Abstract
Background
Syringomyelia (SM) is common in the Cavalier King Charles Spaniel (CKCS). Dogs with syringes express clinical signs or might be clinically silent.
Objectives
To investigate the prevalence and heritability of symptomatic SM, the association between clinical signs and magnetic resonance imaging (MRI) findings, and long‐term outcome.
Animals
All CKCS registered in the Danish Kennel Club in 2001 (n = 240).
Methods
A cross‐sectional questionnaire‐based prevalence study validated by telephone interviews and clinically investigated clinical signs of SM. Dogs were 6 years at the time of investigation. A prospective observational litter study including clinical investigations, MRI and 5‐year follow‐up of symptomatic and asymptomatic siblings. Heritability was estimated based on the scale of liability in the study population and litter cohort.
Results
The cross‐sectional study estimated a prevalence of symptomatic SM at 15.4% in the population. Thirteen symptomatic and 9 asymptomatic siblings participated in the litter study. Spinal cord syringes were confirmed in 21 of 22 littermates (95%). Syrinx diameter and mean syrinx : spinal cord ratio were significantly correlated with clinical signs (P < .01). Estimated heritability of symptomatic SM was 0.81. Symptomatic SM motivated euthanasia in 20%. Dogs with syringes, which expressed no clinical signs at the age of 6, remained asymptomatic in 14/15 cases (93%).
Conclusions and Clinical Importance
The prevalence of symptomatic SM is high and genetics have a high impact on clinical disease expression. Further investigations of factors influencing the outbreak threshold of clinical signs of SM are desirable.
Keywords: Chiari‐like malformation, Dog, Epidemiologic, Genetics, Magnetic resonance imaging
Abbreviations
- CKCS
Cavalier King Charles Spaniel
- CM
chiari‐like malformation
- DICOM
digital imaging and communications in medicine
- DKC
Danish Kennel Club
- DVCAS
Department of Veterinary Clinical and Animal Sciences
- FOV
field of view
- MRI
magnetic resonance imaging
- NEX
number of excitations
- NSAIDs
nonsteroidal anti‐inflammatory drugs
- OME
otitis media with effusion
- SE
spin echo
- SM
syringomyelia
- T1 and T2W
T1 and T2 weighted
- TE
time to echo
- TR
time to repeat
- TSE
turbo spin echo
Syringomyelia (SM) is a neurologic condition occurring in a hereditary form in the Cavalier King Charles Spaniel (CKCS), the Griffon Bruxellois, in other toy brachycephalic breeds as well as in humans.1, 2, 3, 4 The prevalence of SM in the CKCS has not been estimated because of a lack of epidemiologic studies investigating larger populations of dogs. SM is characterized by the development of fluid‐filled cavities (syringes) within the spinal cord parenchyma and is associated with Chiari I malformation in humans and Chiari‐like malformation (CM) in the CKCS.2, 5, 6, 7, 8, 9, 10, 11 The pathophysiology of CM involves a decreased caudal fossa volume with overcrowding of the craniocervical junction and caudal descend of the cerebellum into or through the foramen magnum.5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 The pathogenesis of syrinx formation and the association between CM and SM is not fully understood, but it is believed that a multifactorial etiology including a local obstruction of the subarachnoidal space and abnormal cerebrospinal fluid dynamics are involved.5, 10, 12, 16, 17, 18, 19 Syringes are predominantly found in the cervical region of the spinal cord, but can form in multiple locations.20
In humans, the SM‐associated damage to nociceptive and other sensory pathways of the spinal cord, causes pain to be a prominent feature in 50–90% of adult patients.9, 21, 22 Other common clinical characteristics in humans with SM include dermatomal patterns of mixed loss of thermal sensitivity and paradoxical association of hypersensitivity as well as trophic changes with hyperhidrosis, glossy skin, coldness, and paleness in humans.9, 23 Dogs with SM display characteristic behaviors, such as phantom scratching, unwillingness to be touched or groomed in the head and neck region, and in severe cases, paroxysmal pain manifestations with vocalization, intense scratching, rubbing, and circling on the floor. In dogs with SM, these behaviors have been associated with pain.16, 24, 25, 26 CKCS with a magnetic resonance imaging (MRI)‐confirmed syrinx might be asymptomatic.21 In symptomatic CKCS, a wide syrinx diameter and asymmetric distribution of the syrinx affecting the dorsal horn are strong predictors of pain.25, 27 Middle ear effusion (hyperintense material within the tympanic bulla probably analogous to otitis media with effusion [OME] in humans) is often present in the CKCS and may be detected on T2‐weighted MRI scans.28, 29, 30 The incidence of OME in CKCS has been estimated to 32–54%.28, 29, 30, 31 It has been debated if some of the behaviors associated with pain in CKCS could be related to OME, but this however remains controversial.28, 29, 32, 33 The heritability of SM has been estimated to be 0.37 based on MRI‐confirmed syrinx findings in a cohort of 384 CKCS, whereas the heritability of symptomatic SM has not previously been investigated.1
The aim of this study was to estimate the prevalence and heritability of symptomatic SM in Danish CKCS. Furthermore, to investigate the association between clinical signs and MRI findings and report long‐term outcome in a CKCS litter cohort including symptomatic and asymptomatic siblings.
