Abstract
Background
Closure of PDA can be associated with echocardiographic changes including deterioration of LV systolic function. Although PDA is commonly encountered in dogs, few comprehensive reports of echocardiographic changes in dogs with PDA closure are available.
Objectives
To evaluate the short‐term echocardiographic changes observed after PDA closure in dogs using strain analysis.
Animals
Seventeen client‐owned dogs with left‐to‐right PDA.
Methods
Echocardiographic evaluations, including standard echocardiography and two‐dimensional tissue tracking (2DTT), were performed before and within 3 days of PDA closure.
Results
Preclosure examination showed LV and left atrial dilatation indicating volume overload as a result of PDA. Closure of PDA resulted in significant reduction of LVIDd (<.0001) and LA/Ao (0.01) without change in LVIDs, suggestive of decreased preload. Postclosure LV systolic dysfunction was observed with significant decreased in FS (<.0001) and strain values (P = .0039 for radial strains, P = .0005 for circumferential strains). Additionally, significant LV dyssynchrony (P = .0162) was observed after closure of PDA.
Conclusions and Clinical Importance
Closure of PDA resulted in decreased preload as a result of alleviation of LV volume overload, which in turn caused transient deterioration of LV systolic function. Additionally, this study demonstrated that strain analysis is load dependent. Therefore, care should be taken when interpreting strain measurements as an indicator of LV systolic function.
Keywords: Canine, Congenital heart disease, Strain, Systolic function
Abbreviations
- PDA
patent ductus arteriosus
- LV
left ventricle
- FS
fractional shortening
- 2DTT
two‐dimensional tissue tracking
- LVIDd
left ventricular end‐diastolic diameter
- LVIDs
left ventricular end‐systolic diameter
- LA/Ao
left atrium‐to‐aorta ratio
- LVOT PEP:ET
ratio of pre‐ejection period and ejection time
- LVOT pV
aortic velocity
- E
early ventricular filling velocity
- A
late ventricular filling velocity
- MR
mitral regurgitation
- MRI
magnetic resonance imaging
- STI
synchrony time index
- CRT
cardiac resynchronization therapy
Patent ductus arteriosus (PDA) is 1 of the most common congenital cardiovascular defects in dogs1, 2, 3. It occurs as a result of failure of the ductus arteriosus to close, a normal fetal structure that shunts blood from pulmonary artery to aorta1, 2, 3, 4. After birth, expansion of the lungs causes pulmonary vascular resistance to decrease, which allows blood to flow from the high resistance aorta to the low resistance pulmonary artery through the PDA1, 2, 3, 4. This left‐to‐right shunt results in over‐circulation of the lungs and left side of the heart causing left‐sided volume overload1, 2, 3, 4. Treatment of PDA involving ductus closure is the most effective and is strongly recommended in dogs with left‐to‐right shunting PDA1, 2, 3, 4. However, after PDA closure, transient systolic dysfunction of the left ventricle (LV) is common in both humans and dogs5, 6, 7, 8, 9, 10. Systolic dysfunction is a result of sudden changes in loading conditions, caused by termination of PDA flow, and is not a reflection of actual myocardial contractility5, 6, 7, 8, 9, 10.
Left ventricular fractional shortening (FS) is a parameter that is widely used as a measurement of LV function5. However, FS is affected by loading conditions, thus it is strictly a measurement of LV function rather than a measurement of contractility5. Two‐dimensional tissue tracking (2DTT) is a novel echocardiographic technique, which was developed as an assessment method for myocardial function11, 12, 13, 14. In human, its clinical applications include assessment of coronary artery disease, myocardial infarction and various types of cardiomyopathies11, 12, 13. Two‐dimensional tissue tracking ‐derived strain is a measurement of myocardial deformation over time, and is expressed as the percentage change from its original dimension11, 12, 13, 14. Two‐dimensional tissue tracking provides measurements of segmental strains, which allows evaluation of both global and regional function11, 14. In addition, systolic mechanics occur in radial, circumferential and longitudinal directions, and 2DTT allows measurement of strains in these directions11, 13. Previous reports have demonstrated the influence of loading condition on strain values under various hemodynamic states by manipulating preload and inotropic conditions15, 16, 17. However, 2DTT provides comprehensive evaluation, such as evaluation of myocardial synchronicity, which allows better quantitative assessment of myocardial function18, 19, 20, 21.
