Abstract
Background
Omega‐3 polyunsaturated fatty acid (PUFA) may benefit humans and animals with chronic inflammatory diseases.
Hypothesis
Omega‐3 PUFA supplementation improves clinical signs, lung function, and airway inflammation in horses with recurrent airway obstruction (RAO) and inflammatory airway disease (IAD).
Animals
Eight research horses and 35 client‐owned horses.
Methods
A pilot study examined the dose of PUFA that can alter plasma PUFA composition. Then, a randomized, controlled clinical trial was performed in horses with RAO and IAD. Horses were fed a complete pelleted diet with no hay and randomly assigned to 1 of 3 daily treatments for 2 months: 30 or 60 g of the supplement or 30 g of placebo. Clinical signs, lung function, plasma PUFA composition, and bronchoalveolar lavage fluid (BALF) cytology were evaluated. Data were expressed as median (25–75th percentiles). P < .05 was considered significant.
Results
Polyunsaturated fatty acid supplementation resulted in increased plasma docosahexaenoic acid (DHA) that peaked at 4 weeks. Clinical improvement was noted in all horses involved in the clinical trial, but the group that received PUFA had greater improvement in clinical signs (cough score improved 60%), lung function (respiratory effort decreased 48%), and BALF (neutrophils decreased from 23 to 9%) when compared to placebo (cough score improved 33%, respiratory effort decreased 27%, BALF neutrophils increased from 11 to 17%; P < .05).
Conclusions and Clinical Importance
Feeding horses with RAO and IAD a PUFA supplement containing 1.5–3 g DHA for 2 months provides an additional benefit to low‐dust diet.
Keywords: Cough, Inflammatory airway disease, Poor performance, Recurrent airway obstruction
Abbreviations
- ∆PLmax
maximal change in transpulmonary pressure
- BAL
bronchoalveolar lavage
- Cdyn
dynamic compliance
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- IAD
inflammatory airway disease
- PFT
pulmonary function testing
- PUFA
polyunsaturated fatty acid
- RAO
recurrent airway obstruction
- RL
pulmonary resistance
- VAS
visual analog score
Chronic lower airway inflammatory diseases such as recurrent airway obstruction (RAO) and inflammatory airway disease (IAD) commonly occur in horses with prevalences in the northern hemisphere averaging 14 and 20%, respectively.1, 2 Both IAD‐ and RAO‐affected horses exhibit clinical signs associated with chronic airway inflammation such as coughing and excessive airway mucus, but only horses with RAO during disease exacerbation show increased respiratory effort.3 Confirmation of RAO diagnosis requires demonstration of nonseptic airway inflammation and reversible airway obstruction.3 No definitive cure for RAO exists, but affected horses can be managed long term by a combination of environmental management aimed at minimizing dust exposure (eg, removing hay from the diet), and medical treatment with glucocorticoids and bronchodilators.4, 5 The recurrence rate for IAD in young racehorses is low, and horses suffering from this disease may achieve complete recovery.6 Some studies suggest that environmental changes play a role in horses with IAD.7, 8
Airway inflammation in horses with RAO is associated with increased markers of oxidative stress such as BALF 8‐isoprostane.9 Furthermore, high concentrations of 8‐isoprostane have been reported in BALF of human patients with asthma.10 Measurement of 8‐isoprostane may be the most reliable indicator of oxidant stress and directly reflects free radical formation in vivo.11 Comparison of 8‐isoprostane in BALF between horses with RAO and IAD has not yet been reported.
Alpha‐linolenic acid and linoleic acid are essential polyunsaturated fatty acids (PUFAs) and precursors of the omega‐3 and omega‐6 series of PUFAs, respectively. Linoleic acid found in seeds such as corn and soybean is a precursor to arachidonic acid. Alpha‐linolenic acid originating from seeds such as linseed and walnut is metabolized to eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA).12 Fish oil is a rich source of EPA and DHA, whereas algae contain high concentrations of DHA compared to EPA.13 The incorporation of omega‐3 PUFAs occurs in a dose‐dependent fashion at the expense of arachidonic acid, and as a result, less substrate is available for the synthesis of proinflammatory eicosanoids.12 Additional roles of omega‐3 PUFAs in inflammation include decreasing activation of nuclear factor kappa‐β and, as a result, decreased generation of inflammatory cytokines such as tumor necrosis factor‐α, interleukin‐1β (IL‐1β), IL‐6, IL‐8, and less expression of adhesion molecules.14
The majority of clinical studies investigating the efficacy of omega‐3 PUFAs in people with chronic pulmonary inflammatory conditions have shown beneficial effects, but studies in patients with acute lung injury failed to demonstrate a benefit.15 Omega‐3 PUFAs appear to be beneficial in the management of atopic dermatitis in dogs16 and in cats with experimentally induced asthma.17 In a recent study evaluating immune function of healthy yearling horses, investigators reported that fish oil providing 60 mg of omega‐3 PUFA/kg of body weight increased plasma and red blood cell EPA and DHA concentrations after 35 days of supplementation.18 A randomized, crossover trial in horses diagnosed with RAO and supplemented with sunflower oil (rich in omega‐6 PUFAs) or seal blubber oil (rich in omega‐3 PUFAs) showed a greater reduction in total leukocyte count in BALF after seal blubber oil compared to sunflower oil supplementation, but no effect on clinical score or pulmonary function was detected.19
The purpose of our study was to evaluate the clinical efficacy of an omega‐3 PUFA‐rich equine feed supplement and low‐dust diet in horses affected by chronic lower airway disease, based on changes in clinical score, pulmonary function, and BALF cytology parameters.