Materials and Methods
The study was initiated in 2007, finalized in 2012, and conducted at the Department of Veterinary Clinical and Animal Sciences (DVCAS), University of Copenhagen, Denmark. The study population consisted of all CKCS born and registered in the Danish Kennel Club (DKC) in 2001. The DKC register lists dogs chronologically by date of registration, and to obtain a DKC studbook, dogs must be registered no later than 3 weeks after birth. The study was conducted in 3 phases.
Definitions
In this paper, the terms “symptomatic” and “asymptomatic” have been chosen for simplicity sake although the authors acknowledge that “symptomatic” is a subjective term inappropriate for animals. “Symptomatic” is here defined as being indicative of a specific disease (in this paper, the expression of clinical signs of SM).
Phase I—Prevalence Study
A cross‐sectional design was used to estimate the prevalence of symptomatic SM. The study investigated clinical signs of SM in the study population (n = 240). Dogs born in 2001 (>6 years old) were chosen to secure that clinical signs of SM, if any, would have had their debut at the time of investigation. The first contact to the owners was established with a mailed letter distributed by the DKC. A short introduction of the investigation was accompanied by an invitation to answer an enclosed screening questionnaire with dichotomous alternative questions along with a semiopen component in which comments could be stated. The questions addressed the following clinical signs indicating SM: unilateral/bilateral episodic scratching of the head/neck region, phantom scratching of the head/neck region (the paw does not touch the skin), reluctance to tolerate touching and/or grooming of the head/neck region and/or resists wearing a collar. Signs are worsened if the dog becomes exited, agitated or both, and signs, which may be interpreted as pain often associated with the neck region.10, 34 The owners were asked to return their answers to the DKC in an enclosed stamped envelope along with a written consent confirming their participation and providing contact information. Owners of dogs expressing at least one of the signs listed, or who reported other clinical signs that could raise a suspicion of SM, were subsequently contacted by phone by the investigators and enrolled in an interview. An extended standardized questionnaire was used to provide further information and validate the answers given in the screening questionnaire. The telephone interview was performed by 2 investigators (CSEJ and RMLH), supervised by 2 veterinary neurologists (HG and MB), and the answers were subsequently scrutinized in collaboration with a senior veterinary neurologist (MB). Dogs qualifying as suspected symptomatic SM cases after validating telephone interview were invited to participate in a clinical investigation at the DVCAS Companion Animal University Hospital, including clinical and neurologic examination and standard hematology, biochemical, and thyroid profiles. Based on the definition of “symptomatic” as being indicative of a specific disease (in this case SM),35 a final evaluation procedure served to define (clinical) symptomatic SM cases. The evaluation procedure included the clinical signs reported by the owners subsequently confirmed by the investigators and supported by the results of the clinical investigation (focused on excluding possible differential diagnosis).
Phase II—Litter Cohort Study
Eight litters were selected for further investigation of the association between clinical signs and MRI findings, based on pedigree information and the criteria that at least 1 sibling had been identified as a symptomatic SM case in the prevalence study. The owners were contacted by phone and interviewed using an extended standardized questionnaire addressing signs of SM to report the clinical status of the dogs at the time of inclusion. All dogs were invited to participate in the clinical investigation including an oral investigator‐owner interview, clinical and neurologic examination, standard hematology, biochemical and thyroid profiles, and MRI of the brain and cervical spinal cord (C1–T1). Dogs presenting with a heart murmur were further evaluated with ECG and echocardiography.