In humans, transient changes in LV function in association with closure of PDA have been reviewed extensively6, 7, 8, 9, 10. However, few studies have reported the echocardiographic changes observed with PDA closure in dogs22, 23, 24. The purposes of the present study were to evaluate the short‐term echocardiographic changes in dogs before and after surgical ligation of PDA using both standard echocardiography and 2DTT and to demonstrate the influence of loading conditions on strain analysis including synchronicity and changes observed after surgical ligation of PDA.
Materials and Methods
Study Population
Client‐owned dogs with PDA, presented at the Tokyo University of Agriculture and Technology Animal Medical Centre from August 2010 to August 2013 for the definitive diagnosis and treatment of PDA, were evaluated prospectively. Diagnosis was made based on demonstration of PDA by color Doppler echocardiography in all dogs, and was confirmed by thoracotomy during surgical ligation of the PDA. Dogs were included in the study if they had an uncomplicated left‐to‐right shunting PDA, without concurrent congenital or acquired cardiac disorders and arrhythmias. Dogs with echocardiographic evidence of pulmonary hypertension, bidirectional or reversed (right‐to‐left) PDA, or those with other concurrent cardiac disorders in addition to PDA were excluded.
Study Protocol
Informed consent was obtained from the owners, and the study was approved and conducted in accordance with the standards established by the Tokyo University of Agriculture and Technology Animal Medical Centre. Signalment, body weight and clinical signs at the time of diagnosis as well as type and duration of medical treatments were recorded. Echocardiographic evaluations, including standard echocardiographic and 2DTT examinations, were performed twice, before and after the surgical ligations. Presurgical evaluation was performed at the time of admission, which was the day before surgery, and in order to minimize the effect of anesthetic and analgesic drugs, the postsurgical evaluation was performed at least 24 hours after the last administration of any anesthetic or analgesic, which was no later than 3 days postsurgery. An ultrasonography unit1 equipped with a 5 MHz phased array transducer probe2 was used. A mean of at least 5 measurements was obtained from consecutive cardiac cycles in sinus rhythm for each parameter.
Standard Echocardiography
Examinations were performed in accordance with the methods described by Boon5. Cardiac dimensions were measured from the right parasternal short axis view using M‐mode. Measurements, including LV end‐diastolic (LVIDd) and end‐systolic (LVIDs) diameters, were measured at the level of the papillary muscle, and left atrium‐to‐aorta ratio (LA/Ao) was measured at the heart base level.
Left ventricular systolic function was evaluated using FS and systolic time intervals, which is the ratio of pre‐ejection period and ejection time (LVOT PEP:ET) measured from spectral Doppler aortic velocity (LVOT pV) using the left caudal parasternal 5‐chamber view. Maximal aortic outflow velocities also were obtained. Additionally, diastolic function was assessed by early (E) and late (A) ventricular filling velocity, using E/A ratio from the trans‐mitral flow profile at left parasternal apical 4‐chamber view. Mitral regurgitation (MR) was diagnosed if there was a regurgitating turbulent flow in the left atrium.
