Abstract
Background
Chronic mitral valvular insufficiency (CMVI) in dogs is very common and might cause clinical signs of congestion and poor tissue perfusion.
Hypothesis
Poor tissue perfusion from CMVI causes pancreatitis in dogs, as indicated by serum pancreatic lipase concentrations.
Animals
Sixty‐two client‐owned dogs consisting of 40 dogs with different stages of heart failure from CMVI and 22 age‐matched healthy dogs, based on full cardiac exam and routine laboratory tests.
Methods
Prospective, controlled, observational study. Serum canine pancreatic lipase immunoreactivity (cPLI) concentrations were determined by quantitative cPLI test in healthy and CMVI groups.
Results
Serum cPLI concentrations were 54.0 μg/L (IQR: 38.0–78.8 μg/L) in control, 55.0 μg/L (IQR: 38.3–88.8 μg/L) in ISACHC I, 115.0 μg/L (IQR: 45.0–179.0 μg/L) in ISACHC II and 223.0 μg/L (IQR: 119.5–817.5 μg/L) in ISACHC III. Close correlation to serum cPLI concentration was found in the left atrial to aorta (LA/Ao) ratio (r = 0.597; P = .000) and the severity of heart failure (r = 0.530; P = .000).
Conclusions and Clinical Importance
This study found CMVI is associated with pancreatic injury in congestive heart failure caused by CMVI. Therefore, periodic monitoring on cPLI could be useful in monitoring dogs in heart failure.
Keywords: cPLI, Pancreatic lipase immunoreactivity, Pancreatitis
Abbreviations
- Ao
aorta
- BCS
body condition score
- CHF
congestive heart failure
- CMVI
chronic mitral valve insufficiency
- cPLI
canine pancreatic lipase immunoreactivity
- CRS
cardiorenal syndrome
- ISACHC
International Small Animal Cardiac Health Council
- IQR
interquartile range
- LA
left atrium
- LV
left ventricle
- LVIDd
left ventricular internal dimension in diastole
- LVIDs
left ventricular internal dimension in systole
Chronic mitral valvular insufficiency (CMVI) is the most common cause of congestive heart failure (CHF) in small‐breed dogs and is characterized by progressive myxomatous degeneration of the atrioventricular valves.1 Long standing CHF from CMVI can cause poor body perfusion and potentially induce ischemic injuries to vital organs including kidneys and pancreas.2
Pancreatitis is the most common exocrine pancreatic disease in dogs. Poor tissue perfusion and/or passive congestion from subnormal cardiac output might be responsible for this organ damage. Brain, kidney and pancreas are vulnerable to ischemic injuries.3, 4, 5, 6, 7, 8 Cardiorenal syndrome (CRS) is well known complication from heart failure in humans and is defined as concomitant disorders of the heart and kidneys whereby “acute or chronic dysfunction in one organ might induce acute or chronic dysfunction of the other”.9 Low cardiac output, increase in both intra‐abdominal and central venous pressures, and neurohormonal and inflammatory activation are involved in pathophysiology of CRS. Although the renal failure secondary to poor cardiac function has been well documented in humans and dogs,10, 11, 12 the presence of pancreatic diseases, including pancreatitis, has yet to be studied in dogs with heart failure.
Serum canine pancreatic lipase immunoreactivity (cPLI) assays are widely used for the diagnosis of pancreatitis in dogs.13, 14 Although the hypoxia/ischemia is a known cause for pancreatitis in humans,5, 6, 7, 8 to the authors’ knowledge, the prevalence of pancreatic injuries has not been studied in dogs with CHF. We hypothesized that pancreatic injury occurs in dog with heart disease, as evaluated via serum canine pancreatic lipase immunoreactivity (cPLI) concentrations, in dogs with different stage of heart failure from CMVI.
