation changes within the pancreas. The use of 3‐phase CT angiography provides added beneficial information regarding presence of pancreatic necrosis and vascular thrombi. Published data on the normal CT appearance of the canine pancreas is available, and endoscopic retrograde cholangiopancreotography also has been performed in veterinary patients.19, 20
Therefore, we hypothesized that CT angiography under sedation would represent a rapid and valid means of confirming pancreatitis in dogs, potentially also providing more complete visualization and thorough characterization of pancreatic abnormalities when compared to abdominal ultrasonography. Our main goal in this pilot study was to document the CT characteristics of pancreatitis in dogs and compare those findings to ultrasound results. An additional aim was to compare the CT appearance of the pancreas to the severity of the patients' clinical course.
Materials and Methods
Inclusion Criteria
A prospective pilot case series of 10 dogs with clinical acute pancreatitis was conducted at Colorado State University from August 2012 to December 2013. Dogs were enrolled if history, clinical signs, laboratory findings, and ultrasonography findings were indicative of acute pancreatitis. Clinical findings included, but were not limited to, vomiting, pain on abdominal palpation, and diarrhea. The laboratory findings included a combination of an inflammatory leukogram, hypocalcemia, and an increase in cholestatic liver enzyme activity. The ultrasonographic diagnosis of pancreatitis was based on previous reports and included a hypoechoic, enlarged pancreas with surrounding hyperechoic mesentery with or without adjacent free fluid.21, 22, 23 The study protocol was approved by the Institutional Animal Care and Use Committee (IACUC) and all canine owners gave informed consent. Dogs included in the study also were healthy enough to tolerate a 3‐phase CT angiogram under sedation within 3 days of the ultrasound procedure. Age, breed, sex, and weight were recorded. A concurrent control group with normal appearing pancreas on CT was not evaluated, but comparison to previously published data was made.19 Dogs with known liver and pancreatic neoplasia and evidence of portal hypertension were not entered into the study.
Ultrasound and CT Angiography
All patients were scanned in dorsal recumbency with a B‐mode ultrasound machine.2 A complete abdominal ultrasound examination was performed by either a radiology resident or board‐certified radiologist. Based on clinical and initial ultrasound findings, patients were included in the study. Additional ultrasound images, cine loop recordings, or both the pancreas were obtained by either a board‐certified radiologist (AM) or radiology resident (AA). Next, a 3‐phase CT angiogram under sedation was performed on the same day, or within the following 3 days. The sedation protocol used varied based on the clinician's preference.
The CT was performed using a 16‐slice PET‐CT scanner.3 During CT, the patients were positioned in dorsal recumbency. Two‐millimeter thick precontrast slices of the entire abdomen were acquired in a standard algorithm, scanning cranially to caudally. The locator for the contrast bolus tracer was placed over the aorta at a level between the celiac artery and cranial mesenteric artery. One milliliter per pound body weight of nonionic positive contrast (Iohexol)4 was administered by a CT power injector5 in the cephalic vein at a flow rate of 3 ml/s, if the patient weighed <15 kg and 4 ml/s if the patient weighed ≥15 kg. Arterial and venous phase images were acquired using 1‐mm thick slices reconstructed in a standard algorithm, extending from the level of the midliver to caudal to the left kidney. For the arterial phase, the scan direction was cranial to caudal and for the venous phase the scan direction was caudal to cranial. Approximately 2–3 minutes after contrast injection, delayed phase 2‐mm thick slices of the entire abdomen were acquired in a cranial to caudal scan direction and reconstructed in a standard algorithm. Dorsal and sagittal reformats were created as needed.
Additional Tests
All dogs had a CBC, biochemical profile and cPLI submitted at the time of the imaging studies.6 Negative cPLI results did not exclude patients from the study. After the CT angiogram, ultrasound‐guided aspirates of the pancreas using 22 g needles were obtained for cytologic analysis in an attempt to eliminate a diagnosis of pancreatic neoplasia. A standard Wright–Giemsa stain was used and the cytological samples were reviewed by various board‐certified clinical pathologists.