Two‐Dimensional Tissue Tracking
A right parasternal short axis view was acquired at the level of the papillary muscle at a frame rate of 70‐110 frames/s, and was then analyzed off‐line.3 Strain analysis involved multiple steps. Initially, the endocardium and epicardium were manually traced at end‐systole by placing a number of points at the borders of the myocardium. The software then automatically tracked these points on a frame‐by‐frame basis, and divided the LV into 6 segments (anterior septal, anterior, lateral, posterior, inferior and septal). By tracking the movements of each myocardial segment, analysis of myocardial deformation was performed in terms of strain. From the view used in this study, strain measurements could be made in radial and circumferential directions, and 3 parameters could be measured: (1) regional peak systolic strains of the 6 segments, (2) global peak systolic strain, which is the mean of the 6 segments, and (3) the synchrony time index (STI), which is the difference in timing of peak strains from the earliest to the latest segments used to assess LV synchronicity.
Statistical Analysis
Data are expressed as mean ± standard deviation. The normality of data distribution was shown for each parameter using the D'Agostino and Pearson omnibus normality test. Significant changes before and after closure of the PDA were evaluated using a paired Student's t test for continuous variables. Significant differences were defined as P < .05. Statistical analyses were performed using statistical software.4
Results
Seventeen dogs underwent surgical ligation of their PDA and echocardiographic examinations were performed before and after surgery. Eight breeds were represented with Pomeranians (n = 6), Toy Poodles (n = 4), Chihuahua (n = 2), and 1 each of Jack Russell Terrier, Maltese, Papillon, Penbroke Welsh Corgi and Shetland Sheep Dog. There were 10 females (59%) and 7 males (41%). The average age at the time of diagnosis was 7 months (range: 2–25 months); only 2 dogs were ≧1at the time of diagnosis (12%). Average body weight was 2.5 kg (range: 1.0–6.2 kg). Three dogs were presented for cough, 1 for exercise intolerance and 1 for breathing difficulties, and the remaining 12 dogs did not show any clinical signs. Seven dogs were treated with angiotensin‐converting enzyme inhibitors with or without diuretics (n = 2), pimobendan (n = 2) and a prostacyclin analog (n = 1) for ≧1 week before surgery, and treatment was continued after the surgery until postsurgical evaluation.
Standard Echocardiography
Presurgical examination indicated increased LVIDd, LVIDs and LA/Ao, suggesting LV and left atrial dilatation, and other results were within the reference range (Table 1)5, 25. Surgical ligation of the PDA resulted in a significant decrease in LVIDd and LA/Ao. With the decrease in LVIDd and LA/Ao, LA/Ao returned to within the reference range, but LVIDd and LVIDs still remained above the reference range. Fractional shortening decreased significantly and LV PEP:ET increased significantly, with FS below the reference range indicating LV systolic dysfunction. Additionally, significant decrease in LVOT pV, E and A also were observed. Seven dogs (4 females [57%] and 3 males [43%]) had MR, which persisted postsurgery. In 3 dogs, residual PDA flow of <2.0 m/s was observed, which was considered hemodynamically insignificant.
Table 1.
Standard echocardiographic changes (mean ± SD) observed before and after surgical ligation of PDA
| Before | After | Reference range | P value | |
|---|---|---|---|---|
| LVIDd (mm) | 24.64 ± 5.90 | 20.74 ± 3.87 | 13.05 ‐ 18.61 | <.0001 |
| LVIDs (mm) | 14.83 ± 4.36 | 14.38 ± 3.30 | 7.86 ‐ 13.67 | NS |
| LA/Ao | 1.71 ± 0.40 | 1.28 ± 0.19 | 0.83 ‐ 1.13 | .0143 |
| FS (%) | 40.33 ± 5.81 | 30.85 ± 8.06 | 33.70 ‐ 45.90 | <.0001 |
| LVOT pV (cm/s) | 109.56 ± 26.39 | 68.34 ± 13.32 | <200.00 | .0058 |
| LVOT PEP/ET | 0.19 ± 0.05 | 0.34 ± 0.14 | <0.40 | .0050 |
| E (cm/s) | 74.53 ± 16.86 | 59.89 ± 16.66 | 50.00 ‐ 100.00 | .0037 |
| A (cm/s) | 56.93 ± 14.05 | 34.04 ± 8.70 | 30.00 ‐ 60.00 | .0023 |
LVIDd, left ventricular end‐diastolic diameter; LVIDs, left ventricular end‐systolic diameter; LA/Ao, left atrium‐to‐aorta ratio; FS, fractional shortening; LVOT pV, peak aortic flow velocity; LVOT PEP:ET, left ventricular ejection time to pre‐ejection time ratio; E, early ventricular filling velocity; A, early ventricular filling velocity; NS, nonsignificant.