Materials and Methods
Experimental Design, Animals
Pilot Study
Four healthy horses and 4 RAO‐affected horses housed on dirt lots were selected from the Purdue University Herd to participate in the dose determination study (5 geldings and 3 mares, aged 11–26 years; body weight range, 430–592 kg). Horses were divided into 2 groups: Group 1, consisting of 2 healthy and 2 RAO‐affected horses, was started on omega‐3 PUFA supplementation1 at the lowest dose recommended by the manufacturer (1× = 30 g/day); Group 2, consisting of 2 healthy and 2 RAO‐affected horses, was started on omega‐3 PUFA supplementation at a double dose (2× = 60 g/day). The supplement also contained vitamin C (2 g per 30 g dose), methylsulfonylmethane (5 g per 30 g dose), and a mushroom complex (1 g per 30 g dose) reportedly selected for their antioxidant properties. The supplement was given as a top dressing on complete pelleted feed2 fed at 2% of body weight daily and divided into 2 meals. Each horse underwent a thorough physical examination before enrollment into the study and venous blood samples were collected to determine serum phospholipid profiles.
Horses were monitored daily during the trial for general attitude and appetite with weekly physical examination. Blood was collected every 2 weeks to determine plasma phospholipid profiles. After the first 2 weeks, the dosages were doubled (the group receiving the supplement at a 1× dose was continued at a 2× dose, whereas the group receiving the supplement at a 2× dose was continued at a 4× dose). Horses then were maintained on the same dose and blood samples were collected every 2 weeks to determine plasma phospholipid profiles until <10% change in omega‐3 PUFA ratio was identified compared to previous measurements, suggestive of a plateau effect. Once plateau was reached, supplement administration was discontinued.
Clinical Trial
Sample size calculation based on clinical data from clients applying a visual analog score (VAS)20 showed that ≥32 horses would be needed to allow detection of 30% improvement in VAS (breathing score and cough) posttreatment with 80% power and α = 0.05. The study was performed over a 9‐month period as a randomized, placebo‐controlled, double‐blinded clinical trial. Client‐owned horses were recruited by advertisements and mailings sent out to referring veterinarians and brought to the Purdue University Veterinary Teaching Hospital (PUVTH) for testing. Horses were enrolled if they had a history of chronic respiratory disease of at least a 3‐month duration and exhibited at least one of the following: increased breathing rate or effort at rest, intermittent cough, or poor performance. Horses qualified for the study if they had a VAS ≤70 in at least 1 of the 3 categories (performance, breathing difficulty, and cough) with a score of 100 indicating absence of clinical signs. After obtaining client consent, horses underwent physical examination including clinical scoring, blood collection, pulmonary function testing (PFT), and BALF collection on the day of enrollment (day 0). Only horses with a normal leukogram and no clinical evidence of infectious respiratory disease were included in the study. Horses then returned home after they were randomly assigned to receive 1 of 3 treatments (omega‐3 PUFA supplement 1× or 2×, or placebo) as a daily feed supplement for 8 weeks, whereas their attitude and appetite were monitored daily by their owners. The placebo contained the same matrix as the omega‐3 supplement except that it is was devoid of DHA, methylsulfonylmethane, and mushroom complex. Randomization was performed by an online randomization generator.3 Horses were maintained in the same environment as before enrollment into the study, but their diet was switched to complete pelleted feed2 gradually over a 1‐week period and no exposure to hay was authorized during the study period. All medications were withheld during the study period. Owners were asked to assign a VAS for performance, breathing difficulty, and cough upon enrollment and then once a week (days 7, 14, 21, 28, 35, 42, 49, and 56). At the end of the supplementation period (day 56), horses were brought back to the PUVTH to undergo the same procedures as on day 0.
Horses were diagnosed with IAD based on BAL fluid cytology showing either >1% eosinophils, >2% mast cells, or >5% neutrophils along with characteristic clinical signs (cough or exercise intolerance), and a maximum change in transpulmonary pressure (∆P Lmax) < 15 cmH2O, whereas horses with chronic respiratory disease and ∆P Lmax > 15 cmH2O were diagnosed with RAO. The Purdue University Animal Care and Use Committee approved the study.
Clinical Evaluation
Two clinical scoring systems were used to assess respiratory compromise on days 0 and 56. A long score (range, 0–21) that was based on respiratory rate, respiratory effort, nasal discharge, and presence or absence of cough and abnormal lung sounds, and a short score (range, 2–8) based on assessment of abdominal effort and nostril flaring with lower scores indicating improved function. Owners were instructed on how to quantify performance, breathing difficulty, and cough using a VAS (range, 0–100) and were asked to record it weekly from days 0 to 56.