Magnetic resonance imaging scans were performed using a 0.2 Tesla Esaote Vet‐scan. The standardized protocol included T1‐weighted sagittal images of the neurocranium and cervical spinal cord (C1–C3) (T1‐spin echo [SE] with a field of view [FOV] of 14 cm, time to echo [TE] of 18 milliseconds, time to repeat [TR] of 480–560 milliseconds, slice thickness 4 mm, a slice gap of 0.4 mm, and a number of excitations [NEX] of 3), T1‐weighted transverse SE sequences (FOV: 14 cm, TE: 18 milliseconds, TR: 930 milliseconds, slice thickness 5 mm, slice gap: 0.5 mm and NEX: 3) of the neurocranium, T2‐weighted sagittal sequences (high resolution turbo spin echo [TSE], FOV 14 cm, TE: 80 milliseconds, TR: 2,800 milliseconds, slice thickness 4 mm, a slice gap of 0.4 mm and NEX: 2) and T2‐weighted transverse TSE sequences (FOV 14 cm, TE: 80 milliseconds, TR: 2,800–2,820 milliseconds, slice thickness 5 mm, a slice gap of 0.5 mm and NEX: 2) of the neurocranium and cervical spinal cord (C1–C3). T1 and T2 matrix: 256 × 256. The dogs were placed in sternal recumbency, and the total examination time per patient was 75 minutes. Scans were transferred to the web‐based DICOM image viewing system RemoteEye,1 blinded and subsequently evaluated individually by 2 investigators (CSK & HB) and additionally by an experienced veterinary radiologist (UW).
T2 weighted images were used for syrinx measurements. A syrinx was defined as a fluid‐filled cavity in the parenchyma with a diameter ≥2 mm within the spinal cord. A presyrinx was defined as edema in the spinal cord with a lesion diameter <2 mm with or without central canal dilatation. A SM‐positive case was defined as a CKCS with a MRI‐confirmed syrinx. Descriptive data included syrinx diameter, cranial limit of the syrinx, distribution of the syrinx (symmetric or asymmetric), and spinal cord diameter. The syrinx : spinal cord ratio was calculated. If more than 1 syrinx was detected, only the largest was used in the calculations. The hypothesis of no association between the expression of clinical signs and the presence of a syrinx was tested using Fisher's exact test. The hypotheses of no association between the expression of clinical signs and (1) syrinx diameter and (2) syrinx : spinal cord ratio were tested using Student's t‐tests. Data were analyzed by the statistical software in Excel,2 P < .05 was considered significant. It was recorded if CM, findings consistent with otitis media with effusion (OME), or both were present. CM was diagnosed when cerebellar indentation caused by the supraoccipital bone with or without cerebellar herniation into or through the foramen magnum was identified. In addition, it was recorded if the investigators initiated treatment after the clinical and MRI investigations. In 2007, the treatment used would typically be NSAIDs and/or furosemide (Furix3) alone or in combination and in grave cases, furosemide and prednisolone (Prednisolon DAK3).
Estimation of Heritability
The scale of liability (standard deviation from threshold) in the 2 populations described in phase I and II were used for the estimation of heritability according to methods described for threshold characters.36 The applet available at http://www.ihh.kvl.dk/htm/kc/popgen/genetik/applets/heritt.htm was used for the calculations.
Phase III—5‐year Follow‐up (2012)
A follow‐up telephone interview was conducted in autumn 2012 with the purpose of investigating long‐term outcome for the now 11‐year‐old siblings participating in the 2007 litter cohort. The telephone interview was performed by 1 investigator (CLS) and supervised by a senior veterinary neurologist (MB) to secure a standardized interview. A standardized questionnaire was used to collect data regarding the status of the dogs including information addressing alive/deceased, possible clinical signs, possible progression or remission of clinical signs, development of new clinical signs since 2007, treatment, and for deceased dogs, time and cause of death.
Results
Phase I—Prevalence Study
Of 240 owners contacted by mail, 134 responded, giving a response rate of 56% (Fig 1A). Eleven responders were excluded (6 because the dogs were euthanized before the age of 3, and 5 dogs where questionnaires were returned incomplete), leaving 123 dogs (61 females and 62 males) to be included in the investigation. Nineteen owners reported 1 or more clinical signs of SM in their dogs (positive responders), whereas 104 owners reported that their dog expressed no clinical signs of SM (negative responders). After the subsequent telephone interview and validation procedure, the 19 dogs (positive responders) were categorized as suspected symptomatic SM cases. Fourteen owners agreed to let their dogs participate in the clinical investigation, which revealed no other diagnosis than SM, which could explain the signs expressed, by the dogs. Five dogs did not participate in the clinical investigation, but were evaluated to be symptomatic SM cases based on the expression of multiple signs known to be associated with SM,16, 19, 24, 37 and where the owners reported that there had been no clinical findings related to possible differential diagnosis such as eg, skin disease, ear infection, etc. Thus, based on the clinical signs reported and confirmed by the investigators, and the results of the clinical investigation, 19 dogs (11 females and 8 males) were finally included as symptomatic SM cases. The estimated prevalence of symptomatic SM in 6‐year‐old Danish CKCS born in 2001 was 15.4% (19/123), CI0.95 [9; 22].
Figure 1.