Materials and Methods
Study Population
Our study population consisted of two groups. The normal control group consisted of 22 healthy, small‐breed dogs (1.7–11.0 kg), between 7 and 14 years of age and had no evidence of cardiovascular or disease and other systemic diseases revealed by the physical examination, complete blood count, chemistry panel, chest radiography, and echocardiography. The CMVI age‐matched (Table 1) group included 40 small‐breed dogs (2.3–13.2 kg) aged between 8 and 17 years. All dogs with CMVI had been presented for a cardiology consultation because of previous identification of a heart murmur, because of the presence of clinical signs indicating cardiovascular disorder, including cough and exercise intolerance, or both. The owner's consent for participation of each dog was obtained before enrollment to the study. All dogs underwent physical examination, echocardiography, complete blood count, and chemistry panel. We excluded dogs that had other clinically relevant systemic diseases, such as renal failure and hypertension. The diagnosis of CMVI was made on the basis of clinical signs, chest radiography data, and echocardiographic findings, according to guidelines for the diagnosis of CMVI in dogs.1 The dogs with CMVI were divided by the criteria proposed by the International Small Animal Cardiac Health Council (ISACHC) for the functional classification of heart failure.15 Some dogs with symptomatic CMVI were being medicated for heart disease, depending on its severity, with drugs such as enalapril, furosemide, spironolactone, pimobendan, digoxin, and amlodipine. We evaluated diet, as well as body condition scores (BCS), using visual inspection and palpation, for each dog enrolled in this study. Because of concern for obesity and high‐fat diets contributing to the presence of pancreatitis, only BCS 3–6/9 dogs and dogs not receiving high‐fat diets (eg, prescription diets) were included in this study
Table 1.
Demographic characteristics of the study population
| ISACHC Class | ||||
|---|---|---|---|---|
| Control | Class I | Class II | Class III | |
| n (62) | 22 | 14 | 11 | 15 |
| Age (years) | 10.0 ± 2.3 | 11.5 ± 1.8 | 10.1 ± 2.3 | 11.5 ± 2.4 |
| Sex | M (10), F (12) | M (6), F (8) | M (6), F (5) | M (10, F (5) |
| BW (kg) | 4.6 ± 2.4 | 4.4 ± 2.1 | 4.4 ± 2.4 | 5.1 ± 3.0 |
| BCS (9‐pt) | 5.1 ± 1.0 | 5.4 ± 0.9 | 5.1 ± 0.5 | 5.1 ± 1.1 |
| Breeds | ||||
| Beagle | 1 | |||
| Cocker Spaniel | 2 | |||
| Cross‐breed | 2 | 1 | 1 | |
| Dachshund | 1 | 1 | ||
| Maltese | 8 | 4 | 3 | 7 |
| Miniature Pinscher | 1 | |||
| Miniature Schnauzer | 1 | 1 | ||
| Pekingese | 1 | |||
| Pomeranian | 1 | 1 | ||
| Poodle | 2 | |||
| Shih Tzu | 6 | 3 | 4 | 3 |
| Yorkshire Terrier | 1 | 2 | 2 | 2 |
All data are expressed with the mean value (±SD).
BW, body weight; BCS, body condition score; M, male; F, female; ISACHC, International Small Animal Cardiac Health Council.
Analysis of Serum cPLI Concentration
All dogs were fasted for 12 hours before blood collection from the jugular vein. The serum was separated from collected blood samples within 30 minutes by centrifugation at 8,450 g for 3 minutes. Aliquots of serum were stored at −80°C freezer before analysis. The serum cPLI concentration was measured using a commercial kit,1, 2 with analyses completed within 72 hours of sample arrival at the reference laboratory. Because some data could not be recorded exactly because they fell outside the maximum measured value of 1,000 μg/L and minimum measured value of 30 μg/L. These results were recorded as 1,000 μg/L when over maximum cut‐off value and as 30 μg/L when under minimum cut‐off value for statistical analysis in this study.
Echocardiography
Echocardiographic examinations were conducted in accordance with recommended standards for dogs. M‐mode, Doppler, and 2‐dimensional echocardiography3 were performed in left and right lateral recumbency. M‐mode echocardiography was used to measure left ventricular dimension at systole (LVIDs) and diastole (LVIDd). 2‐D echocardiography was used to measure left atrium (LA) and proximal aortic (Ao) diameter from the right parasternal short axis at the aortic valve level. These measurements were used to determine the LA to proximal Ao diameter (LA/Ao) ratio and LVIDd to Ao diameter (LVIDd/Ao) ratio.
Statistical Analysis
Statistical analyses were performed using commercially available statistical software.4 Descriptive statistics were calculated for quantitative variables by study group and analyzed for normality using the Kolmogorov‐Smirnov test. Differences in serum cPLI concentration among groups were evaluated using Kruskal‐Wallis one way ANOVA. Pearson's coefficient of bivariate correlation analysis was used to test the strength of association between serum cPLI concentration and other echocardiographic indices used for evaluation of the severity, and between serum cPLI concentration and age/body weight/sex of dogs. Median values and interquartile (IQR) ranges are reported. In all comparisons, a probability value of P < .05 was considered statistically significant, unless stated otherwise.
