Abstract
Background
Myocardial injury can be detected by cardiac troponin I (cTnI) concentration, which appears to be a predictor of short‐term death in critically ill patients. It is unknown if the best prognostic indicator of short‐term survival is cTnI measurement at admission or at later time points.
Hypothesis/Objectives
Measuring cTnI with a high‐sensitivity (HS) test at different time points after admission may be a better short‐term prognostic indicator than a single cTnI measurement at admission in dogs with systemic inflammatory response syndrome (SIRS).
Animals
Prospective, observational clinical study of 60 dogs with SIRS.
Methods
Cardiac troponin I concentration was measured in 133 serum samples, collected at days 1, 2, 3, and 5. Additionally, the acute patient physiologic and laboratory evaluation (APPLE) fast score was evaluated at admission. Prognostic capabilities of cTnI measurement and APPLE fast score for 28‐day mortality were assessed by receiver operating characteristic curve analysis.
Results
Forty‐one dogs with SIRS that survived 28 days had significantly lower serum cTnI concentrations at admission (median, 0.09 ng/mL; P = .004) and at the peak time point (median, 0.23 ng/mL; P = .01) compared to 19 nonsurvivors (median at admission, 0.63 ng/mL; median at peak, 1.22 ng/mL). Area under the curve to predict survival, using cTnI was similar at admission (0.732) and at peak (0.708), and was 0.754 for the APPLE fast score.
Conclusions and Clinical Importance
Increased cTnI concentration in dogs with SIRS is associated with poor outcome. Daily follow‐up measurement of cTnI concentration provides no additional prognostic information for short‐term mortality.
Keywords: Biomarker, Canine, Myocardial injury, Outcome, Prognostic factor, Systemic inflammatory response syndrome
Abbreviations
- ACS
acute coronary syndrome
- APPLE
acute patient physiologic and laboratory evaluation
- AUC
area under the curve
- cTnI
cardiac troponin I
- cTnT
cardiac troponin T
- ECG
electrocardiogram
- GDV
gastric dilatation volvulus
- HS
high‐sensitivity
- ICU
intensive care unit
- ROC
receiver operating characteristic
- SIRS
systemic inflammatory response syndrome
Myocardial injury has been reported to occur in critically ill humans and animals.1, 2, 3, 4, 5, 6, 7 Several noncardiac diseases can lead to secondary myocardial injury, which accentuates the importance of monitoring cardiac function. Myocardial injury can be detected by measuring cardiac troponin I (cTnI) or cardiac troponin T (cTnT) concentrations, and has been reported in dogs with gastric dilatation volvulus (GDV),4, 6 ehrlichiosis,8, 9 babesiosis,10, 11, 12 leishmaniasis,13 pyometra,14, 15 snake envenomation,16 kidney diseases,17, 18 noncardiac causes of respiratory distress,19 and canine parvovirosis.20
Troponins are physiologic regulatory proteins and part of the contractile apparatus of skeletal and cardiac muscle tissue. They are essential for the calcium‐mediated regulation of muscle contraction. The troponin complex consists of 3 different proteins (troponin I, T, and C).21 Each troponin protein has specific functions that are essential for muscle contraction.22 Troponin I has 3 isoforms; 2 are present in skeletal muscle. cTnI has a molecular weight of 24,000 Da and is present only in cardiac muscle.2, 23, 24 The majority of cTnI is structurally bound to the contractile apparatus. Approximately, 2–4% of cTnI remains free in the cytosol.25, 26
During myocardial injury, loss of membrane integrity because of destruction of cardiac myocytes causes release of cTnI into the circulation.27 An increase in serum cTnI concentration is indicative of cardiac myocyte injury.7, 28
Increased plasma cTnI concentrations have been described in dogs with cardiac diseases such as cardiomyopathy, mitral valve disease or subvalvular aortic stenosis.29 In clinically normal Doberman Pinschers, increased cTnI concentrations indicate myocardial injury during early phases of the disease when no echocardiography and Holter‐ECG abnormalities are present.30
The optimal time point of cTnI measurement to detect myocardial injury still is a matter of debate. In humans, cTnI is detectable in blood 3–12 hours after cardiac injury. Concentrations begin to increase and peak at 12–48 hours and then decrease after 5–10 days.31 A canine model of acute myocardial infarction showed similar release times, but the peak occurred earlier (range, 10–16 hours).32 A study of dogs with GDV identified peak serum cTnI concentrations between 48 and 72 hours, after surgery. Some of the dogs had progressive increases in serum cTnI concentrations and at each measurement time cTnI concentrations were significantly higher in dogs that died than in dogs that survived, suggesting that progressively increasing serum cardiac troponin concentrations may have prognostic value.6
Development of secondary myocardial injury in critically ill patients is associated with poor outcome in humans and animals.1, 4, 5, 6, 33, 34 To determine prognosis in affected patients, myocardial injury must be detected as soon as possible. Therefore, an early and sensitive test for use in dogs with severe systemic diseases is warranted.