Diagnostic Testing
Jugular blood was collected 3–4 hours after morning feeding in evacuated tubes containing EDTA for CBC and plasma was harvested and stored at −80°C until fatty acid analysis.
Fatty acid analysis of plasma was carried out on freshly thawed samples after extraction of lipids by the Folch method,21 isolation of phospholipids by solid phase extraction using silica cartridges,22 methylation utilizing boron trifluoride and gas chromatography.23
Horses were restrained in stocks without sedation and instrumentation for PFT carried out as previously described.24 Lung resistance (R L) and dynamic compliance (C dyn) were computed from 10 representative breaths and average values for each horse at each time point were used in the analyses.
After completion of PFT, horses were sedated with a combination of IV detomidine (0.02 mg/kg) and butorphanol (0.02 mg/kg) and BAL performed with a tube using 500 mL of sterile isotonic saline.24 The BAL fluid was immediately placed on ice and processed within 20 minutes of collection. Cytological specimens were prepared by centrifugation and processed with Wright's stain. For 8‐isoprostane concentrations, aliquots of 2‐mL BAL fluid were treated with 0.005% butylated hydroxytoluene and stored at −80°C until analysis by an enzyme immunoassay kit4 validated in the horse.9
Data Analysis
Data distribution was assessed using the Shapiro‐Wilk test for normality. The effect of dose and disease status (healthy versus RAO) was tested by analysis of covariance (ANCOVA) using posttreatment variable as outcome variable and pretreatment variable as covariate.25 Posthoc analysis was conducted using Friedman analysis of variance (ANOVA) when comparing repeated measurements between week 0 and 8 or Mann‐Whitney U‐test when comparing between groups at a given time point.
Owner‐assigned scores (VAS) between week 0 and 8 were compared using Friedman ANOVA. Posthoc paired comparisons of VAS scores between week 0 and subsequent weeks were performed using Wilcoxon matched pairs tests with Bonferroni adjusted P‐value (0.05/7 = .0071; P < .0071).
All data were expressed as median (25–75% quartiles). P < .05 was considered significant.
Results
Pilot Study
All horses (n = 8) remained healthy throughout the experiments. Data from both groups were pooled because there were no differences between physical examination findings and plasma lipid proportions between the healthy and RAO‐affected horses.
Horses receiving the 2× dose of omega‐3 PUFA for 2 weeks followed by 4× dose were only supplemented for 4 weeks, because their PUFA profile did not differ from the lower dose group after 4 weeks. The relative amount of plasma DHA (expressed as % of total fatty acids) increased significantly between week 0 (0% [0–0]) and 4 (0.89% [0.62–1.04]) of supplementation with 2× dose and then reached a plateau between week 4 and 8 (0.70% [0.4–0.83]; P = .012).
Efficacy Study
Horses
Thirty‐five horses were evaluated for the dose efficacy study. One horse diagnosed with upper respiratory tract obstruction on the initial evaluation was not enrolled, and 32 horses completed the trial. One of the horses died of colic approximately 1 month after enrollment in the study. No necropsy was performed, but the horse was reported to be clinically well up until the onset of colic, and according to the VAS scoring sheet, showed clinical improvement in respiratory signs. Therefore, we assumed that the colic was unrelated to the trial. Another horse was excluded from the final data analysis because the owner disclosed that they started feeding hay again after the second week of the trial.
The sex distribution was 18 geldings, 11 mares, and 3 stallions (Table 1). The age of the horses ranged between 6 and 22 years with a variety of breeds being represented. There was no statistically significant difference in age or body weight between IAD and RAO horses (Table 1).
Table 1.
Comparison of clinical, lung function, and bronchoalveolar lavage fluid cytology variables at enrollment between horses with recurrent airway obstruction (RAO; n = 14) and inflammatory airway disease (IAD; n = 18)
| Variable | IAD | RAO |
|---|---|---|
| Clinical scores | ||
| Age | 14.0 [10.0–19.0] | 15.5 [12.0–17.0] |
| Sex | 12 geldings, 1 female, 5 males | 7 geldings, 3 females, 4 males |
| Body weight (kg) | 494 [454–590] | 486 [445–517] |
| Long score | 7.5 [4.0–12.0] | 9.0 [7.0–11.0] |
| Short score | 4.0 [3.0–5.0] | 5.0 [4.0–6.0] |
| VAS cough | 40 [20–50] | 40 [30–80] |
| VAS respiratory effort | 50 [40–80] | 40 [20–50] |
| VAS performance | 50 [30–90] | 40 [0–60] |
| Pulmonary function | ||
| ∆P Lmax (cmH2O) | 8.1 [7.0–10.3] | 22.3 [17.4–33.1]a |
| R L (cmH2O/L/s) | 0.6 [0.4–0.9] | 1.5 [1.4–1.9]a |
| C dyn (L/cmH2O) | 1.7 [1.4–2.5] | 1.3 [0.7–1.5]b |
| Bronchoalveolar lavage cytology | ||
| Total nucleated cells (/μl) | 342 [187–501] | 255 [137–530] |
| Macrophages (%) | 36 [24–43] | 25 [18–34]b |
| Neutrophils (%) | 11 [7–25] | 31 [19–55]b |
| Lymphocytes (%) | 38 [25–42] | 31 [21–48] |
| Eosinophils (%) | 0 [0–1] | 0 [0–0] |
| Mast cells (%) | 2 [1–3] | 1 [0–3] |
Data displayed as median [25–75% quartiles].