Results
The mean age, body weight, sex, body condition score, and breeds of each group are summarized in Table 1. Clinically obvious pancreatitis was not noticed in control or CMVI groups. Serum cPLI concentration were 54.0 μg/L (IQR: 38.0–78.8 μg/L) in control, 55.0 μg/L (IQR: 38.3–88.8 μg/L) in ISACHC I, 115.0 μg/L (IQR: 45.0–179.0 μg/L) in ISACHC II, and 223.0 μg/L (IQR: 119.5–817.5 μg/L) in ISACHC III, respectively. The number of dogs with serum cPLI concentrations in the questionable range (201–399 μg/L), based on reference laboratory guidelines, was 2/22 in control, 1/14 in ISACHC I, 2/11 in ISACHC II and 5/15 in ISACHC III dogs, respectively. Frank pancreatitis (≥400 μg/L) was only noticed in 5/15 ISACHC III dogs. Serum cPLI concentrations were statistically significantly higher in dogs with overt heart failure (Fig 1). An association of serum cPLI concentration to LA/Ao ratio (r = 0.597; P = .000), LVIDd/Ao ratio (r = 0.464; P = .003) and ISACHC stages (r = 0.530; P = .000) were found in this study. Age, sex, and body weight were not significantly correlated serum cPLI concentration.
Figure 1.
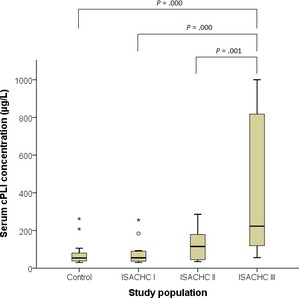
Serum canine pancreatic lipase immunoreactivity (cPLI) concentrations in this study population. Serum cPLI concentrations were statistically significantly higher in dogs with overt heart failure. Statistically significant differences were only obtained between control and chronic mitral valve insufficiency (CMVI) group classified by international small animal cardiac health council (ISACHC) in dogs. Bar = median, box = interquartile range (IQR, 25–75% range). O represents upper fence (not drawn) 1.5 times IQR above 75th percentile, * represents upper fence (not drawn) 2.0 times IQR above 75th percentile.
Discussion
This study clearly found the increase in cPL in dogs with advanced heart failure, suggesting that the risk of pancreatitis might be increased with the worsening of heart failure signs in dogs. The number of dogs with borderline or frank laboratory indication of pancreatitis (201–399 μg/L and ≥400 μg/L of cPLI, respectively) was significantly higher in ISACHC II and III dogs, suggesting the risk of pancreatitis to be higher in dogs with more advanced heart disease and failure. LA/Ao ratio and LVIDd/Ao ratio are closely related to the severity of heart failure in dogs.16 These two echodiagnostic indices were significantly correlated to the cPLI concentration in this study population. One interesting finding was that no dogs in this study had clinically obvious pancreatitis, although 10/15 dogs with ISACHC III had borderline or frank enzymatic change suggestive of pancreatitis. This is particularly important to clinicians, as we may be overlooking the risk of pancreatitis in dogs in the advanced stages of heart failure. At least, we believed that the borderline or diagnostic pancreatitis in these dogs can be the contributing factor for deteriorating the clinical signs and the risk factor for transforming stable heart failure to unstable heart failure.
Although several factors have been implicated in the development of pancreatitis after ischemic injury in humans,5 tissue hypoperfusion was found to a major factor involved in its pathogenesis. A mouse study found that ischemia might be a contributing factor in the pathogenesis of acute pancreatitis.17 A human study also found acinar cell injury was progressive, even in heart failure of brief duration.5 Even though most acinar cell injuries were subclinical, some cases presented as severe, acute pancreatitis.
There were several limitations to this study. First, the study population was small and may not have provided sufficient statistical power to adequately reflect the correlation of cPLI to the severity of heart failure in CMVI dogs. Second, the influence on renal dysfunction by cardiac medication and/or heart failure was not assessed in our study population and diminished renal clearance can cause the increase in cPLI in dog with more advanced stage of heart failure. Thirdly, many dogs diagnosed as pancreatitis by cPLI test have never been confirmed by the histopathological exam, although the diagnostic value of the cPLI on pancreatitis has been clearly proven in dogs.13, 14 Fourthly, the dogs showing subclinical pancreatitis were not more carefully assessed for malassimilation, yet maldigestion and malabsorption are known to occur in dogs with advanced stages of heart failure.18 Finally, we did not demonstrate the presence of gastrointestinal findings with increased serum PLI concentrations, thereby weakening any conclusions that clinical pancreatitis may arise as a complication of heart failure in dogs with CMVI. Despite these study limitations, our study results clearly suggest that the increased serum PLI concentration, to levels accepted as indicating pancreatitis, is a common comorbidity with congestive heart failure. Therefore, regular check‐up for serum PLI level is warranted for early detection of pancreatitis from chronic heart diseases.