The SIRS classification was introduced to describe the clinical response to systemic inflammation and has been applied to patients with inflammatory disease.35, 36, 37 The SIRS classification is based on basic clinical and laboratory parameters. A recent study found a significant relationship between cTnI and short‐term outcome in critically ill dogs with systemic inflammation. cTnI contributed significantly to the prognostic power of another scoring system for dogs with severe systemic diseases, the physiologic and laboratory evaluation (APPLE) full score in those patients.38 Using immunoassays with high sensitivity for cTnI improved the sensitivity for detection of myocardial injury as compared to older generation cTnI assays.7, 39, 40, 41
The aim of this prospective observational clinical study was to determine if measurement of cTnI using a high‐sensitivity (HS) test at different time points after admission is a better short‐term prognostic indicator than a one‐time cTnI measurement at admission in dogs with SIRS.
Material and Methods
The study was approved by the institutional animal use and care committee of the veterinary faculty of the LMU University, Munich, Germany.
Animals and Selection Criteria
The study was performed as a prospective cohort study with 28 days of follow‐up. Dogs presented to the medical Emergency and Critical Care Service with SIRS, and that required Intensive Care Unit (ICU) hospitalization afterward, were prospectively included in this observational clinical study. The diagnosis of SIRS was based upon fulfillment of ≥2 of the established SIRS criteria as described in a previous publication:36
Hypo‐or hyper‐thermia (<37.8°C or >39.7°C)
Tachycardia (>160 heart rate/min)
Tachypnea (>40 breaths/min)
PCO2 (≤32 mmHg)
Leukopenia or leukocytosis (<4.0 × 109/L or >12.0 × 109/L)
Immature (band) neutrophils >10%
Dogs were excluded if they had a relevant cardiac disease that could increase serum troponin concentration or if their cardiac status was unclear. An echocardiographic examination was performed and a 3‐minute electrocardiogram (ECG) was recorded and analyzed by a board‐certified cardiologist or cardiology resident. Dogs, in which evidence of structural primary heart disease (symptomatic or asymptomatic) was identified, were excluded. Likewise, dogs recently treated with a known cardiotoxic drug (eg, doxorubicin) were excluded. Additionally, 10 healthy control dogs were included in the study. Health status was validated by physical examination, CBC, clinical bio‐chemistry, a 3‐minute ECG, and echocardiography. Clinical outcome was defined as survival or death 28 days postadmission and was determined by follow‐up visits or telephone contact with the owner.
Data Collection and Analysis
Data collection included a history of underlying diseases and duration of clinical signs before admission based upon owner information, duration of hospitalization in the ICU and 28‐day outcome, as well as physical examination findings at admission and at all time points used for statistical analysis. Additionally, an APPLE fast score (based on lactate, albumin, platelets, glucose and mentation) of each dog with SIRS was determined at admission.42
Blood samples for cTnI measurement were collected in serum tubes at admission (day 1) and days 2, 3, and 5 until death or discharge from the ICU. Samples were centrifuged for 5 minutes at 4,000 × g after being allowed to clot for 30 minutes. Serum was separated and stored in Eppendorf tubes at −80°C until being shipped on dry ice for analysis at an external commercial laboratory using a HS chemiluminescence immunoassay.1
Statistical Analysis
Data were analyzed using commercial statistical software.2 Testing for normality was performed by the D'Agostino & Pearson omnibus normality test. Normally distributed data are reported as mean ± SD. These data for age, weight, and APPLE fast score were compared by an unpaired t‐test. Nonparametric, not normally distributed data, are reported as median and range. For analysis of variance of cTnI concentrations, the Mann‐Whitney U‐test was applied for comparison of 2 groups and the Kruskal‐Wallis test for comparison of multiple groups. The prognostic value of cTnI and the APPLE fast score for 28‐day mortality was assessed using receiver operating characteristic (ROC) curve analysis. Area under the curve (AUC) was compared using the method of DeLong et al.43 Statistical significance was defined as P < .05.