P < .001.
P < .05.
Eleven horses received placebo feed supplement, 10 were fed the supplement at the recommended label dose (1×; DHA dose range, 2.5–3.8 mg/kg) and 11 horses received twice the labeled dose (2×; DHA dose range, 5.1–9.1 mg/kg). None of the owners reported any adverse effects related to the supplementation, and all horses consumed the supplement or placebo well.
Baseline Comparisons
Fourteen horses were diagnosed with RAO and 18 with IAD. Horses with RAO exhibited significant pulmonary dysfunction consistent with airway obstruction, whereas horses with IAD had lung mechanics parameters within normal limits (Table 1). In addition, the neutrophil percentage in the BAL fluid was significantly higher in RAO horses compared to IAD horses (Table 1). The fatty acid profiles and 8‐isoprostane concentration in the BAL fluid were not different between IAD and RAO horses at baseline. In addition, none of the variables were significantly different between placebo and treatment groups at enrollment.
Treatment Effect
No difference in treatment effect was detected between horses with RAO and IAD. Therefore, data analysis subsequently was performed on data pooled for all horses with chronic respiratory disease.
Visual analog scoring of cough improved significantly (ie, increased score) in all 3 treatment groups (placebo, 1×, 2×), but horses treated with the 1× dose of omega‐3 PUFA supplement exhibited a significantly higher VAS cough score 2 months later compared to horses receiving placebo (P = .043; Fig 1). Because no additional effect associated with the different doses of supplementation was noted, data from horses treated with 1× and 2× dose of omega‐3 PUFA supplement were pooled and compared to data from horses treated with placebo.
Figure 1.
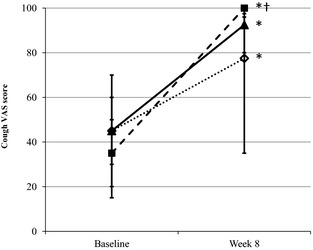
Visual analog score (VAS) for cough before and 2 months after daily feeding low‐dust diet and supplementing with placebo (open diamonds) or 1× (solid square) or 2× (solid triangle) dose of the omega‐3 supplement. *Significantly different from baseline (P < .05). †Significantly different from placebo at week 8 (P = .043).
Overall, both clinician‐assigned scores (long and short) and owner‐assigned scores (VAS) improved after 8 weeks in the trial (P < .01; Table 2). Clinician‐assigned scores of horses treated with the omega‐3 PUFA supplement improved significantly (P < .001), whereas the improvement in placebo‐treated horses did not reach statistical significance. However, scores at the end of supplementation were not different between the placebo and treatment groups (Table 2). All of the owner‐assigned VAS scores improved significantly in the placebo‐ and omega‐3‐treated horses over time. However, VAS for cough and respiratory effort 2 months after treatment showed a significantly greater improvement in horses given the omega‐3 PUFA supplement compared to placebo (P = .031; Table 2). Horses in the placebo group displayed greater variability in VAS cough score than horses in the omega‐3 supplement group toward the end of the trial because 6 of 11 placebo‐treated horses had final VAS cough scores <80 compared to only 2 of 21 supplement‐treated horses.
Table 2.
Summarized data from the effect of low‐dust diet and 8‐week supplementation with placebo or omega‐3 PUFA in horses with chronic respiratory disease
| Placebo | Treatment | |||
|---|---|---|---|---|
| Before Supplementation | After Supplementation | Before Supplementation | After Supplementation | |
| Long score | 9.0 [4.5–11.0] | 3.5 [3.0–7.0] | 8.5 [5.5–12.0] | 3.0 [2.0–4.5]b |
| Short score | 5.0 [4.0–5.5] | 3.0 [2.5–5.0] | 4.0 [4.0–6.0] | 2.5 [2.0–4.0]b |
| VAS cough | 45 [20–50] | 77.5 [35–95]b | 40 [20–70] | 100 [90–100]a , b |
| VAS respiratory effort | 50 [30–50] | 75 [50–90]b | 50 [20–80] | 93 [90–100]a , b |
| VAS performance | 45 [15–60] | 65 [40–95]b | 50 [20–90] | 90 [75–100]b |
| ∆P Lmax (cmH2O) | 9.1 [7.5–16.9] | 6.6 [5.9–9.3] | 14.5 [7.5–23.9] | 7.5 [6.0–13.3]b |
| R L (cmH2O/L/s) | 0.7 [0.5–1.3] | 0.4 [0.2–1.1] | 1.3 [0.7–1.6] | 0.6 [0.29–1.1]b |
| C dyn (L/cmH2O) | 1.9 [1.3–2.8] | 2.2 [1.2–3.6] | 1.4 [0.8–1.5] | 1.7 [1.3–3.5] |
| BAL Neutrophil% | 11 [7–32] | 17 [6–29] | 23 [13–54] | 9 [3–20]b |
| DHA % in plasma | 0.09 [0.0–0.18] | 0.15 [0.0–0.37] | 0.04 [0.0–0.17] | 0.43 [0.33–0.57]b |
| 8‐Isoprostane (pg/mL) | 13.4 [10.5–20.7] | 13.9 [6.9–27.6] | 14.3 [9.0–24.0] | 13.4 [8.1–28.0] |
Statistically significant difference between placebo and treatment after supplementation.