Data Analysis
The ultrasound and CT angiography images were reviewed by a board‐certified radiologist (AM) and a third‐year radiology resident (AA) to establish a consensus of the findings. The radiologists knew every dog had a clinical diagnosis of pancreatitis, but they were blinded to the ultrasound findings. They also were blinded to the ultrasound findings when evaluating the CT angiography findings. The following parameters were assessed and compared: portion of visible pancreas (left limb, body, right limb, all), affected limb, pancreatic size (measured at the largest thickness) and margin (smooth versus irregular), pancreatic parenchyma (hypo‐, iso‐, or hyperechoic/attenuating), presence of peripancreatic changes (eg, hyperattenuating mesentery, free peritoneal fluid) and (on CT only) contrast enhancement pattern during each phase (homogenous, heterogenous, ring‐like). In addition, the duodenum, stomach, portal vein, adjacent lymph nodes, pancreatic duct, and common bile duct were evaluated if visible.
Mean, median, and range were calculated for pancreatic size. Comparative statistics between ultrasound and CT angiography under sedation were not performed because of small sample size. Imaging results were compared with clinical patient outcome to determine whether CT angiography under sedation allowed assessment of severity of disease.
Results
Clinical Findings
Four female spayed and 6 male castrated dogs with an age range of 6.5–12 years (mean, 9.25; median, 9) were entered in the study. Three dogs were Miniature Schnauzers, with 1 each of a Pug, Welsh Corgi, mixed breed, Keeshound, Miniature Pinscher, Italian Greyhound and Cocker Spaniel. Two dogs with positive ultrasound imaging findings, clinical signs, and laboratory findings were not enrolled because of the critical status of the patients. The weight ranged from 6 to 24.16 kg (mean, 15.25; median, 15.45 kg). On the CBC, an inflammatory leukogram was present in 6/10 dogs, 5/10 had left shift, 1/10 had a left shift with normal mature neutrophils, 3/10 patients had toxic neutrophils, 1/10 had thrombocytosis, and 2/10 patients had a normal CBC. On bio‐chemistry evaluation, 5/10 dogs had hypercholesterolemia, 4/10 hyperglycemia, 3/10 hypocalcemia, 5/10 hypoalbuminemia, 1/10 hyperglobulinemia, 3/10 hyperbilirubinemia, 1/10 hypertriglyceridemia (the only patient for which this test was ordered), 7/10 high cholestatic liver enzyme activity and 4/10 elevated hepatocellular liver enzyme activity. One of the dogs had a previous body and left limb pancreatectomy for possible insulinoma, which was not confirmed on histology. No cytologic abnormalities, but normal pancreatic cells, were seen in 3/10 cases, inflammation and pancreatic cells were present in 4/10 and 3/10 cases, respectively, with 1/4 having leukocytes and 3/4 having neutrophils, and 3/10 were nondiagnostic samples. The cPLI was abnormal in 6/10 dogs (>400 μg/L), 1/10 was equivocal (201–399 μg/L) and 3/10 were normal (<200 μg/L).
Ultrasound imaging Results
Ultrasound and sedated CT angiography results are summarized in Table 1. On US, 6/10 pancreata were visualized in their entirety. In 1/4, the left limb and body (because of partial pancreatectomy); in 1/4, the left limb; and, in 1/4, the body was not identified. In 1/4 dogs, the right limb and body were visualized in the entirety, but just the cranial portion of the left limb was seen. The visualized portion of this left limb was evaluated and included in the subsequent description. Ultrasonographically, 5/7 left limbs, 7/7 bodies, and 9/10 right limbs were abnormal, enlarged, and hypoechoic. A summary of the ultrasonographic pancreatic height measurements of the affected pancreata is presented in Table 2. The surrounding mesentery was hyperechoic in 4/7 left limbs, 6/7 bodies, and 9/10 right limbs (Fig 1A). Eight of 10 dogs had a fluid‐ and gas‐distended stomach. One patient had a cholelith. Ultrasonography did not identify the common bile duct or pancreatic duct in any dog. The duodenum was corrugated in 1 patient and was normal in the remaining 9 patients.
Table 1.