Results
Study Population Characteristics
Sixty dogs (20 male; 6 male castrated; 10 female; 24 female spayed) with mean age of 82.1 ± 58.5 months and mean weight of 22.2 ± 14 kg and SIRS were included in the study. The most common breeds were mixed breed (n = 14), Doberman Pinscher (4), Golden Retriever (3), Dachshund (3), Yorkshire Terrier (3), and Australian Shepherd (3). Other breeds were represented with fewer than 3 dogs.
The control group consisted of 10 healthy dogs (1 male, 4 male castrated, 1 female, 4 female spayed), consisting of 7 mixed breed dogs, 2 Golden Retrievers, 1 Boxer and 1 Labrador Retriever with a mean age of 75 ± 43.8 months and a mean weight of 24.6 ±7.3 kg. Age (P = .633) and weight (P = .72) of the control group were not significantly different from those of the SIRS group.
Median duration of clinical signs before admission as noted by the owners was 1 day (range, 0–202 days). Median time of hospitalization in the ICU was 3 days (range, 0–12 days). Forty‐one dogs survived, 17 were euthanized, 1 died and 1 was euthanized because of recurrence of underlying disease within 28 days after presentation.
Cardiac troponin I concentration was measured in 133 serum samples. At the time of blood collection at admission, the dogs mean heart rate was 139.9 ± 32.3 beats per minute, median respiratory rate was 44 breath per minute (range, 12–120 breath per minute), and median body temperature was 39.1°C (range, 33.4–42.4°C). Values below the reference ranges of the SIRS criteria are possible because only 2 of the SIRS criteria had to be fulfilled for inclusion.
Troponin Concentration
Median cTnI concentration of dogs with SIRS at admission was 0.16 ng/mL (range, 0.01–50 ng/mL). In 35 of the 60 dogs with SIRS, including 15 nonsurvivors admission, cTnI concentrations were above the reference range of healthy control dogs (Table 1).
Table 1.
Serum cardiac troponin I (cTnI) concentrations (ng/mL) over time in survivors, nonsurvivors, whole study population, and control dogs
| cTnI day 1 (ng/mL) | cTnI day 2 (ng/mL) | cTnI day 3 (ng/mL) | cTnI day 5 (ng/mL) | cTnI Peak (ng/mL) | cTnI Controls (ng/mL) | |
|---|---|---|---|---|---|---|
| All | ||||||
| N | 60 | 39 | 24 | 8 | 60 | 10 |
| Median | 0.16 | 0.23 | 0.22 | 0.06 | 0.34 | 0.03 |
| Range | 0.01–50 | 0.1–50 | 0.01–17.3 | 0.03–10.7 | 0.01–50 | 0.01–0.09 |
| Survivor | ||||||
| N | 41 | 30 | 17 | 7 | 41 | |
| Median | 0.09 | 0.23 | 0.21 | 0.06 | 0.23 | |
| Range | 0.01–20.04 | 0.01–50 | 0.01–4.86 | 0.03–0.12 | 0.01–50 | |
| Nonsurvivor | ||||||
| N | 19 | 9 | 7 | 1 | 19 | |
| Median | 0.63 | 0.16 | 0.34 | 10.7 | 1.22 | |
| Range | 0.05–50 | 0.06–46.11 | 0.04–17.3 | — | 0.05–50 | |
| P | .004 | .526 | .465 | — | .01 | .003 |
Nonsurvivors had significantly higher cTnI concentrations at admission compared to survivors (P = .004; Mann‐Whitney U‐test; Fig 1) and control dogs (P = .003; Mann‐Whitney U‐test; Table 1). Survivors had significantly higher cTnI concentrations than did control dogs (P = .02; Mann‐Whitney U‐test).
Figure 1.
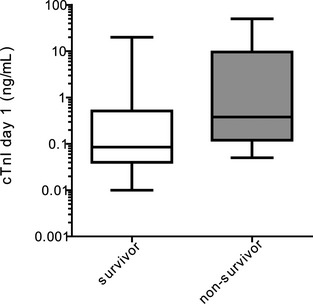
Serum cardiac troponin I (cTnI) concentrations (ng/mL) in survivors and nonsurvivors (60 dogs) at admission. Medians of cTnI concentrations are shown as horizontal lines.
Troponin concentrations were evaluated at day 2 in 39, at day 3 in 24, and at day 5 in 8 dogs (Table 1). There was no significant difference in the cTnI concentrations among the different days of hospitalization (P = .197; Kruskal‐Wallis test).