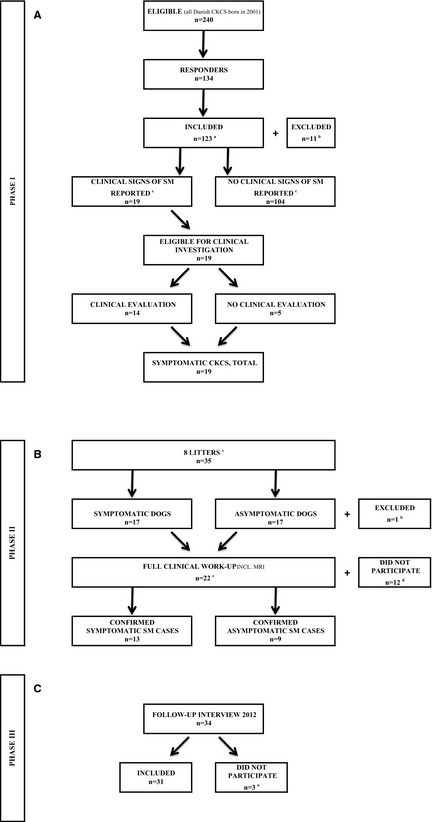
(A) Flow diagram, prevalence study—study phase I. aSix responders excluded because of euthanazia of the dogs before the age of 6, 5 because of incomplete questionnaires. bSixty‐one females and 62 males. cClinical signs reported by the owners, validated on subsequent telephone interview. (B) Flow diagram, litter cohort study—study phase II. aIdentified in study phase I (of all eligible Danish CKCS born in 2001). bExcluded because of euthanazia in 2001. cIncluding 11 dogs identified and validated in study phase I. dTen dogs excluded because of reluctant owners, 1 because of euthanazia, 1 because of severe heart disease. (C) Flow diagram of the 2012 long‐term follow‐up study—study phase III. aTwo owners declined to participate, 1 answer was excluded.
Phase II—Litter Cohort Study
Eight litters were selected for further investigation based on the criteria that at least 1 sibling had been identified as a symptomatic SM case in the prevalence study. The 8 litters comprised a total of 35 dogs (Fig 1B). Seventeen dogs expressed clinical signs of SM, whereas 17 dogs expressed no signs of SM. One dog was excluded as it had been euthanized at the age of 15 weeks because of severe seizures.
Twenty‐two of 34 dogs (63%), including 11 dogs identified as symptomatic SM cases in the prevalence study, were allowed by the owners to participate in full clinical work‐up including MRI. Ten owners declined to let their dogs participate in the MRI investigation, primarily because of concerns associated with anesthesia. One 5‐year‐old dog had been euthanized the year before because of expression of severe signs consistent with SM and could therefore not be investigated, and the investigators excluded 1 dog because of severe heart disease.
Thirteen of the 22 dogs (59%) were classified as symptomatic SM cases, whereas 9 dogs displayed no clinical signs of SM. MRI scans, however, revealed syringes in the cervical spinal cord in 21 of 22 dogs (95%); the remaining dog (expressing no clinical signs of SM) had a presyrinx (Table 1). Thus, the MRI investigation revealed that 8 dogs were clinically silent (asymptomatic SM‐positive cases), although having a syrinx.
Table 1.
MRI findings in 22 dogs participating in the litter cohort study
| In All CKCS (n = 22) | In Asymptomatic SM Cases (n = 9) | In Symptomatic SM Cases (n = 13) | P‐Value | |
|---|---|---|---|---|
| Syrinx present/total (%) | 21/22 (95) | 8/9 (89) | 13/13 (100) | |
| Syrinx diameter T2W (cm), mean (range) | 0.55 (0.3–0.8) | 0.4 (0.3–0.8) | 0.63 (0.3–0.8) | .01 |
| Spinal cord diameter (cm), mean (range) | 0.94 (0.7–1.1) | 0.9 (0.8–1.0) | 0.96 (0.7–1.1) | .08 |
| Syrinx : spinal cord ratio, mean (range) | 0.58 (0.3–0.86) | 0.43 (0.3–0.8) | 0.66 (0.33–0.86) | .01 |
| Asymmetric syrinx/total (%) | 6/21 (29) | 1/8 (13) | 5/13 (38) | .19 |
| Cerebellar herniation/total (%) | 21/21 (100) | 8/8 (100) | 13/13 (100) |
MRI, magnetic resonance imaging; CKCS, Cavalier King Charles Spaniel; SM, syringomyelia; T2W, T2 weighted.