Summary of selected results
| Criteria | US | CT |
|---|---|---|
| Entire visible pancreas | 6/10 | 10/10 |
| Signs of inflammation (enlargement with hypoechogenicity and altered attenuation) |
5/7 left limbs 7/7 bodies 9/10 right limbs |
4/9 left limbs 9/9 bodies 10/10 right limbs |
| Homogeneity of parenchyma |
7/7 left limbs 5/7 bodies 7/10 right limbs |
9/9 left limbs 8/9 bodies 9/10 right limbs |
| Ill‐defined borders |
5/7 left limbs 7/7 bodies 8/10 right limbs |
3/9 left limbs 7/9 bodies 7/10 right limbs |
| Hyperechoic/hyperattenuating mesentery |
4/7 left limbs 6/7 bodies 9/10 right limbs |
3/9 left limbs 4/9 bodies 5/10 right limbs |
| Surrounding free fluid |
1/7 left limbs 1/7 bodies 5/10 right limbs |
2/9 left limbs 2/9 bodies 3/10 right limbs |
| Distended stomach | 8/10 | 7/10 |
| Portal vein thrombus detection | 0/10 | 3/10 |
| Cholelith detection | 1/10 | 2/10 |
Table 2.
US height measurements of the affected pancreata
| Dogs with Pancreatitis | Normal Dogsa | ||||
|---|---|---|---|---|---|
| Mean Height (cm) | Median Height (cm) | Range Height (cm) | Mean Height (cm) | SD Height (cm) | |
| Left limb | 1.79 | 1.38 | 1.2–3.3 | 0.65 | 0.4–0.8 |
| Body | 1.87 | 1.59 | 1.2–3.7 | 0.63 | 0.4–0.7 |
| Right limb | 2.07 | 1.93 | 1.2–4.3 | 0.81 | 0.6–9.9 |
Normal data republished from Penninck et al.28
Figure 1.
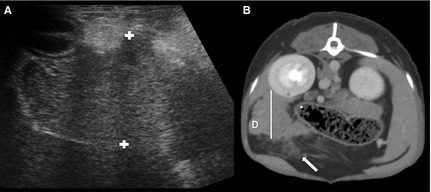
Transverse ultrasound (A) and delayed phase transverse CT (B) image of the right pancreatic limb of the same patient. On both images, the pancreas is irregularly marginated and surrounded by hyperechoic and attenuating parenchyma. The pancreas is hypoechoic on the ultrasound image. Calipers show the height measurements on the ultrasound image and the white line represents the height measurements on the CT image. Note the surrounding free fluid visualized on the CT image (arrow). D, duodenum.
CT imaging results
Overall, the CT angiographic procedure under sedation was successful in all patients with good vascular phase separation. Drugs used included a combination of at least 2 of the following: buthorphanol, midazolam, fentanyl, ketamine, propofol, and buprenorphine. The sedation protocols were deemed equally effective, but a combination of propofol with another drug was commonly used. The overall scan and sedation time was approximately 20 minutes per patient. All studies were of adequate diagnostic quality and motion artifact was minimal. There were no complications in any dog. Ultrasonography and CT angiography results are summarized in Table 1. On CT, all pancreata were visualized in their entirety; however, 1 right limb was visualized only on postcontrast images and 2 left limbs were better visualized on postcontrast images. A summary of the CT pancreatic height measurements of the affected pancreata is presented in Table 3. The surrounding mesentery was hyperattenuating in 3/9 left limbs, 4/9 bodies, and 5/10 right limbs (Fig 1B). The contrast enhancement pattern in all 3 phases was homogenous in 7/9 left limbs and bodies, and 9/10 right limbs. In 4 patients with heterogenous contrast enhancement, the delayed phase was best for visualizing ring‐like and patchy enhancement. Figure 2 shows the different types of contrast enhancement. Seven of 10 dogs had a fluid‐distended stomach. CT‐identified thrombi in the portal vein in 3/10 dogs (Fig 3), none of the thrombi were identified on US, and a cholelith in 2 dogs, 1 of which was identified on US. The pancreatic duct was visualized on CT in 3 patients, and these ducts were considered normal. The common bile duct was visualized in 5/10 dogs and 2 ducts were considered dilated. The duodenum was fluid‐distended in 1 patient, with the remainder of the patients being normal.