There was no significant difference between the serum cTnI concentration of 28‐day survivors and nonsurvivors at days 2 (P = .526) and 3 (P = .465). Day 5 was not analyzed due to the low number of dogs (Table 1).
Serum cTnI concentrations from day 1 to 2 increased in 23 dogs (18 survivors and 5 nonsurvivors), decreased in 15 dogs (11 survivors, 4 nonsurvivors) and were unchanged in 1 dog from admission to day 2 with no significant difference between survivors and nonsurvivors (P = .714; Mann‐Whitney U‐test).
Cardiac troponin I concentrations from day 1 to 3 increased in 11 dogs (7 survivors, 4 nonsurvivors), decreased in 12 (9 survivors, 3 nonsurvivors) and were unchanged in 1 dog with no significant difference between survivors and nonsurvivors (P = .611; Mann‐Whitney U‐test).
Peak cTnI concentrations were found on day 1 in 34 dogs (20 survivors, 14 nonsurvivors), on day 2 in 21 dogs (18 survivors, 3 nonsurvivors) and on day 3 in 5 dogs (3 survivors, 2 nonsurvivors). Nonsurvivors had significantly higher peak cTnI concentrations than did survivors (P = .01; Mann‐Whitney U‐test; Table 1).
Receiver operating characteristic analysis at admission showed an AUC of 0.732 (95% CI, 0.601–0.863) and at peak cTnI concentration showed an AUC of 0.708 (95% CI, 0.571–0.845). The difference between these AUC was not significant (P = .505).
Based on ROC analysis, the optimal predictive cutoff for cTnI at admission was >0.14 ng/mL (sensitivity, 78.95%; 95% CI, 54.4–93.9; specificity, 58.54%; 95% CI, 42.1–73.7) and for peak cTnI concentration >1.12 ng/mL (sensitivity, 52.63%; 95% CI, 28.9–75.6; specificity, 80.49%; 95% CI, 65.1–91.2). A dot diagram with optimal cutoff values for cTnI at admission or using the peak cTnI concentration to differentiate between survivor and nonsurvivor dogs is shown in Figure 2.
Figure 2.
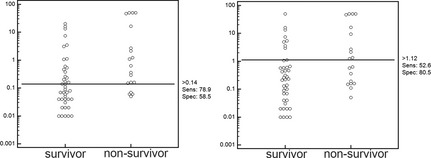
Dot diagram with optimal cutoff values (based on receiver operating characteristic analysis) for cardiac troponin I (cTnI) at admission (A) or using the peak cTnI concentration (B) to differentiate between survivor and nonsurvivor dogs.
The mean APPLE fast score of the 60 dogs at admission was 25.3 ± 4.59. Nonsurvivors (28.11 ± 4.18) had significantly higher APPLE fast scores than did survivors (24 ± 4.21; P < .001; unpaired 2‐tailed t‐test).
Receiver operating characteristic analysis showed an optimal predictive cutoff of 26 (sensitivity, 68.42%; specificity, 75.61%) for the APPLE fast score. Graphing the APPLE fast score (AUC–ROC, 0.754; 95% CI, 0.623–0.884) against the cTnI concentration at admission (AUC–ROC, 0.732; 95% CI, 0.601–0.862) showed decreased false positive results when both tests were combined (Fig 3).
Figure 3.
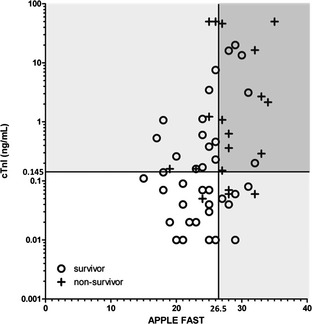
Serum cardiac troponin I (ng/mL) and the acute patient physiologic and laboratory evaluation (APPLE) fast score in 60 dogs with systemic inflammatory response syndrome. The vertical line represents the optimal predictive cutoff for the APPLE fast score. Dogs to the left of this line were predicted to survive by the APPLE fast score, those to the right were predicted to die. The horizontal line represents the optimal predictive cutoff for cardiac troponin I (cTnI). Dogs below this line were predicted to survive by cTnI, and those above were predicted to die. Both cutoff values were identified by receiver operating characteristic (ROC) analysis. The dark gray zone represents dogs predicted to die by cTnI and APPLE fast score, the white zone those predicted to survive and the light gray zone those predicted to die, by either cTnI or APPLE fast score (adapted from Langhorn et al).38
Discussion
This study shows that cTnI concentrations at admission and at the peak time point of daily cTnI measurements are significantly higher in nonsurvivor dogs with SIRS compared to dogs that survived beyond 28 days. However, daily follow‐up measurement of cTnI provides no additional prognostic information for short‐term mortality.