Statistically significant difference between before and after supplementation.
The effect of omega‐3 PUFA supplementation and pelleted feed on VAS scores was noticeable during the first 2 weeks of treatment and reached maximal benefit between weeks 2 and 5 for coughing, weeks 5 and 6 for respiratory effort and weeks 3 and 5 for poor performance (Fig 2). In contrast, VAS for cough and respiratory effort in placebo‐fed horses tended to improve only after week 5, and the effect never reached statistical significance.
Figure 2.

Weekly change in visual analog score (VAS) for cough (A) and respiratory effort (B) in horses fed a daily placebo (open diamonds) or the omega‐3 supplement (closed squares). *Significantly different from baseline (P < .01).
Omega‐3 PUFA supplementation for 2 months resulted in a significant decrease in the relative proportion of neutrophils in the BAL fluid, and this effect was not observed in horses fed the placebo (Table 2). Isoprostane concentrations in BAL fluid were not significantly affected by treatment (Table 2).
Two months of supplementation and pelleted feed improved lung function (ie, decreased ΔP Lmax and R L) in both placebo‐ and omega‐3 PUFA‐treated horses, but the effect only reached statistical significance in horses fed the omega‐3 PUFA supplement (P < .01; Table 2).
The only fatty acid that was significantly affected by omega‐3 PUFA treatment was plasma DHA, for which 2 months of supplementation resulted in a >10‐fold increase (P < .001; Table 2).
Discussion
Results from the clinical trial showed that administration of the omega‐3 PUFA supplement to horses with RAO or IAD fed a complete pelleted feed provided additional clinical benefits when compared to the control group fed a complete pelleted feed and placebo.
In this study, we elected to use both horses diagnosed with RAO and IAD because we wanted to see if there would be a difference in response to omega‐3 PUFA administration based on disease severity. At baseline evaluation, a difference in disease severity was clearly seen in PFT and BAL fluid cytology, but overall clinical assessment was not different between the RAO and IAD groups. These differences in lung function and BALF were expected, because horses with IAD do not have detectable evidence of airway obstruction based on standard lung mechanics24 and often have mastocytic or eosinophilic inflammation present in BAL fluid1 compared to RAO horses that have increased ΔP Lmax and R L and severe neutrophilic inflammation in BAL fluid.26 However, we anticipated that clinical signs would be more severe in RAO horses compared to IAD, in particular respiratory efforts, but they were not. This finding may be because of the fact that increased respiratory efforts are associated not only with the degree of airway obstruction but also with the severity of neutrophilic inflammation.27 The lack of differentiation between RAO and IAD phenotypes based on clinical signs warrants caution when conducting studies using only clinical parameters or scores as enrollment criteria.
A previous study evaluated omega‐3 PUFA supplementation in horses with RAO,19 but failed to detect a significant treatment effect on PFT. The supplement used was seal blubber oil which provided a higher dosage of DHA (2.5 g/day or 5.3 mg/kg/day) compared to the DHA dosage used in our study (1.5 g/day or 3.0 mg/kg/day). In that experiment, however, horses were sedated (detomidine and butorphanol) before lung function testing, which may have contributed to the overall negative results, because the sedatives used result in bronchodilatation.28, 29 In addition, horses were fed hay throughout the study period, and thus they were continuously exposed to a dusty environment which would likely diminish any potential benefit provided by PUFA supplementation. In this study, horses receiving omega‐3 PUFA supplementation for 2 months exhibited improvement in clinical signs and PFT and decreased airway inflammation. Limiting dust exposure in the environment by removing hay from the diet results in clinical and lung function improvement in horses with RAO within 6–8 weeks.20 Thus some degree of improvement was expected in all treatment groups just by switching the horses' diet from hay to pelleted feed. We thought this design would allow a more accurate evaluation of the effect of the supplement, because RAO horses in disease exacerbation continuously exposed to hay would be unlikely to show clinical improvement just with omega‐3 PUFA supplementation. This experimental design also standardized the influence of dietary fatty acids and other nutrients, and represented the typical management change that practitioners would recommend in the field.