Two‐dimensional tissue tracking
Global radial and circumferential strains before closure of the PDA were 33.47 ± 15.68 (reference range: 22.30–71.10) and −14.43 ± 2.79 (reference range not available), respectively26. Closure of the PDA resulted in a decrease in strains in both directions (P = .0039 for radial strains, P = .0005 for circumferential strains), with the radial strains below the reference range (Fig 1). Regional strain analysis had indicated decreases of strains in all segments in both directions with the exception of cranial and inferior segments in radial directions (Fig 2). Radial STI (P = .0162) was increased just above the reference range after PDA closure (radial STI reference range: 0–45 ms)26.
Figure 1.
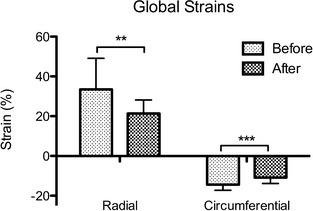
Changes of global strains (mean ± SD) in radial and circumferential directions in dogs with PDA (n = 17) before and after the surgical ligation of PDA. Significant differences (**P < .005, ***P < .001) in strains were observed in association with attenuation of PDA flow.
Figure 2.
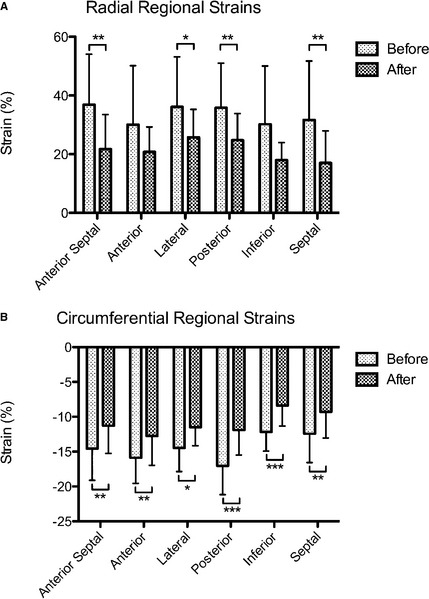
Changes in regional strains (mean ± SD) observed in (A) radial and (B) circumferential directions in dogs with PDA (n = 17) before and after the surgical ligation of PDA. Significant differences (*P < .01, **P < .005, ***P < .001) in strains were observed in association with attenuation of PDA flow.
Discussion
This study illustrated a several points. First, before closure of the PDA, the LV was under a state of volume overload, and closure of the PDA resulted in significant decreases in LVIDd and LA/Ao without a change in LVIDs, indicating decreased preload. This point is supportive of the previous findings that systolic dysfunction was the result of sudden changes in loading conditions and may not be a reflection of actual myocardial contractility5, 6, 7, 8, 9, 10. Secondly, this postclosure left atrial systolic dysfunction also was observed with significant decrease in strain values, indicating that strain analysis is load dependent. Lastly, significant LV dyssynchrony was observed after closure of the PDA, and was caused by the sudden decrease in preload.
This study demonstrated the presence of LV volume overload in dogs with PDA, indicated by LV and left atrial dilatation, and the diagnosis of MR in 43% of the cases. Attenuation of PDA flow resulted in an immediate decrease in LVIDd and LA/Ao, and decrease in mitral inflow and aortic outflow velocities, all suggestive of decreased preload. Additionally, deterioration of LV systolic function, indicated by the significant decrease in FS and LV PEP:ET, was observed. Such a decrease in preload and accompanying deterioration of LV systolic function have been well documented after attenuation of hemodynamically relevant PDA flow6, 7, 8, 9, 10.