Several studies in human and veterinary medicine have demonstrated that serum cardiac troponin concentrations are increased in various cardiac and noncardiac diseases.4, 19, 30, 33, 38, 44, 45
In humans, myocardial injury is common in critically ill medical patients and is associated with significantly higher mortality rates.33, 34 Previous studies in veterinary patients found similar results.6, 12, 15, 20 In a study of 85 dogs with GDV, 87% had detectable concentrations of cTnI and cTnI was increased in all dogs that did not survive. cTnI also was significantly higher at each measurement time in the 16 nonsurviving dogs compared to dogs that survived.6 In 34 dogs with babesiosis, 3 dogs died and those dogs had markedly increased cTnI concentrations.12
In most previous studies, cTnI was measured at admission,8, 10, 38 but some studies examined cTnI concentration over an extended period in dogs with specific diseases. Two studies in dogs with GDV detected peak cTnI concentration at later time points, 1 between 48 and 72 hours,6 and the other found significantly higher cTnI concentrations after 24 and 48 hours compared with concentrations at presentation.4 In dogs with pyometra, increased cTnI concentrations pre‐ and postsurgery were reported.14, 15 Comparing the cTnI concentrations at admission, day 2, day 3, and day 5 in this study showed no significant differences among the various measurement time points.
When comparing the ROC curves of cTnI at admission and peak cTnI concentrations, no significant difference between the 2 time points was found. The similarity in AUC does not support any benefit of measuring cTnI at different time points. However, the optimal cutoff value to predict poor outcome for cTnI at admission and for peak cTnI was different (Fig 2). The cutoff value for peak cTnI was higher compared to the cutoff value at admission.
It may be reasonable to assume that with disease duration or progression, myocardial injury had increased and therefore peak cTnI in general was higher than at admission. This increase in cTnI likely reflects the vicious cycle of disease severity affecting the heart, which again causes disease progression. In addition, the time of commencement of treatment determines the time that cTnI peaks. The individual diseases of the study population may also influence the peak time. According to previous studies, a study population consisting of dogs with 1 disease complex would probably simplify finding a common peak time point.
Some dogs of the survivor group had peak cTnI concentrations as high as those of nonsurvivors (Fig 2). Therefore, a high cTnI concentration is not always associated with poor outcome. A high cTnI concentration rather should be used as an indicator of high risk, and such patients should be monitored more closely or treated more intensively. Furthermore, it is important to treat the root cause of the disease and to monitor cardiac function to ensure the best patient outcome.
Based on the results of this study, serial cTnI measurement showed no increased prognostic value. cTnI can be an additional tool for the assessment of disease severity, but it is not reliable to distinguish between survival and nonsurvival.
A recent study of 42 dogs with systemic inflammation demonstrated a correlation between cTnI and survival and APPLE full score and survival. Nonsurvivors had significantly higher APPLE full scores as well as cTnI concentrations.38 This study supports those results. Nonsurvivors had significantly higher serum cTnI concentrations and APPLE fast scores at admission compared to dogs that died or were euthanized. Combined use of cTnI measurement and the APPLE fast score at admission decreases the rate of false positives (survivors predicted to die) and seems to be meaningful.
Several mechanisms have been proposed for increased cTnI concentrations in patients with renal insufficiency, but a conclusion has not been reached until now. Uremic myocarditis and pericarditis as well as silent myocardial necrosis, hypertension and decreased renal clearance are contributory.17, 18, 46, 47 Based on this information, we did not exclude dogs with renal failure. The study should include all feasible medical ICU patients, excluding those with cardiac disease, but renal failure as well as age29, 30, 48 and extensive physical strain should be kept in mind as differentials for increased cTnI concentrations.49
An advantage of this study compared to some previous investigations is that it excluded dogs with cardiac diseases not only by clinical examination but also by echocardiography. False positive cTnI concentrations thus were minimized.
Our study had some limitations. To minimize bias, all dogs that fulfilled the inclusion criteria and required hospitalization in the ICU were enrolled in the study. Therefore, a mixed study population with different diseases resulted, which might complicate comparability of cTnI concentrations.