Owner assessment of clinical signs in horses with chronic airway disease has been previously reported to be useful in judging efficacy of dexamethasone treatment.20 The magnitude of improvement in the VAS for cough and respiratory effort is comparable between this previous study and our current study, suggesting that low‐dust diet along with omega‐3 PUFA supplementation of horses with chronic airway disease for 8 weeks have similar clinical benefits as a 3‐week course of dexamethasone administration (0.1 mg/kg q24h PO) without diet modification.
Inflammation during an acute RAO crisis has been associated with increased concentrations of markers of oxidative stress in the airways, including 8‐isoprostane.30 Interestingly, 8‐isoprostane concentrations in BAL fluid were unaffected by treatment in our study. Peroxidation of arachidonic acid in cell membranes results in formation of F2‐isoprostanes such as 8‐isoprostane via nonenzymatic pathways, whereas conversion of arachidonic acid via cyclooxygenase and 5‐lipooxigenase lead to prostanoid and leukotriene synthesis, respectively.31 One possible explanation is that oxidative stress in the airways was not directly affected by the treatment, and clinical improvement may have resulted mainly from omega‐3 PUFAs shifting enzymatic conversion of arachidonic acid toward less inflammatory compounds.15 Another possibility is that 8‐isoprostane concentrations did not accurately reflect the redox state of the horses in the study, and measurements of other markers of oxidative stress such as reduced or oxidized glutathione would have been useful.30
Measurement of fatty acid classes in plasma identified a significant increase in plasma DHA concentrations in the treatment group when compared to baseline, which was not observed in the placebo group. These results were expected because the supplement contained a DHA‐rich ingredient and suggest that the increase in DHA concentrations in blood was responsible for the clinical improvement in supplemented horses. The DHA dose used in this study was based on the recommendations of the product manufacturer and was designed to provide approximately 150% of the suggested minimum human dose based on metabolic body weight equivalency. According to label, the daily recommended dose of 30 g of the supplement contains 1.5 g of DHA. Interestingly, we found that a single dose of the supplement (30 g) appeared to be as beneficial as the double dose (60 g). Incorporation of omega‐3 PUFAs into the inflammatory cell membrane occurs in a dose‐dependent fashion,32, 33 and therefore, greater improvement would be expected with the higher dose. The dose of 1.5 g DHA/day (3.0 mg/kg/day) altered the plasma phospholipid classes, and resulted in a significant increase in the plasma DHA % in both the pilot and clinical trials, although the DHA dose used in this study was lower than reported previously in horses (6–21 g/day or 13–44 mg/kg/day).18, 33, 34 According to our findings, feeding the 2× dose did not proportionally increase plasma concentrations of DHA. It is possible that there is a limit on how much omega‐3 PUFA can be absorbed and integrated in the horse's plasma, and higher doses of supplementation may not confer additional health benefits. Another possible explanation is that the source of the omega‐3 PUFA makes a difference. The supplement used in our study was from an algae source, and we are not aware of any previous report of its use in horses.
Other ingredients in the supplement (eg, vitamin C, methylsulfonylmethane, mushroom complex) may have contributed to decreasing inflammation. Methylsulfonylmethane has been shown to improve clinical signs of inflammation in people with hay fever,35 whereas vitamin C may decrease exercise‐induced bronchoconstriction in people.36 However, vitamin C also was included in the placebo product. According to the manufacturer, the proprietary mushroom complex is supposed to provide additional antioxidant properties to the supplement. Because oxidative stress measurement using 8‐isoprostane was unchanged, it is unlikely that clinical benefits observed in the treatment group were because of the mushroom complex rather than the DHA supplementation.
When evaluating a feed supplement to incorporate in daily practice for lifelong management, palatability, and ease of administration are crucial factors. Horses in this study readily accepted the supplement, making it feasible for long‐term administration. The combination of low‐dust diet and omega‐3 PUFAs supplementation resulted in a 65% decrease in “long” clinical score that is similar to the 61% decrease observed in another study, where horses with RAO were treated with inhaled corticosteroids and low‐dust diet for 4 weeks.4 When ease of administration is taken into consideration, horse owners are more likely to be compliant with adding a supplement to the feed than with administrating medications to the horse on a daily basis (oral or inhaled). Furthermore, enhancing dietary DHA would decrease the potential adverse effects associated with long‐term steroid administration.37
Horses suffering from RAO benefit greatly from decreasing dust in the environment, but data on IAD are scant.3, 38 Furthermore, some RAO horses even on strict environmental control may experience flares up that require rescue medications. Based on this study, omega‐3 PUFA supplementation could be an additional option to help better manage both IAD and RAO horses when compared to a low‐dust environment by itself. Future studies should determine the optimal duration and dose of supplementation for treatment and prevention of chronic airway disease.
Acknowledgments
The study was supported by Arenus, Purina Mills, and by the State of Indiana and the Purdue University College of Veterinary Medicine research account funded by the total wager tax. The authors thank Donna Griffey, Meghan Benson, Casey Cromer, Lisa Lawrence, Ryan Louer, Lexi Mechem, and Katie Smith for technical assistance and referring veterinarians and horse owners for their support of the study.