There was no statistical association between expression of clinical signs and the presence of syringes (P = .41). A positive predictive value of the MRI scan was calculated to 0.62 (the probability that a dog would express clinical signs if it had a syrinx). Syrinx diameter and syrinx : spinal cord ratio was evaluated on T2W transverse sequences in 19 dogs. Two dogs were excluded because the MRI studies were incomplete. The syrinx diameter varied from 0.3 to 0.8 cm. The total mean of the maximum syrinx diameter was 0.55 cm. The mean maximum syrinx diameter was 0.63 cm in symptomatic dogs and 0.4 cm in asymptomatic dogs. The data demonstrated that symptomatic dogs had a significantly wider syrinx diameter than asymptomatic dogs (P < .01). The total mean syrinx : spinal cord ratio was 0.58. Symptomatic dogs had a significantly greater mean syrinx : spinal cord ratio (0.66), when compared to asymptomatic dogs, which had a mean syrinx : spinal ratio of 0.43 (P < .01). The cranial limit of the syrinx was C2 in 17 of the 21 dogs (81%), C3 in 3 dogs (14%), and C1 in 1 dog (5%). Syrinx asymmetry (ie, unilateral dorsolateral deviation of the cavity) was observed in 6 (5 symptomatic and 1 asymptomatic) dogs. Fisher's exact test of the association between symmetry versus asymmetry and expression of clinical signs (yes/no) was nonsignificant (P = .19). CM was identified in 22 of 22 dogs (100%). OME was present in 11 dogs (5 symptomatic and 6 asymptomatic SM cases).
Estimation of Heritability
Heritability of symptomatic SM in dogs >6 years of age was calculated using information on difference in mean liability within the population and within related individuals to an affected animal, respectively.36 Using the data presented (Table 2), the heritability was calculated at 0.81. In support of the high heritability, the difference between prevalence of symptomatic SM in the population and in the ones that were related to an affected full sibling was statistically significant (chi‐square = 9.3; df = 1; P < .05).
Table 2.
Prevalence of symptomatic syringomyelia used for heritability estimation
| No. Non‐affected Individuals | No. Affected Individuals | Frequency | |
|---|---|---|---|
| In the population | 104 | 19 | 0.1544 |
| In full sib families | 17 | 9a | 0.3461 |
| In remaining populationb | 87 | 10 | 0.1031 |
The 9 individuals represent the 17 affected dogs minus the 8 index cases/propositus used for the selection of the families.
Used for calculation of chi‐square.
Phase III—5‐year Follow‐up (2012)
Thirty‐one of 34 dogs from the 2007 litter cohort (16 symptomatic and 15 asymptomatic dogs) participated in follow‐up in 2012, where the dogs were 11 years old (Fig 1C). Two owners declined and 1 dog was excluded because of an incomplete interview. Eleven of the 31 dogs (35%) were alive, while 20 (65%) had been euthanized. Euthanasia was directly related to signs of SM in 4 dogs (20%). Thirteen of 16 symptomatic dogs remained symptomatic during the study period (Table 3A). With respect to expression of signs, 4 dogs experienced progression, 5 dogs remained status quo, 4 dogs experienced regression (3 with treatment) and 3 dogs became asymptomatic (1 with treatment) during the study period. Fourteen of 15 asymptomatic dogs remained asymptomatic during the study period, whereas 1 dog (with a MRI‐confirmed syrinx in 2007) developed clinical signs of SM from 2007 to 2012.
Table 3.
Long‐term outcome in (A) 34 and (B) 22 symptomatic and asymptomatic siblings identified in the 2007‐litter cohort study
| 2007 | 2012 | ||||
|---|---|---|---|---|---|
| Status Quo | Deterioration | Improvement | Unknown | ||
| A | |||||
| Symptomatic SM cases | 17 | 5 | 4 | 7a | 1 |
| Asymptomatic SM cases | 17 | 14 | 1b | 2 | |
| B | |||||
| Symptomatic SM cases | 12 | 3 | 4 | 4c | 1 |
| Asymptomatic SM cases | 10 | 8 | 1b | 1 | |
Three dogs changed status from symptomatic to asymptomatic.
One dog changed status from asymptomatic to symptomatic.
One dog changed status from symptomatic to asymptomatic.
When evaluating the 22 dogs participating in the clinical and MRI investigation in 2007 separately, the following results were obtained (Table 3B): Twenty of 22 dog owners participated in the 2012 follow‐up, including 11 symptomatic SM cases and 9 asymptomatic SM cases (8 dogs with a MRI‐confirmed syrinx and 1 dog with a presyrinx). Ten of 11 symptomatic SM cases remained symptomatic, whereas 1 previously symptomatic SM case became asymptomatic during the study period (without treatment). With respect to the expression of clinical signs in the 10 dogs, which remained symptomatic, 4 dogs deteriorated, 3 dogs remained status quo, and 3 dogs improved (with treatment) during the study period. Eight of 9 asymptomatic SM cases remained asymptomatic, whereas 1 dog developed clinical signs of SM during the study period.