To minimize individual variance at inclusion, we used the SIRS criteria. However, those criteria are nonspecific, because very excited or stressed animals can fulfill almost any parameter of the SIRS criteria without suffering from severe systemic disease. Nevertheless, the SIRS criteria are most applicable on emergency service and during after hours for which reason we chose them.
It was not possible to use standardized diagnostic tests or treatments, as those were different based on the various underlying diseases. It would have been interesting to get histologic examinations of the heart from the dogs that died, and correlate location and level of myocardial injury with cTnI concentrations. Unfortunately, this was not possible because owners had refused postmortem examinations. Furthermore, it would have been interesting to compare serum cTnI concentrations of euthanized dogs with those of deceased dogs. However, this was not possible because only 1 dog died and all others were euthanized because of grave prognosis. Potentially, some of the euthanized dogs would have survived with intensive treatment, but this was not believed to cause relevant bias because euthanasia was the decision of the owners and clinicians, who were unaware of the cTnI concentrations.
This study showed, that serum cTnI is increased in dogs with SIRS and is associated with poor outcome. A combination of cTnI measurement and APPLE fast score seems meaningful. cTnI measurements over time did not increase prognostic value compared to cTnI measurement at admission. The study establishes a foundation for follow‐up studies and additional applications for cTnI measurement in management of dogs with SIRS.
Acknowledgment
The authors thank IDEXX Germany for the financial support for cTnI analysis.
Conflict of Interest Declaration: The study was performed at the Clinic of Small Animal Medicine of the LMU University Munich, Germany. Analysis of cTnI was performed free of charge by IDEXX Germany.
Off‐label Antimicrobial Declaration: The authors declare no off‐label use of antimicrobials.
Footnotes
ADVIA Centaur XP TnI‐Ultra; Siemens Healthcare, Munich, Germany
Graph Pad Prism 5; GraphPad Software, San Diego, CA
References
- 1. Arlati S, Brenna S, Prencipe L, et al. Myocardial necrosis in ICU patients with acute non‐cardiac disease: A prospective study. Intensive Care Med 2000;26:31–37. [DOI] [PubMed] [Google Scholar]
- 2. Babuin L, Jaffe AS. Troponin: The biomarker of choice for the detection of cardiac injury. CMAJ 2005;173:1191–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Berlot G, Vergolini A, Calderan C, et al. Acute myocardial infarction in non‐cardiac critically ill patients: A clinical‐pathological study. Monaldi Arch Chest Dis 2010;74:164–171. [DOI] [PubMed] [Google Scholar]
- 4. Burgener IA, Kovacevic A, Mauldin GN, et al. Cardiac troponins as indicators of acute myocardial damage in dogs. J Vet Intern Med 2006;20:277–283. [DOI] [PubMed] [Google Scholar]
- 5. Edouard AR, Benoist JF, Cosson C, et al. Circulating cardiac troponin I in trauma patients without cardiac contusion. Intensive Care Med 1998;24:569–573. [DOI] [PubMed] [Google Scholar]
- 6. Schober KE, Cornand C, Kirbach B, et al. Serum cardiac troponin I and cardiac troponin T concentrations in dogs with gastric dilatation‐volvulus. J Am Vet Med Assoc 2002;221:381–388. [DOI] [PubMed] [Google Scholar]
- 7. Apple FS, Smith SW, Pearce LA, et al. Use of the Centaur TnI‐Ultra assay for detection of myocardial infarction and adverse events in patients presenting with symptoms suggestive of acute coronary syndrome. Clin Chem 2008;54:723–728. [DOI] [PubMed] [Google Scholar]
- 8. Diniz PP, de Morais HS, Breitschwerdt EB, et al. Serum cardiac troponin I concentration in dogs with ehrlichiosis. J Vet Intern Med 2008;22:1136–1143. [DOI] [PubMed] [Google Scholar]