Conflict of Interest Declaration: The authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: The authors declare no off‐label use of antimicrobials.
The work was conducted at Purdue University College of Veterinary Medicine.
The study was presented as a research report at the 2013 ACVIM Forum in Seattle, WA.
This article was published online on 10 October 2014. An error was subsequently identified in Figure‐2. This notice is included in the online and print versions to indicate that both have been corrected on 26 November 2014.
Footnotes
Aleira, Arenus, Fort Collins, CO
Equine Senior, Purina Mills LLC, St Louis, MO
Cayman Chemical Co, Ann Arbor, MI
References
- 1. Couëtil LL, Hoffman AM, Hodgson J, et al. Inflammatory airway disease of horses. J Vet Intern Med 2007;21:356–361. [DOI] [PubMed] [Google Scholar]
- 2. Hotchkiss JW, Reid SW, Christley RM. A survey of horse owners in Great Britain regarding horses in their care. Part 2: Risk factors for recurrent airway obstruction. Equine Vet J 2007;39:301–308. [DOI] [PubMed] [Google Scholar]
- 3. Robinson NE, Karmaus W, Holcombe SJ, et al. Airway inflammation in Michigan pleasure horses: Prevalence and risk factors. Equine Vet J 2006;38:293–299. [DOI] [PubMed] [Google Scholar]
- 4. Couëtil LL, Chilcoat CD, DeNicola DB, et al. Randomized, controlled study of inhaled fluticasone propionate, oral administration of prednisone, and environmental management of horses with recurrent airway obstruction. Am J Vet Res 2005;66:1665–1674. [DOI] [PubMed] [Google Scholar]
- 5. Bertin FR, Ivester KM, Couëtil LL. Comparative efficacy of inhaled albuterol between two hand‐held delivery devices in horses with recurrent airway obstruction. Equine Vet J 2011;43:393–398. [DOI] [PubMed] [Google Scholar]
- 6. Wood JL, Newton JR, Chanter N, Mumford JA. Inflammatory airway disease, nasal discharge and respiratory infections in young British racehorses. Equine Vet J 2005;37:236–242. [DOI] [PubMed] [Google Scholar]
- 7. Holcombe SJ, Jackson C, Gerber V, et al. Stabling is associated with airway inflammation in young Arabian horses. Equine Vet J 2001;33:244–249. [DOI] [PubMed] [Google Scholar]
- 8. Ivester KM, Couëtil LL, Moore GE, et al. Environmental exposures and airway inflammation in young thoroughbred horses. J Vet Intern Med 2014;28:918–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kirschvink N, Smith N, Fiévez L, et al. Effect of chronic airway inflammation and exercise on pulmonary and systemic antioxidant status of healthy and heaves‐affected horses. Equine Vet J 2002;34:563–571. [DOI] [PubMed] [Google Scholar]
- 10. Xie J, Zhang Q, Zhong N, Lai K. BAL fluid 8‐isoprostane concentrations in‐eosinophilic bronchitis and asthma. J Asthma 2009;46:712–715. [DOI] [PubMed] [Google Scholar]
- 11. Kadiiska MB, Gladen BC, Baird DD, et al. Biomarkers of oxidative stress study II: Are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radic Biol Med 2005;38:698–710. [DOI] [PubMed] [Google Scholar]
- 12. Calder PC. n‐3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr 2006;83(6 Suppl):1505S–1519S. [DOI] [PubMed] [Google Scholar]
- 13. Lane K, Derbyshire E, Li W, Brennan C. Bioavailability and potential uses of vegetarian sources of omega‐3 fatty acids: A review of the literature. Crit Rev Food Sci Nutr 2014;54:572–579. [DOI] [PubMed] [Google Scholar]
- 14. Kang JX, Weylandt KH. Modulation of inflammatory cytokines by omega‐3 fatty acids. Subcell Biochem 2008;49:133–143. [DOI] [PubMed] [Google Scholar]
- 15. Giudetti AM, Cagnazzo R. Beneficial effects of n‐3 PUFA on chronic airway inflammatory diseases. Prostaglandins Other Lipid Mediat 2012;99:57–67. [DOI] [PubMed] [Google Scholar]
- 16. Mueller RS, Fettman MJ, Richardson K, et al. Plasma and skin concentrations of polyunsaturated fatty acids before and after supplementation with n‐3 fatty acids in dogs with atopic dermatitis. Am J Vet Res 2005;66:868–873. [DOI] [PubMed] [Google Scholar]
- 17. Leemans J, Cambier C, Chandler T, et al. Prophylactic effects of omega‐3 polyunsaturated fatty acids and luteolin on airway hyperresponsiveness and inflammation in cats with experimentally‐induced asthma. Vet J 2010;184:111–114. [DOI] [PubMed] [Google Scholar]