Table 3.
CT height measurements of the affected pancreata
| Dogs with Pancreatitis | Normal Dogsa | ||||
|---|---|---|---|---|---|
| Mean Height (cm) | Median Height (cm) | Range Height (cm) | Median Height (cm) | Range Height (cm) | |
| Left limb | 2.1 | 2.05 | 1.5–2.8 | 1.2 | 0.8–1.9 |
| Body | 2.01 | 1.97 | 1.4–4.1 | 1.4 | 0.8–2.5 |
| Right limb | 2.36 | 2.26 | 1.6–3.3 | 1.6 | 1.2–2.1 |
Normal data republished from Cáceres et al.19
Figure 2.
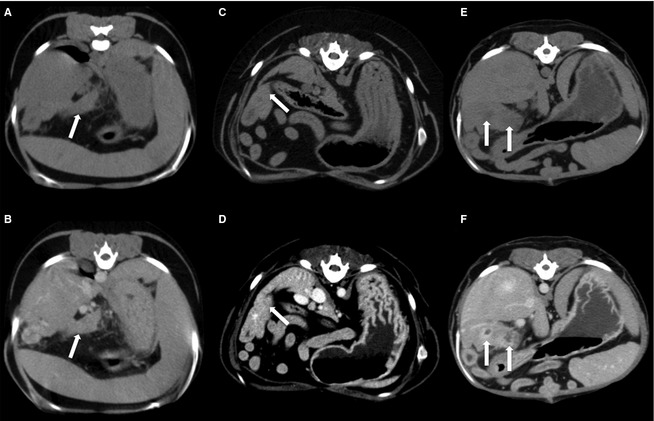
Transverse precontrast images of 3 patients (A, C, E) Transverse delayed phase CT images of the same 3 patients (B, D, F). (B) The left limb of the pancreas has homogenous contrast enhancement (arrow). (D) The right limb of the pancreas has heterogenous contrast enhancement. Note the multifocal noncontrast enhancing areas throughout the right limb (arrow). (F) The right limb of the pancreas has a ring‐like contrast enhancement pattern (arrows).
Figure 3.
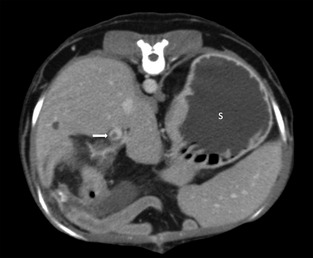
Transverse delayed phase CT image. A round, well‐defined filling defect is visualized in the portal vein (arrow), representing a thrombus. Note also the fluid‐distended stomach (S).
Outcome
The medical records of the patients had follow‐up information ranging from between 1 day and 12 month poststudy. Of the patients with heterogenous contrast enhancement pattern based on CT imaging, 1 died, 2 had recurrent pancreatitis, and 1 recovered uneventfully. Of the 2 patients that had recurrent pancreatitis, 1 had recurrent pancreatitis at 5 months and 9.5 months poststudy and the other had recurrent pancreatitis at 16 and 29 days after the study. The first dog was followed up for 11 months and the second dog was followed up for 7 months. The remaining 6 patients with homogenous contrast enhancement pattern recovered uneventfully. Of the 3 patients with portal vein thrombi, 1 patient, that also showed heterogenous contrast enhancement, had recurrent pancreatitis, and 2 recovered uneventfully.
Discussion
Pancreatitis in dogs is the most frequently reported disease process of the canine exocrine pancreas, but the diagnosis often is difficult, relying on a combination of clinical and laboratory findings and imaging studies as well as clinical experience and judgment.1, 3 The most commonly accepted definitive diagnosis of pancreatitis requires histopathology, but this is rarely performed in clinical situations. Although severe acute disease can be detected by US, there are inherent limitations in evaluating the pancreas sonographically. In this small pilot study in the majority of cases, CT under sedation allowed a more complete evaluation of pancreatic changes than did US. An enlarged, homogenously to heterogeneously attenuating and contrast‐enhancing pancreas with ill‐defined borders on CT was consistent with pancreatitis. Based on the outcome of the dogs enrolled in this study, heterogeneous contrast enhancement may be a negative prognostic indicator. Three of 10 dogs also were diagnosed with portal vein thrombosis, which could alter clinical management and outcome.