- 9. Koutinas CK, Mylonakis ME, O'Brien PJ, et al. Serum cardiac troponin I concentrations in naturally occurring myelosuppressive and non‐myelosuppressive canine monocytic ehrlichiosis. Vet J 2012;194:259–261. [DOI] [PubMed] [Google Scholar]
- 10. Lobetti R, Kirberger R, Keller N, et al. NT‐ProBNP and cardiac troponin I in virulent canine babesiosis. Vet Parasitol 2012;190:333–339. [DOI] [PubMed] [Google Scholar]
- 11. Lobetti RG. Cardiac involvement in canine babesiosis. J S Afr Vet Assoc 2005;76:4–8. [DOI] [PubMed] [Google Scholar]
- 12. Lobetti R, Dvir E, Pearson J. Cardiac troponins in canine babesiosis. J Vet Intern Med 2002;16:63–68. [DOI] [PubMed] [Google Scholar]
- 13. Silvestrini P, Piviani M, Alberola J, et al. Serum cardiac troponin I concentrations in dogs with leishmaniasis: Correlation with age and clinicopathologic abnormalities. Vet Clin Pathol 2012;41:568–574. [DOI] [PubMed] [Google Scholar]
- 14. Pelander L, Hagman R, Haggstrom J. Concentrations of cardiac troponin I before and after ovariohysterectomy in 46 female dogs with pyometra. Acta Vet Scand 2008;50:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hagman R, Lagerstedt AS, Fransson BA, et al. Cardiac troponin I levels in canine pyometra. Acta Vet Scand 2007;49:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Langhorn R, Persson F, Ablad B, et al. Myocardial injury in dogs with snake envenomation and its relation to systemic inflammation. J Vet Emerg Crit Care 2014;24:174–181. [DOI] [PubMed] [Google Scholar]
- 17. Sharkey LC, Berzina I, Ferasin L, et al. Evaluation of serum cardiac troponin I concentration in dogs with renal failure. J Am Vet Med Assoc 2009;234:767–770. [DOI] [PubMed] [Google Scholar]
- 18. Porciello F, Rishniw M, Herndon WE, et al. Cardiac troponin I is elevated in dogs and cats with azotaemia renal failure and in dogs with non‐cardiac systemic disease. Aust Vet J 2008;86:390–394. [DOI] [PubMed] [Google Scholar]
- 19. Payne EE, Roberts BK, Schroeder N, et al. Assessment of a point‐of‐care cardiac troponin I test to differentiate cardiac from noncardiac causes of respiratory distress in dogs. J Vet Emerg Crit Care 2011;21:217–225. [DOI] [PubMed] [Google Scholar]
- 20. Kocaturk M, Martinez S, Eralp O, et al. Tei index (myocardial performance index) and cardiac biomarkers in dogs with parvoviral enteritis. Res Vet Sci 2012;92:24–29. [DOI] [PubMed] [Google Scholar]
- 21. Kittleson M, Kienle R. Small Animal Cardiovascular Medicine. St. Louis, MO: Mosby Inc.; 1998. [Google Scholar]
- 22. Wells SM, Sleeper M. Cardiac troponins. J Vet Emerg Crit Care 2008;18:235–245. [Google Scholar]
- 23. Filatov VL, Katrukha AG, Bulargina TV, et al. Troponin: Structure, properties, and mechanism of functioning. Biochemistry Biokhimiia 1999;64:969–985. [PubMed] [Google Scholar]
- 24. Goldmann BU, Christenson RH, Hamm CW, et al. Implications of troponin testing in clinical medicine. Curr Control Trials Cardiovasc Med 2001;2:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Katus H, Remppis A, Scheffold T, et al. Intracellular compartmentation oc cardiac troponin T and its release kinetics in patients with reperfused and nonreperfused myocardial infarction. Am J Cardiol 1991;67:1360–1367. [DOI] [PubMed] [Google Scholar]
- 26. Adams JI, Schechtman K, Landt Y, et al. Comparable detection of acute myocardial infarction by creatine kinase MB isoenzyme and cardiac troponin I. Clin Chem 1994;40:1291–1295. [PubMed] [Google Scholar]
- 27. Kostin S, Pool L, Elsasser A, et al. Myocytes die by multiple mechanisms in failing human hearts. Circ Res 2003;92:715–724. [DOI] [PubMed] [Google Scholar]
- 28. Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med 2009;361:858–867. [DOI] [PubMed] [Google Scholar]
- 29. Oyama MA, Sisson DD. Cardiac troponin‐I concentration in dogs with cardiac disease. J Vet Intern Med 2004;18:831–839. [DOI] [PubMed] [Google Scholar]
- 30. Wess G, Simak J, Mahling M, et al. Cardiac troponin I in Doberman Pinschers with cardiomyopathy. J Vet Intern Med 2010;24:843–849. [DOI] [PubMed] [Google Scholar]