- 18. Vineyard KR, Warren LK, Kivipelto J. Effect of dietary omega‐3 fatty acid source on plasma and red blood cell membrane composition and immune function in yearling horses. J Anim Sci 2010;88:248–257. [DOI] [PubMed] [Google Scholar]
- 19. Khol‐Parisini A, van den Hoven R, Leinker S, et al. Effects of feeding sunflower oil or seal blubber oil to horses with recurrent airway obstruction. Can J Vet Res 2007;71:59–65. [PMC free article] [PubMed] [Google Scholar]
- 20. Gerber V, Schott Ii HC, Robinson NE. Owner assessment in judging the efficacy of airway disease treatment. Equine Vet J 2011;43:153–158. [DOI] [PubMed] [Google Scholar]
- 21. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 1957;226:497–509. [PubMed] [Google Scholar]
- 22. Juaneda P, Rocquelin G. Rapid and convenient separation of phospholipids and non phosphorus lipids from rat heart using silica cartridges. Lipids 1985;20:40–41. [DOI] [PubMed] [Google Scholar]
- 23. Antalis CJ, Stevens LJ, Campbell M, et al. Omega‐3 fatty acid status in attention‐deficit/hyperactivity disorder. Prostaglandins Leukot Essent Fatty Acids 2006;75:299–308. [DOI] [PubMed] [Google Scholar]
- 24. Couëtil LL, Rosenthal FS, DeNicola DB, Chilcoat CD. Clinical signs, evaluation of bronchoalveolar lavage fluid, and assessment of pulmonary function in horses with inflammatory respiratory disease. Am J Vet Res 2001;62:538–546. [DOI] [PubMed] [Google Scholar]
- 25. Van Breukelen GJP. ANCOVA versus change from baseline had more power in randomized studies and more bias in nonrandomized studies. J Clin Epidemiol 2006;59:920–925. [DOI] [PubMed] [Google Scholar]
- 26. Leclere M, Lavoie‐Lamoureux A, Lavoie JP. Heaves, an asthma‐like disease of horses. Respirology 2011;16:1027–1046. [DOI] [PubMed] [Google Scholar]
- 27. Robinson NE, Berney CB, Eberhart S, et al. Coughing, mucus accumulation, airway obstruction, and airway inflammation in control horses and horses affected with recurrent airway obstruction. Am J Vet Res 2003;64:550–557. [DOI] [PubMed] [Google Scholar]
- 28. Lavoie JP, Phan ST, Blais D. Effects of a combination of detomidine and butorphanol on respiratory function in horses with or without chronic obstructive pulmonary disease. Am J Vet Res 1996;57:705–709. [PubMed] [Google Scholar]
- 29. Broadstone RV, Gray PR, Robinson NE, Derksen FJ. Effects of xylazine on airway function in ponies with recurrent airway obstruction. Am J Vet Res 1992;53:1813–1817. [PubMed] [Google Scholar]
- 30. Art T, Kirschvink N, Smith N, Lekeux P. Indices of oxidative stress in blood and pulmonary epithelium lining fluid in horses suffering from recurrent airway obstruction. Equine Vet J 1999;31:397–401. [DOI] [PubMed] [Google Scholar]
- 31. Kubáň P, Foret F. Exhaled breath condensate: Determination of non‐volatile compounds and their potential for clinical diagnosis and monitoring. A review. Anal Chim Acta 2013;17:1–18. [DOI] [PubMed] [Google Scholar]
- 32. Healy DA, Wallace FA, Miles EA, et al. Effect of low‐to‐moderate amounts of dietary fish oil on neutrophil lipid composition and function. Lipids 2000;35:763–768. [DOI] [PubMed] [Google Scholar]
- 33. King SS, Abughazaleh AA, Webel SK, Jones KL. Circulating fatty acid profiles in response to three levels of dietary omega‐3 fatty acid supplementation in horses. J Anim Sci 2008;86:1114–1123. [DOI] [PubMed] [Google Scholar]
- 34. Brinsko SP, Varner DD, Love CC, et al. Effect of feeding a DHA‐enriched nutriceutical on the quality of fresh, cooled and frozen stallion semen. Theriogenology 2005;63:1519–1527. [DOI] [PubMed] [Google Scholar]
- 35. Barrager E, Veltmann JR, Schauss AG, Schiller RN. A multicentered, open‐label trial on the safety and efficacy of methylsulfonylmethane in the treatment of seasonal allergic rhinitis. J Altern Complement Med 2002;8:167–173. [DOI] [PubMed] [Google Scholar]
- 36. Tecklenburg SL, Mickleborough TD, Fly AD, et al. Ascorbic acid supplementation attenuates exercise‐induced bronchoconstriction in patients with asthma. Respir Med 2007;101:1770–1778. [DOI] [PubMed] [Google Scholar]
- 37. Bailey SR. Corticosteroid‐associated laminitis. Vet Clin North Am Equine Pract 2010;26:277–285. [DOI] [PubMed] [Google Scholar]
- 38. Derksen FJ. Chronic obstructive pulmonary disease (heaves) as an inflammatory condition. Equine Vet J 1993;25:257–258. [DOI] [PubMed] [Google Scholar]