Discussion
This study estimated a prevalence of 15.4% of symptomatic SM in Danish CKCS born in 2001 (>6 years old) and demonstrated that genetic factors strongly influence the clinical expression of SM.36 The study furthermore reported that only a minority of asymptomatic dogs with a MRI‐confirmed syrinx at 6 years develop clinical signs later in life.
The dogs under investigation were older than 6 years of age, which served to secure that clinical signs of SM would have evolved at the time of inclusion. For a cross‐sectional study based on mailed questionnaires, a response rate of 56% is considered to be good.38 It is possible that the prevalence estimate is biased by the fact that owners with experience of SM might be more inclined to reply, whereas others may neglect signs of SM. Had all 240 owners returned the mailed questionnaire and had no new symptomatic cases been identified; the minimum prevalence had been 7.9% (19/240). The prevalence estimate was based on owner‐reported clinical signs of SM, a telephone validation procedure, and a clinical evaluation, which served to further confirm the presence of clinical signs and rule out potential differential diagnosis. Five of the 19 dogs, which contributed to the prevalence estimate, did not participate in the clinical evaluation, and although nothing in the dogs' history indicated other diagnosis than SM, this is a weakness to the study, as we cannot exclude that some dogs were included as false‐positive cases. However, 11 of the 19 dogs (60%) which participated both in the prevalence study and litter cohort study all had a MRI‐confirmed syrinx and this supports that the dogs which expressed clinical signs of SM were true SM cases.
This study identified a substantial number of clinically silent dogs although having a syrinx and having symptomatic littermates. Symptomatic SM cases had a significantly greater syrinx diameter and a larger syrinx : spinal cord ratio compared to the group of asymptomatic SM cases which confirms previous reports.25
Our data allowed us to estimate heritability of the expression of symptomatic SM in CKCS 6 years of age, which has not previously been done. The heritability of SM diagnosed by a single MRI diagnosis without follow‐up on clinical status has been estimated at 0.37,1 whereas heritability of symptomatic SM was estimated at 0.81 in this study. It is apparent that the development of a syrinx does not always result in a clinical expression of signs of SM, and thus there may not be a discrepancy between these results. Although the heritability estimate for symptomatic SM might be an overestimation because of the fact that there is a potential bias in the prevalence study (dog owners with affected dogs might be more inclined to participate), the heritability is clearly very high. Per definition heritability is measured by estimating the relative contribution of genetic and nongenetic differences to the total phenotypic variation in a population.36 The estimated heritability of symptomatic SM indicates that genetics explain most of the total phenotypic variance in the population.
As this study found an equal number of symptomatic and asymptomatic dogs with OME, and CM was confirmed in all MRI scanned dogs, it was not possible to examine if CM, OME alone, or both could have contributed to some of the clinical signs reported by the owners.
Finally, we acknowledge that the results of this study are affected by the drawbacks inevitably associated with epidemiologic and clinical studies, where it is seldom possible to motivate all owners to allow their dogs to participate in all planned procedures. It lies within the design of the study that all dogs should undergo the same standardized investigations as described in details in the materials and methods section. Being an epidemiologic study where the investigators did contact the owners, we must however respect that in some cases, owners do not want to participate in full work‐up. In that case, the investigators must thoroughly determine for each dog if it can be defined as a true case based on the collected information. We also acknowledge that some of the choices made in 2007 regarding the study design may have influenced the results, eg, that measuring syrinx size on T2W images obtained from a low field system was suboptimal.39 MRI identification of a syrinx is presently the gold standard for diagnosing SM. The results of this study provides information which necessitate further examination of factors contributing to disease expression and progression, and to the threshold of outbreak of clinical signs.
Conclusions
This study of Danish CKCS estimated a high prevalence of symptomatic SM and found a high impact of genetics on the clinical expression of the disease. Dogs with a MRI‐confirmed syrinx and clinical signs are at risk of euthanasia, whereas dogs with a MRI‐confirmed syrinx which are asymptomatic by the age of 6, seem to have a good chance of never developing such signs. It is of interest to conduct further studies investigating factors, which may influence the threshold of outbreak of clinical signs of SM.
Acknowledgments
This study was supported by grants donated by the Danish Kennel Club and the Danish Cavalier King Charles Club who are gratefully acknowledged.
Conflict of Interest Declaration: The authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: The authors declare no off‐label use of antimicrobials.
The study was conducted at the Department of Veterinary Clinical and Animal Science, University of Copenhagen, Denmark.
Poster presentation of preliminary results at the ECVN/ESVN 26th Symposium, September 26–28th 2013, Paris.