A study by Barlow et al compared LV contractility of infants with clinically relevant PDA to control infants using the load independent parameter of LV contractility, the rate‐corrected velocity of fiber shortening, and their results failed to show differences between the control and affected infants27. Additionally, Takahashi et al evaluated the changes in LV contractility by mean normalized systolic ejection rate, another load independent parameter, and demonstrated that it did not change although EF decreased with closure of the PDA8. These studies strongly suggest that LV contractility is not impaired in the presence of volume overload, and postclosure systolic dysfunction is the result of decreased preload.
Two‐dimensional tissue tracking derived strain analysis was developed as an assessment method for myocardial function, and it has been validated with tagged magnetic resonance imaging (MRI), which is considered to be the gold standard for assessment of LV contractility11, 12, 13, 14. However, recent reports have indicated that strain may not only reflect LV contractility12, 15, 17. Several studies have compared strain values to invasive measurements during various hemodynamic and inotropic conditions, and have demonstrated that strain is closely related to stroke volume and thus influenced by loading conditions15, 17. In this study, global analysis identified decrease in strains in both radial and circumferential directions after closure of the PDA in similar fashion to FS, indicating the influence of preload. Additionally, regional analysis identified significant changes in almost all of the segments in both directions, suggesting the influence of loading condition to be uniform throughout the myocardium.
Left ventricular mechanical dyssynchrony is known to be a sensitive indicator of myocardial dysfunction, and it has been shown to relate more closely to hemodynamic alterations than EF12. For this reason, it has been widely used for patient selection and assessment of the response to cardiac resynchronization therapy (CRT)11, 12, 13. Normally, LV mechanical contraction is synchronous, with the septal segments contracting slightly earlier than the lateral and caudal segments18. The results of this study showed a similar coordinated pattern of contraction before attenuation of PDA flow, with the septal segment showing earlier time‐to‐peak in comparison to the lateral and caudal segments (Fig 3). On the other hand, an uncoordinated pattern of contraction was observed after PDA ligation, which indicates radial dyssynchrony. Studies on CRT have demonstrated that LV systolic dysfunction is related to increased radial dyssynchrony18, 19. Additionally, changes in loading conditions can result in significant LV dyssynchrony28. Similarly, the slight LV dyssynchrony observed in this study after attenuation of PDA flow was a reflection of transient myocardial dysfunction caused bydecreased preload.
Figure 3.

Pattern of contraction observed (A) before and (B) after the PDA ligation in dogs with PDA (n = 17).
This study had a number of limitations. First, the Teichholz equation was the only method used to assess the volume of the LV. The lack of strain rate analysis was another limitation. Strain rate is the rate of myocardial deformation, and like strain, it may be load dependent, but some studies suggest that strain rate is less influenced by loading conditions15, 16, 17. Another limitation includes lack of follow‐up to evaluate the long‐term outcome of surgical ligation of the PDA. The immediate LV systolic dysfunction observed after occlusion of the PDA is known to be transient, but, LV dysfunction may persist with delayed closure (e.g., in older patients)10. Therefore, although most of the cases were <1 year old, long‐term follow‐up should be done to evaluate the recovery of LV systolic dysfunction using echocardiographic parameters including 2DTT.
This study demonstrated that surgical ligation of the PDA causes a decrease in preload by alleviation of LV volume overload, which in turn results in transient deterioration of LV systolic function observed by significant echocardiographic changes. Additionally, this study showed that strain analysis is influenced by acute changes in loading conditions as demonstrated by previous reports. Therefore, care should be taken when interpreting strain measurements as an indicator of LV systolic function.
Acknowledgments
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
This prospective clinical study was carried out at Tokyo University of Agriculture and Technology Animal Medical Centre. This study did not receive support of any kind, and it has not been presented at any meetings. There are no acknowledgements.