The signalment in these dogs correlated well with previous descriptions of pancreatitis in dogs.4, 6, 24 Three dogs had normal cPLI assay results. Even though serum cPLI concentrations are considered highly sensitive (with approximately 91% sensitivity when cPLI values are >200 μL/L), false negative results are possible in approximately 25% of dogs with acute pancreatitis. Only 4 patients had cytologic evidence of pancreatitis and, although these negative cytologic results may have been accurate; these patients had other findings consistent with pancreatitis and were considered to have pancreatic inflammation despite the negative cytologic results. This low number could be because of the sampling method or the patchy nature of the disease.25
Previously described ultrasonographic changes consistent with acute pancreatitis including pancreatic enlargement (>1–2 cm in size), hypoechoic parenchyma, adjacent hyperechoic mesentery, and peritoneal fluid26 also were identified in this study.26, 27 Comparing the ultrasonographic size measurements from this study with the previously published normal reference range,28 it is shown that the mean values were up to 3 times higher. When comparing the size measurements of CT and US, the CT measurements were higher, which likely is because of magnification and the physics of the different modalities. The lack of visualization of some of the pancreatic limbs or body and the common bile duct were secondary to the presence of a gas‐distended stomach or abdominal pain limiting the US examination. In this study, the majority of dogs had a large fluid‐distended stomach, which hindered thorough sonographic evaluation of the pancreas. A large fluid‐filled stomach may be secondary to ileus from the adjacent pancreatic inflammation.
The results of this study supported our hypotheses that CT angiography would allow evaluation of the entire pancreas and identify more pancreatic and extrapancreatic abnormalities than US, similar to the experience with pancreatitis in humans, but, because this is a pilot study, enrolling more dogs in the future is necessary. Some of the most salient advantages of CT angiography under sedation for evaluating pancreatitis in dogs is visualization of the entire pancreas because of the lack of superimposition of gastrointestinal gas and gastric fluid distention, identification of contrast enhancement pattern, and full evaluation of the portal vein. This modality also is less operator dependent than ultrasonography which may improve repeatability. Affected dogs had an enlarged, homogenously to heterogeneously attenuating and contrast‐enhancing pancreas with ill‐defined borders, as has been reported in humans.9, 10, 11, 13, 14, 17, 18 Comparing the CT height measurements of this study with the previously published normal reference range,19 mean values were nearly 2 times higher. Contrast enhancement patterns, particularly ring‐like contrast enhancement, was best visualized on the delayed phase images. The thrombi also were visualized on the delayed phase images. Because the aterial and venous phases did not add additional information, the delayed phase appears to be adequate for evaluation of acute pancreatitis.
The small number of dogs with heterogeneous contrast enhancement had a poorer outcome compared to patients with homogenous contrast enhancement. This might indicate that heterogenous contrast enhancement is associated with poorer outcome, although a larger study would be required to confirm our observation. Pancreatic necrosis is present in 96% of dogs with fatal acute pancreatitis.27 Acute pancreatitis in humans is divided into edematous or necrotizing subcategories, which can be distinguished based on CT findings.10 Edematous pancreatitis enhances homogenously whereas necrotizing pancreatitis has parenchymal areas that do not enhance.16, 29 The heterogenous pattern visualized in this study may correlate with necrotic or fibrotic nonenhancing regions in the pancreas. Necrotizing pancreatitis in humans has a more guarded prognosis.10, 30, 31, 32, 33, 34 In dogs, areas of noncontrast enhancement on CT also have been associated with pancreatic necrosis.29 Heterogenous contrast enhancement may be a negative prognostic indicator, but larger studies are needed to support this observation.