Footnotes
Microsoft Excel 2008 for Mac Version 12.3.6
Takeda Pharma A/S, Langebjerg 1, DK‐4000 Roskilde, Denmark
References
- 1. Lewis T, Rusbridge C, Knowler P, et al. Heritability of syringomyelia in Cavalier King Charles spaniels. Vet J 2010;183:345–347. [DOI] [PubMed] [Google Scholar]
- 2. Boyles AL, Enterline DS, Hammock PH, et al. Phenotypic definition of Chiari type I malformation coupled with high‐density SNP genome screen shows significant evidence for linkage to regions on chromosomes 9 and 15. Am J Med Genet A 2006;140:2776–2785. [DOI] [PubMed] [Google Scholar]
- 3. Rusbridge C, Knowler SP. Hereditary aspects of occipital bone hypoplasia and syringomyelia (Chiari type I malformation) in cavalier King Charles spaniels. Vet Rec 2003;153:107–112. [DOI] [PubMed] [Google Scholar]
- 4. Rusbridge C, Knowler SP, Pieterse L, et al. Chiari‐like malformation in the Griffon Bruxellois. J Small Anim Pract 2009;50:386–393. [DOI] [PubMed] [Google Scholar]
- 5. Rusbridge C. Chiari‐like malformation and syringomyelia. EJCAP 2013;23:1–20. [Google Scholar]
- 6. Milhorat TH, Nishikawa M, Kula RW, et al. Mechanisms of cerebellar tonsil herniation in patients with Chiari malformations as guide to clinical management. Acta Neurochir 2010;152:1117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cerda‐Gonzalez S, Olby NJ, McCullough S, et al. Morphology of the caudal fossa in Cavalier King Charles spaniels. Vet Radiol Ultrasound 2009;50:37–46. [DOI] [PubMed] [Google Scholar]
- 8. Cappello R, Rusbridge C. Report from the Chiari‐like malformation and syringomyelia working group round table. Vet Surg 2007;36:509–512. [DOI] [PubMed] [Google Scholar]
- 9. Ducreux D, Attal N, Parker F, et al. Mechanisms of central neuropathic pain: A combined psychophysical and fMRI study in syringomyelia. Brain 2006;129:963–976. [DOI] [PubMed] [Google Scholar]
- 10. Rusbridge C, Greitz D, Iskandar BJ. Syringomyelia: Current concepts in pathogenesis, diagnosis, and treatment. J Vet Intern Med 2006;20:469–479. [DOI] [PubMed] [Google Scholar]
- 11. Milhorat TH, Capocelli AL Jr, Anzil AP, et al. Pathological basis of spinal cord cavitation in syringomyelia: Analysis of 105 autopsy cases. J Neurosurg 1995;82:802–812. [DOI] [PubMed] [Google Scholar]
- 12. Driver CJ, Volk HA, Rusbridge C, et al. An update on the pathogenesis of syringomyelia secondary to Chiari‐like malformations in dogs. Vet J 2013;198:551–559. [DOI] [PubMed] [Google Scholar]
- 13. Shaw TA, McGonnell IM, Driver CJ, et al. Caudal cranial fossa partitioning in Cavalier King Charles spaniels. Vet Rec 2013;172:341. [DOI] [PubMed] [Google Scholar]
- 14. Carrera I, Dennis R, Mellor DJ, et al. Use of magnetic resonance imaging for morphometric analysis of the caudal cranial fossa in Cavalier King Charles spaniels. Am J Vet Res 2009;70:340–345. [DOI] [PubMed] [Google Scholar]
- 15. Driver CJ, Rusbridge C, Cross HR, et al. Relationship of brain parenchyma within the caudal cranial fossa and ventricle size to syringomyelia in cavalier King Charles spaniels. J Small Anim Pract 2010;51:382–386. [DOI] [PubMed] [Google Scholar]
- 16. Plessas IN, Rusbridge C, Driver CJ, et al. Long‐term outcome of Cavalier King Charles spaniel dogs with clinical signs associated with Chiari‐like malformation and syringomyelia. Vet Rec 2012;171:501. [DOI] [PubMed] [Google Scholar]
- 17. Levine DN. The pathogenesis of syringomyelia associated with lesions at the foramen magnum: A critical review of existing theories and proposal of a new hypothesis. J Neurol Sci 2004;220:3–21. [DOI] [PubMed] [Google Scholar]
- 18. Bilston LE, Stoodley MA, Fletcher DF. The influence of the relative timing of arterial and subarachnoid space pulse waves on spinal perivascular cerebrospinal fluid flow as a possible factor in syrinx development. J Neurosurg 2010;112:808–813. [DOI] [PubMed] [Google Scholar]