Footnotes
ALOKA prosound α 10, Hitachi Aloka Medical, Ltd, Tokyo, Japan
UST52108, Hitachi Aloka Medical, Ltd
DAS‐RS1 software 1.1v, Hitachi Aloka Medical, Ltd
Prism 5.0v, GraphPad Software Inc, La Jolla, CA and JMP 10.0.2, SAS Institute Inc, Cary, NC
References
- 1. Bonagura JD, Lehmkuhl LB. Congenital Heart Disease In: Fox PR, Sisson D, Moise NS, eds. Textbook of Canine and Feline Cardiology: Principles and Clinical Practice, 2nd ed Philadelphia: W.B. Saunders; 1999:471–535. [Google Scholar]
- 2. Broaddus K, Tillson M. Patent ductus arteriosus in dogs. Compend Contin Educ Vet 2010;32:E3. [PubMed] [Google Scholar]
- 3. Buchanan JW. Patent ductus arteriousus morphology, pathogenesis, types and treatment. J Vet Cardiol 2001;3:7–16. [DOI] [PubMed] [Google Scholar]
- 4. Strickland K. Congenital Heart Disease In: Tilley LP, Smith FWK, Jr, Oyama MA, Sleeper MM, eds. Manual of Canine and Feline Cardiology (4th ed.), 4th ed. ed. Saint Louis: W.B. Saunders; 2008:223–247. [Google Scholar]
- 5. Boon J. The M‐mode and Doppler examination In: Boon J, ed. Veterinary Echocardiography, 2nd ed Chichester: Wiley‐Blackwell; 2011:101–112. [Google Scholar]
- 6. Gupta SK, Krishnamoorthy K, Tharakan JA, et al. Percutaneous closure of patent ductus arteriosus in children: Immediate and short‐term changes in left ventricular systolic and diastolic function. Ann Pediatr Cardiol 2011;4:139–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Galal MO, Amin M, Hussein A, et al. Left ventricular dysfunction after closure of large patent ductus arteriosus. Asian Cardiovasc Thorac Ann 2005;13:24–29. [DOI] [PubMed] [Google Scholar]
- 8. Takahashi Y, Harada K, Ishida A, et al. Changes in left ventricular volume and systolic function before and after the closure of ductus arteriosus in full‐term infants. Early Hum Dev 1996;44:77–85. [DOI] [PubMed] [Google Scholar]
- 9. Kim YH, Choi HJ, Cho Y, et al. Transient left ventricular dysfunction after percutaneous patent ductus arteriosus closure in children. Korean Circ J 2008;38:596–600. [Google Scholar]
- 10. Jeong YH, Yun TJ, Song JM, et al. Left ventricular remodeling and change of systolic function after closure of patent ductus arteriosus in adults: Device and surgical closure. Am Heart J 2007;154:436–440. [DOI] [PubMed] [Google Scholar]
- 11. Geyer H, Caracciolo G, Abe H, et al. Assessment of myocardial mechanics using speckle tracking echocardiography: Fundamentals and clinical applications. J Am Soc Echocardiogr 2010;23:351–369. [DOI] [PubMed] [Google Scholar]
- 12. Dandel M, Hetzer R. Echocardiographic strain and strain rate imaging–clinical applications. Int J Cardiol 2009;132:11–24. [DOI] [PubMed] [Google Scholar]
- 13. Nesbitt GC, Mankad S, Oh JK. Strain imaging in echocardiography: Methods and clinical applications. Int J Cardiovasc Imaging 2009;25(Suppl 1):9–22. [DOI] [PubMed] [Google Scholar]
- 14. Artis NJ, Oxborough DL, Williams G, et al. Two‐dimensional strain imaging: A new echocardiographic advance with research and clinical applications. Int J Cardiol 2008;123:240–248. [DOI] [PubMed] [Google Scholar]