- 31. Eisenman A. Troponin assays for the diagnosis of myocardial infarction and acute coronary syndrome: Where do we stand? Expert Rev Cardiovasc Ther 2006;4:509–514. [DOI] [PubMed] [Google Scholar]
- 32. Cummins B, Cummins P. Cardiac specific troponin‐I release in canine experimental myocardial infarction: Development of a sensitive enzyme‐linked immunoassay. J Mol Cell Cardiol 1987;19:999–1010. [DOI] [PubMed] [Google Scholar]
- 33. Reynolds T, Cecconi M, Collinson P, et al. Raised serum cardiac troponin I concentrations predict hospital mortality in intensive care unit patients. Br J Anaesth 2012;109:219–224. [DOI] [PubMed] [Google Scholar]
- 34. Quenot JP, Le Teuff G, Quantin C, et al. Myocardial injury in critically ill patients: Relation to increased cardiac troponin I and hospital mortality. Chest 2005;128:2758–2764. [DOI] [PubMed] [Google Scholar]
- 35. Hauptman J, Walshaw R, Olivier N. Evaluation of the sensitivity and specificity of diagnostic criteria for sepsis in dogs. Vet Surg 1997;26:393–397. [DOI] [PubMed] [Google Scholar]
- 36. Okano S, Yoshida M, Fukushima U, et al. Usefulness of systemic inflammatory response syndrome criteria as an index for prognosis judgement. Vet Rec 2002;150:245–246. [DOI] [PubMed] [Google Scholar]
- 37. Gebhardt C, Hirschberger J, Rau S, et al. Use of C‐reactive protein to predict outcome in dogs with systemic inflammatory response syndrome or sepsis. J Vet Emerg Crit Care 2009;19:450–458. [DOI] [PubMed] [Google Scholar]
- 38. Langhorn R, Oyama MA, King LG, et al. Prognostic importance of myocardial injury in critically Ill dogs with systemic inflammation. J Vet Intern Med 2013;27:895–903. [DOI] [PubMed] [Google Scholar]
- 39. Zimmerman J, Fromm R, Meyer D, et al. Diagnostic marker cooperative study for the diagnosis of myocardial infarction. Circulation 1999;99:1671–1677. [DOI] [PubMed] [Google Scholar]
- 40. Tucker JF, Collins RA, Anderson AJ, et al. Early diagnostic efficiency of cardiac troponin I and troponin T for acute myocardial infarction. Acad Emerg Med 1997;4:13–21. [DOI] [PubMed] [Google Scholar]
- 41. Newby LK, Storrow AB, Gibler WB, et al. Bedside multimarker testing for risk stratification in chest pain units: The chest pain evaluation by creatine kinase‐MB, myoglobin, and troponin I (CHECKMATE) study. Circulation 2001;103:1832–1837. [DOI] [PubMed] [Google Scholar]
- 42. Hayes G, Mathews K, Doig G, et al. The acute patient physiologic and laboratory evaluation (APPLE) score: A severity of illness stratification system for hospitalized dogs. J Vet Intern Med 2010;24:1034–1047. [DOI] [PubMed] [Google Scholar]
- 43. DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988;44:837–845. [PubMed] [Google Scholar]
- 44. Guglielmini C, Civitella C, Diana A, et al. Serum cardiac troponin I concentration in dogs with precapillary and postcapillary pulmonary hypertension. J Vet Intern Med 2010;24:145–152. [DOI] [PubMed] [Google Scholar]
- 45. Fonfara S, Loureiro J, Swift S, et al. Cardiac troponin I as a marker for severity and prognosis of cardiac disease in dogs. Vet J 2010;184:334–339. [DOI] [PubMed] [Google Scholar]
- 46. Freda BJ, Tang WW, Van Lente F, et al. Cardiac troponins in renal insufficiencyReview and clinical implications. J Am Coll Cardiol 2002;40:2065–2071. [DOI] [PubMed] [Google Scholar]
- 47. Jeremias A, Gibson CM. Narrative review: Alternative causes for elevated cardiac troponin levels when acute coronary syndromes are excluded. Ann Intern Med 2005;142:786–791. [DOI] [PubMed] [Google Scholar]
- 48. O'Brien PJ, Smith DE, Knechtel TJ, et al. Cardiac troponin I is a sensitive, specific biomarker of cardiac injury in laboratory animals. Lab Anim 2006;40:153–171. [DOI] [PubMed] [Google Scholar]
- 49. Wakshlag JJ, Kraus MS, Gelzer AR, et al. The influence of high‐intensity moderate duration exercise on cardiac troponin I and C‐reactive protein in sled dogs. J Vet Intern Med 2010;24:1388–1392. [DOI] [PubMed] [Google Scholar]