- 19. Cerda‐Gonzalez S, Olby NJ, Broadstone R, et al. Characteristics of cerebrospinal fluid flow in Cavalier King Charles spaniels analyzed using phase velocity cine magnetic resonance imaging. Vet Radiol 2009;50:467–476. [DOI] [PubMed] [Google Scholar]
- 20. Loderstedt S, Benigni L, Chandler K, et al. Distribution of syringomyelia along the entire spinal cord in clinically affected Cavalier King Charles spaniels. Vet J 2011;190:359–363. [DOI] [PubMed] [Google Scholar]
- 21. Gamache FW Jr, Ducker TB. Syringomyelia: A neurological and surgical spectrum. J Spinal Disord 1990;3:293–298. [PubMed] [Google Scholar]
- 22. Hida K, Iwasaki Y, Koyanagi I, et al. Surgical indication and results of foramen magnum decompression versus syringosubarachnoid shunting for syringomyelia associated with Chiari I malformation. Neurosurgery 1995;37:673–678; discussion 678–679. [DOI] [PubMed] [Google Scholar]
- 23. Todor DR, Mu HT, Milhorat TH. Pain and syringomyelia: A review. Neurosurg Focus 2000;8:E11. [DOI] [PubMed] [Google Scholar]
- 24. Rutherford L, Wessmann A, Rusbridge C, et al. Questionnaire‐based behaviour analysis of Cavalier King Charles spaniels with neuropathic pain due to Chiari‐like malformation and syringomyelia. Vet J 2012;194:294–298. [DOI] [PubMed] [Google Scholar]
- 25. Rusbridge C, Carruthers H, Dube MP, et al. Syringomyelia in cavalier King Charles spaniels: The relationship between syrinx dimensions and pain. J Small Anim Pract 2007;48:432–436. [DOI] [PubMed] [Google Scholar]
- 26. Rusbridge C. Persistent scratching in Cavalier King Charles spaniels. Vet Rec 1997;141:179. [PubMed] [Google Scholar]
- 27. Hu HZ, Rusbridge C, Constantino‐Casas F, et al. Histopathological investigation of syringomyelia in the Cavalier King Charles spaniel. J Comp Pathol 2012;146:192–201. [DOI] [PubMed] [Google Scholar]
- 28. Owen MC, Lamb CR, Lu D, et al. Material in the middle ear of dogs having magnetic resonance imaging for investigation of neurologic signs. Vet Radiol Ultrasound 2004;45:149–155. [DOI] [PubMed] [Google Scholar]
- 29. Hayes GM, Friend EJ, Jeffery ND. Relationship between pharyngeal conformation and otitis media with effusion in Cavalier King Charles spaniels. Vet Rec 2010;167:55–58. [DOI] [PubMed] [Google Scholar]
- 30. McGuinness SJ, Friend EJ, Knowler SP, et al. Progression of otitis media with effusion in the Cavalier King Charles spaniel. Vet Rec 2013;172:315. [DOI] [PubMed] [Google Scholar]
- 31. Lu D, Lamb CR, Pfeiffer DU, et al. Neurological signs and results of magnetic resonance imaging in 40 cavalier King Charles spaniels with Chiari type 1‐like malformations. Vet Rec 2003;153:260–263. [DOI] [PubMed] [Google Scholar]
- 32. Volk HA, Davies ES. Middle ear effusions in dogs: An incidental finding? Vet J 2011;188:256–257. [DOI] [PubMed] [Google Scholar]
- 33. Rusbridge C. Primary secretory otitis media in Cavalier King Charles spaniels. J Small Anim Pract 2004;45:222; author reply 222. [PubMed] [Google Scholar]
- 34. Rusbridge C. Neurological diseases of the Cavalier King Charles spaniel. J Small Anim Pract 2005;46:265–272. [DOI] [PubMed] [Google Scholar]
- 35. Blood D, Studdert V. Saunders Comprehensive Veterinary Dictionary, 2nd ed London: London Harcourt Publishers Ltd; 1999:1106. [Google Scholar]
- 36. Falconer DS, Mackay TFC. Introduction to Quantitative Genetics, 4th ed Harlow: Longman; 1996:464. [Google Scholar]
- 37. Rusbridge C, Jeffery ND. Pathophysiology and treatment of neuropathic pain associated with syringomyelia. Vet J 2008;175:164–172. [DOI] [PubMed] [Google Scholar]
- 38. Asch DA, Jedrziewski MK, Christakis NA. Response rates to mail surveys published in medical journals. J Clin Epidemiol 1997;50:1129–1136. [DOI] [PubMed] [Google Scholar]
- 39. Akiyama Y, Koyanagi I, Yoshifuji K, et al. Interstitial spinal‐cord oedema in syringomyelia associated with Chiari type 1 malformations. J Neurol Neurosurg Psychiatry 2008;79:1153–1158. [DOI] [PubMed] [Google Scholar]