- 15. Burns AT, La Gerche A, D'Hooge J, et al. Left ventricular strain and strain rate: Characterization of the effect of load in human subjects. Eur J Echocardiogr 2010;11:283–289. [DOI] [PubMed] [Google Scholar]
- 16. Culwell NM, Bonagura JD, Schober KE. Comparison of echocardiographic indices of myocardial strain with invasive measurements of left ventricular systolic function in anesthetized healthy dogs. Am J Vet Res 2011;72:650–660. [DOI] [PubMed] [Google Scholar]
- 17. Weidemann F, Jamal F, Sutherland GR, et al. Myocardial function defined by strain rate and strain during alterations in inotropic states and heart rate. Am J Physiol Heart Circ Physiol 2002;283:H792–799. [DOI] [PubMed] [Google Scholar]
- 18. Friedberg MK, Slorach C. Relation between left ventricular regional radial function and radial wall motion abnormalities using two‐dimensional speckle tracking in children with idiopathic dilated cardiomyopathy. Am J Cardiol 2008;102:335–339. [DOI] [PubMed] [Google Scholar]
- 19. Labombarda F, Blanc J, Pellissier A, et al. Health‐e‐Child project: Mechanical dyssynchrony in children with dilated cardiomyopathy. J Am Soc Echocardiogr 2009;22:1289–1295. [DOI] [PubMed] [Google Scholar]
- 20. Tops LF, Suffoletto MS, Bleeker GB, et al. Speckle‐tracking radial strain reveals left ventricular dyssynchrony in patients with permanent right ventricular pacing. J Am Coll Cardiol 2007;50:1180–1188. [DOI] [PubMed] [Google Scholar]
- 21. Yoshikawa H, Suzuki M, Tezuka N, et al. Differences in left ventricular dyssynchrony between high septal pacing and apical pacing in patients with normal left ventricular systolic function. J Cardiol 2010;56:44–50. [DOI] [PubMed] [Google Scholar]
- 22. Van Israel N, French AT, Dukes‐McEwan J, et al. Review of left‐to‐right shunting patent ductus arteriosus and short term outcome in 98 dogs. J Small Anim Pract 2002;43:395–400. [DOI] [PubMed] [Google Scholar]
- 23. Stauthammer CD, Tobias AH, Leeder DB, et al. Structural and functional cardiovascular changes and their consequences following interventional patent ductus arteriosus occlusion in dogs: 24 cases (2000‐2006). J Am Vet Med Assoc 2013;242:1722–1726. [DOI] [PubMed] [Google Scholar]
- 24. Achen SE, Miller MW, Gordon SG, et al. Transarterial ductal occlusion with the Amplatzer vascular plug in 31 dogs. J Vet Intern Med 2008;22:1348–1352. [DOI] [PubMed] [Google Scholar]
- 25. Fuentes L. Echocardiography and Doppler ultrasound In: Tilley LP, Smith FWK, Jr, Oyama MA, Sleeper MM, eds. Manual of Canine and Feline Cardiology, 4th ed Saint Louis: W.B. Saunders; 2008:87–107. [Google Scholar]
- 26. Chetboul V, Serres F, Gouni V, et al. Radial strain and strain rate by two‐dimensional speckle tracking echocardiography and the tissue velocity based technique in the dog. J Vet Cardiol 2007;9:69–81. [DOI] [PubMed] [Google Scholar]
- 27. Barlow AJ, Ward C, Webber SA, et al. Myocardial contractility in premature neonates with and without patent ductus arteriosus. Pediatr Cardiol 2004;25:102–107. [DOI] [PubMed] [Google Scholar]
- 28. Park HE, Chang SA, Kim HK, et al. Impact of loading condition on the 2D speckle tracking‐derived left ventricular dyssynchrony index in nonischemic dilated cardiomyopathy. Circ Cardiovasc Imaging 2010;3:272–281. [DOI] [PubMed] [Google Scholar]