Acknowledgments
This study was supported by Human Resource Training Project for Regional Innovation from National research foundation of Korea (NRF). The authors thank Prof. Clarke Atkins (North Carolina State University) for advice on manuscript preparation.
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Footnotes
Spec® cPL™ kit, IDEXX
IDEXX laboratory, Seongnam, Korea
Acuson X‐300, Siemens, Mountain view, CA
SPSS, SPSS Inc., Chicago, IL
References
- 1. Atkins C, Bonagura J, Ettinger S, et al. Guidelines for the diagnosis and treatment of canine chronic valvular heart disease. J Vet Intern Med 2009;23:1142–1150. [DOI] [PubMed] [Google Scholar]
- 2. Borgarelli M, Häggström J. Canine degenerative myxomatous mitral valve disease: Natural history, clinical presentation and therapy. Vet Clin North Am Small Anim Pract 2010;40:651–663. [DOI] [PubMed] [Google Scholar]
- 3. Lee JM, Grabb MC, Zipfel GJ, et al. Brain tissue responses to ischemia. J Clin Invest 2000;106:723–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. El Sabbahy M, Vaidya VS. Ischemic kidney injury and mechanisms of tissue repair. Wiley Interdiscip Rev Syst Biol Med 2011;3:606–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gullo L, Cavicchi L, Tomassetti P, et al. Effects of ischemia on the human pancreas. Gastroenterology 1996;111:1033–1038. [DOI] [PubMed] [Google Scholar]
- 6. Sakorafas GH, Tsiotos GG, Sarr MG. Ischemia/reperfusion‐induced pancreatitis. Dig Surg 2000;17:3–14. [DOI] [PubMed] [Google Scholar]
- 7. Hackert T, Hartwig W, Schneider L, et al. Ischemic acute pancreatitis: Clinical features, diagnosis. Therapy and Outcome. Pancreas 2007;35:406. [Google Scholar]
- 8. Warshaw AL, O'Hara PJ. Susceptibility of the pancreas to ischemic injury in shock. Ann Surg 1978;188:197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ronco C, McCullough P, Anker SD, et al. Cardio‐renal syndromes: Report from the consensus conference of the acute dialysis quality initiative. Eur Heart J 2010;31:703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bongartz LG, Cramer MJ, Doevendans PA, et al. The severe cardiorenal syndrome: ‘Guyton revisited’. Eur Heart J 2005;26:11–17. [DOI] [PubMed] [Google Scholar]
- 11. Nicolle AP, Chetboul V, Allerheiligen T, et al. Azotemia and glomerular filtration rate in dogs with chronic valvular disease. J Vet Intern Med 2007;21:943–949. [DOI] [PubMed] [Google Scholar]
- 12. Ronco C, Haapio M, House AA, et al. Cardiorenal syndrome. J Am Coll Cardiol 2008;52:1527–1539. [DOI] [PubMed] [Google Scholar]
- 13. Steiner JM, Teague SR, Williams DA. Development and analytic validation of an enzyme‐linked immunosorbent assay for the measurement of canine pancreatic lipase immunoreactivity in serum. Can J Vet Res 2003;67:175–182. [PMC free article] [PubMed] [Google Scholar]
- 14. Steiner JM, Williams DA. Development and validation of a radioimmunoassay for the measurement of canine pancreatic lipase immunoreactivity in serum of dogs. Am J Vet Res 2003;64:1237–1241. [DOI] [PubMed] [Google Scholar]
- 15. Fox PR, Sisson D, Moise NS. International Small Animal Cardiac Health Council 1999. Appendix A—Recommendations for diagnosis of heart disease and treatment of heart failure in small animals In: Fox PR, Sisson D, Moise NS, eds. Textbook of Canine and Feline Cardiology. Philadelphia, PA: W.B. Saunders; 1999. [Google Scholar]
- 16. Chetboul V, Tissier R. Echocardiographic assessment of canine degenerative mitral valve disease. J Vet Cardiol 2012;14:127–148. [DOI] [PubMed] [Google Scholar]
- 17. Kyogoku T, Manabe T, Tobe T. Role of ischemia in acute pancreatitis. Hemorrhagic shock converts edematous pancreatitis to hemorrhagic pancreatitis in rats. Dig Dis Sci 1992;37:1409–1417. [DOI] [PubMed] [Google Scholar]
- 18. Moneva‐Jordan A. Dietary considerations for the cardiac patient. In Practice 2012;38:28–29. [Google Scholar]