A complication in humans with acute pancreatitis is venous thrombosis, which is seen in 30% of severe and 57% of necrotizing pancreatitis cases.35 Portal vein thrombosis previously has been reported in dogs with several conditions, including pancreatits.36 Three of the dogs in this study had CT‐identified portal vein thrombi. One dog had recurrent pancreatitis and 2 recovered uneventfully. None of the thrombi diagnosed with CT were identified ultrasonographically, but no color doppler ultrasound was performed over the portal vein in this study because of inability to properly visualize the portal vein as a consequence of gastrointestinal superimposition and because of patient discomfort. Additional studies are needed to further evaluate the prognostic value of portal vein thrombosis in dogs with pancreatitis.
Computed tomography angiography under sedation with a multislice CT scanner allows excellent evaluation of the cranial abdomen. Another benefit may be to evaluate patients with suspected pancreatitis that have negative ultrasound imaging findings, because CT may be more sensitive to less severe pancreatic inflammation.
Sedation was used in this study, because general anesthesia can be considered a relative contraindication for performing CT in patients with pancreatitis.29 The sedation protocols allowed for an adequate imaging study with no adverse effects secondary to contrast administration. CT angiography is technically challenging and requires time and effort to develop. It is unknown what CT angiography image quality under sedation would be in a single slice CT scanner. The vascular phases may not be adequately separated, decreasing the quality of the study.
Limitations of this study include the small number of dogs and the inherent bias of the reviewers who were intentionally not blinded. In addition, the cases for this study were preselected based on clinical signs, appropriate hematologic and biochemical abnormalities and US findings, and therefore there was a bias to cases with positive imaging findings. Dogs with pancreatitis and normal ultrasound findings therefore were not included, and CT appearance might be different for those patients. However, the main goal of this small pilot study was to determine the CT changes associated with pancreatitis in a group of dogs with evidence of clinical pancreatitis. An additional limitation was the lack of histological confirmation of pancreatitis. Pancreatic biopsies are rarely obtained to confirm pancreatitis because of the need for general anesthesia and the invasive nature of pancreatic biopsy procurement in sick clinical patients. A large prospective study is needed to determine the specificity, sensitivity, and accuracy of CT angiography compared with ultrasonography.
In summary, the CT findings of suspected pancreatitis in dogs are an enlarged, homogeneously to heterogeneously attenuating and contrast‐enhancing pancreas with ill‐defined borders. Sedated CT angiography allowed the entire pancreas to be examined and a more complete evaluation of pancreatic and peripancreatic changes than did US. The contrast enhancement of the pancreas additionally showed clinically important features that were not seen with US. Identification of heterogeneous contrast enhancement of the pancreas may be a negative prognostic indicator. There is potential for portal vein thrombosis to occur in dogs with pancreatitis, and identification of a thrombus may influence clinical management. Ultrasonography may be a first line screening examination for dogs with suspected pancreatitis because of availability and cost, but, based on this study, CT angiography under sedation should be considered for evaluation of severe or recurrent pancreatitis in patients suspected to have pancreatitis in which ultrasound imaging is negative, and to evaluate for portal vein thrombosis.
Acknowledgments
The authors thank Billie K. Arceneaux and Jeff Stewart for excellent CT technical work.
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
This work was performed at Colorado State University. The study was supported by funds from the Colorado State University College Research Council. It was presented as an abstract in form of a poster at the American College of Veterinary Radiology Annual Meeting, Savannah, GA, October 2013.
Footnotes
Steiner J. Serum canine pancreatic lipase immunoreactivity (cPLi) concentrations in dogs with spontaneous pancreatitis. J Vet Intern Med 2001 May;15:274
Antares Siemens; Siemens Medical Solutions, Mountain View, CA
Gemini TF Big Bore System; Philips Medical System, Cleveland, OH
350 mgI/mL Omnipaque; GE Healthcare Inc, Princeton, NJ
Stellant Dual Head Power Injector; Medrad, Indianola, PA
IDEXX Laboratories, Westbrook, ME or Gastrointestinal Laboratory, College Station, TX
References
- 1. Hänichen T, Minkus G. Retrospektive Studie zur Pathologie der Erkrankungen des exokrinen Pankreas bei Hund und Katze. Tierärztliche Umschau 1990;45:363–368. [Google Scholar]
- 2. Newman SJ, Steiner JM, Woosley K, et al. Histologic assessment and grading of the exocrine pancreas in the dog. J Vet Diagn Invest 2006;18:115–118. [DOI] [PubMed] [Google Scholar]
- 3. Watson PJ, Roulois AJA, Scase T, et al. Prevalence and breed distribution of chronic pancreatitis at post‐mortem examination in first‐opinion dogs. J Small Anim Pract 2007;48:609–618. [DOI] [PubMed] [Google Scholar]
- 4. Steiner J. Is it pancreatitis? Vet Med 2006;101:158–166. [Google Scholar]
- 5. Steiner JM, Rutz GM, Williams DA. Serum lipase activities and pancreatic lipase immunoreactivity concentrations in dogs with exocrine pancreatic insufficiency. Am J Vet Res 2006;67:84–87. [DOI] [PubMed] [Google Scholar]
- 6. Xenoulis PG, Suchodolski JS, Ruaux CG, Steiner JM. Association between serum triglyceride and canine pancreatic lipase immunoreactivity concentrations in miniature schnauzers. J Am Anim Hosp Assoc 2010;46:229–234. [DOI] [PubMed] [Google Scholar]
- 7. McCord K, Morley PS, Armstrong J, et al. A multi‐institutional study evaluating the diagnostic utility of the Spec cPL™ and SNAP® cPL™ in clinical acute pancreatitis in 84 dogs. J Vet Intern Med 2012;26:888–896. [DOI] [PubMed] [Google Scholar]
- 8. Hess RSR, Saunders HMH, Van Winkle TJT, et al. Clinical, clinicopathologic, radiographic, and ultrasonographic abnormalities in dogs with fatal acute pancreatitis: 70 cases (1986‐1995). J Am Vet Med Assoc 1998;213:665–670. [PubMed] [Google Scholar]
- 9. Kim DH, Pickhardt PJ. Radiologic assessment of acute and chronic pancreatitis. Surg Clin NA 2007;87:1341–1358. [DOI] [PubMed] [Google Scholar]
- 10. Scaglione M, Casciani E, Pinto A, et al. Imaging assessment of acute pancreatitis: A review. Semin Ultrasound CT MR 2008;29:322–340. [DOI] [PubMed] [Google Scholar]
- 11. Frossard J‐L, Steer ML, Pastor CM. Acute pancreatitis. Lancet 2008;371:143–152. [DOI] [PubMed] [Google Scholar]
- 12. Cappell MS. Acute pancreatitis: Etiology, clinical presentation, diagnosis, and therapy. Med Clin North Am 2008;92:889–923. [DOI] [PubMed] [Google Scholar]
- 13. Wahab SS, Khan RAR, Ahmad II, Wahab AA. Imaging and clinical prognostic indicators of acute pancreatitis: A comparative insight. Acta Gastroenterol Latinoam 2010;40:283–287. [PubMed] [Google Scholar]
- 14. Choueiri NE, Balci NC, Alkaade S, Burton FR. Advanced imaging of chronic pancreatitis. Curr Gastroenterol Rep 2010;12:114–120. [DOI] [PubMed] [Google Scholar]
- 15. De Waele JJJ, Delrue LL, Hoste EAE, et al. Extrapancreatic inflammation on abdominal computed tomography as an early predictor of disease severity in acute pancreatitis: Evaluation of a new scoring system. Pancreas 2007;34:185–190. [DOI] [PubMed] [Google Scholar]
- 16. Arvanitakis M, Koustiani G, Gantzarou A, et al. Staging of severity and prognosis of acute pancreatitis by computed tomography and magnetic resonance imaging‐a comparative study. Dig Liver Dis 2007;39:473–482. [DOI] [PubMed] [Google Scholar]
- 17. Sheu YY, Furlan AA, Almusa OO, et al. The revised Atlanta classification for acute pancreatitis: A CT imaging guide for radiologists. Emerg Radiol 2012;19:237–243. [DOI] [PubMed] [Google Scholar]
- 18. Remer EM, Baker ME. Imaging of chronic pancreatitis. Radiol Clin North Am 2002;40:1229–1242. [DOI] [PubMed] [Google Scholar]
- 19. Cáceres AV, Zwingenberger AL, Hardam E, et al. Helical computed tomographic angiography of the normal canine pancreas. Vet Radiol Ultrasound 2006;47:270–278. [DOI] [PubMed] [Google Scholar]
- 20. Spillmann T, Schnell‐Kretschmer H, Dick M, et al. Endoscopic retrograde cholangio‐pancreatography in dogs with chronic gastrointestinal problems. Vet Radiol Ultrasound 2005;46:293–299. [DOI] [PubMed] [Google Scholar]
- 21. Murtaugh RJ, Herring DS, Jacobs RM, DeHoff WD. Pancreatic ultrasonography in dogs with experimentally induced acute pancreatitis*. Vet Radiol 1985;2:27–32. [Google Scholar]
- 22. Nyland TG, Mulvany MH, Strombeck DR. Ultrasonic features of experimentally induced, acute pancreatitis in the dog. Vet Radiol Ultrasound 1983;24:260–266. [Google Scholar]
- 23. Lamb CR, Simpson KW. Ultrasonographic findings in cholecystokinin‐induced pancreatitis in dogs. Vet Radiol Ultrasound 1995;36:139–145. [Google Scholar]
- 24. Cook AK, Breitschwerdt EB, Levine JF, et al. Risk factors associated with acute pancreatitis in dogs: 101 cases (1985‐1990). J Am Vet Med Assoc 1993;203:673–679. [PubMed] [Google Scholar]
- 25. Newman S, Steiner J, Woosley K, et al. Localization of pancreatic inflammation and necrosis in dogs. J Vet Intern Med 2004;18:488–493. [DOI] [PubMed] [Google Scholar]
- 26. Saunders HM. Ultrasonography of the pancreas. Probl Vet Med 1991;3:583–603. [PubMed] [Google Scholar]
- 27. Hess RSR, Kass PHP, Shofer FSF, et al. Evaluation of risk factors for fatal acute pancreatitis in dogs. J Am Vet Med Assoc 1999;214:46–51. [PubMed] [Google Scholar]
- 28. Penninck DG, Zeyen U, Taeymans ON, Webster CR. Ultrasonographic measurement of the pancreas and pancreatic duct in clinically normal dogs. Am J Vet Res 2013;74:433–437. [DOI] [PubMed] [Google Scholar]
- 29. Jaeger JQ, Mattoon JS, Bateman SW, Morandi F. Combined use of ultrasonography and contrast enhanced computed tomography to evaluate acute necrotizing pancreatitis in two dogs. Vet Radiol Ultrasound 2003;44:72–79. [DOI] [PubMed] [Google Scholar]
- 30. Bradley EL. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, GA, September 11 through 13, 1992. 1993. pp. 586–590. [DOI] [PubMed] [Google Scholar]
- 31. Mortele KJ, Wiesner W, Intriere L, et al. A modified CT severity index for evaluating acute pancreatitis: Improved correlation with patient outcome. Am J Roentgenol 2004;183:1261–1265. [DOI] [PubMed] [Google Scholar]
- 32. Steinberg W, Tenner S. Acute pancreatitis. N Engl J Med 1994;330:1198–1210. [DOI] [PubMed] [Google Scholar]
- 33. de Beaux AC, Palmer KR, Carter DC. Factors influencing morbidity and mortality in acute pancreatitis; an analysis of 279 cases. Gut 1995;37:121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hylands R. Veterinary diagnostic imaging. Can Vet J 2006;47:1214–1217. [PMC free article] [PubMed] [Google Scholar]
- 35. Dörffel Y, Wruck U, Rückert RI, et al. Vascular complications in acute pancreatitis assessed by color duplex ultrasonography. Pancreas 2000;21:126–133. [DOI] [PubMed] [Google Scholar]
- 36. Respess M, O'Toole TE, Taeymans O, et al. Portal vein thrombosis in 33 dogs: 1998–2011. J Vet Intern Med 2012;26:230–237. [DOI] [PubMed] [Google Scholar]


