Abstract
Background
Synthetic colloids are often used during fluid resuscitation and affect coagulation.
Objective
To compare the effects of an isotonic crystalloid and synthetic colloid on coagulation in healthy dogs and dogs with systemic inflammation.
Animals
Sixteen adult purpose‐bred Beagles.
Methods
Randomized, placebo‐controlled, blinded study. Dogs were randomized into one of two groups receiving fluid resuscitation with either 40 mL/kg IV 0.9% NaCl or tetrastarch after administration of lipopolysaccharide or an equal volume of placebo. After a 14‐day washout period, the study was repeated such that dogs received the opposite treatment (LPS or placebo) but the same resuscitation fluid. Blood samples were collected at 0, 1, 2, 4, and 24 hours for measurement of coagulation variables.
Results
Administration of either fluid to healthy dogs and dogs with systemic inflammation resulted in similar increases in prothrombin time and activated clotting time. In comparison to saline administration, tetrastarch administration resulted in significantly decreased R (P = .017) in healthy dogs, as well as significantly increased activated partial thromboplastin time (P ≤ .016), CL30% (P ≤ .016), and K (P < .001) and significantly decreased platelet count (P = .019), α (P ≤ .001), MA (P < .001), and von Willebrand factor antigen (P < .001) and collagen binding activity (P ≤ .003) in both healthy dogs and dogs with systemic inflammation.
Conclusions and Clinical Importance
Tetrastarch bolus administration to dogs with systemic inflammation resulted in a transient hypocoagulability characterized by a prolonged activated partial thromboplastin time, decreased clot formation speed and clot strength, and acquired type 1 von Willebrand disease.
Keywords: Lipopolysaccharide, Tetrastarch, Thromboelastography, von Willebrand factor
Abbreviations
- aPTT
activated partial thromboplastin time
- HES
hydroxyethyl starch
- LPS
lipopolysaccharide
- MW
molecular weight
- PT
prothrombin time
- TEG
thromboelastography
- vWF
von Willebrand factor
- α
angle
- ACT
activated clotting time
- CL30%
clot lysis index at 30 minutes
- CL60%
clot lysis index at 60 minutes
- FVIII
factor VIII
- G
global clot strength
Fluid therapy is a common treatment for hospitalized patients although there is debate regarding optimal fluid selection. Although crystalloids and colloids are both routinely recommended for use in critically ill animals, indications and adverse effects of these fluids have not been extensively investigated in dogs. General indications for colloid therapy include intravascular volume expansion for animals with hypovolemic or distributive shock, as well as animals with hypoalbuminemia and decreased oncotic pressure.1 Adverse effects have been associated with the use of certain synthetic colloids in humans including the induction of coagulopathies, impaired kidney function, and prolonged tissue storage resulting in pruritus.2, 3, 4 Several human studies have compared crystalloids and colloids used during fluid resuscitation, but have failed to identify a survival advantage for either type of fluid.5, 6, 7
Different synthetic colloids are available, but hydroxyethyl starches (HES) are the products routinely used in North America.5 Tetrastarch, a third generation HES, has a smaller molecular weight (MW) and lower degree of molar substitution, thereby exhibiting more rapid elimination and lack of accumulation compared to other HES.4, 8 Reports support a possible lesser impact of tetrastarch on coagulation compared to other larger MW HES products.3, 4, 9 Adverse effects of HES on hemostasis in humans are characterized by decreased factor VIII (FVIII) and von Willebrand factor (vWF) activity, dilution of clotting factors, decreased expression of integrin αIIbβ3 on activated platelets leading to decreased aggregation, decreased fibrinogen polymerization, acquired fibrinogen deficiency, and impaired fibrinolysis.3, 4, 10 Studies in vitro and in vivo using thromboelastography (TEG) and similar viscoelastic devices have been performed in humans to evaluate the effect of HES on hemostasis.10, 11, 12 Progressive in vitro hemodilution with tetrastarch produces a hypocoagulable TEG profile in people.13, 14, 15 To date, there are no published veterinary studies investigating the in vivo effects of HES on coagulation in dogs using TEG.
The primary objective of this study was to compare the effects of an isotonic crystalloid (0.9% NaCl) and synthetic colloid (tetrastarch) on coagulation in healthy dogs and dogs with systemic inflammation. The secondary objective was to determine the correlation between TEG variables and traditional coagulation test results. The authors hypothesized that an IV bolus of tetrastarch would produce a hypocoagulable TEG profile compared to 0.9% NaCl administration in both healthy dogs and dogs with systemic inflammation. The authors also hypothesized that TEG variables would be correlated with traditional coagulation test results.
Materials and Methods
Animals
Sixteen healthy adult purpose‐bred Beagles were included in this study. All dogs were deemed healthy on the basis of a normal physical examination, CBC, biochemistry profile, prothrombin time (PT), and partial thromboplastin time (aPTT). The institution Animal Care Committee approved the study. Dogs were housed and handled according to the guidelines of the Canadian Council on Animal Care, the requirements of the Animals for Research Act Revised Statutes of Ontario, and the institution Animal Care Policy.
Experimental Design
A randomized, placebo‐controlled, blinded study was performed. Dogs were randomized into one of two fluid resuscitation groups for the entire study: 8 dogs received an isotonic crystalloid (0.9% NaCl)1 and 8 dogs received a synthetic colloid (tetrastarch).2 Dogs were randomized to first receive either 5 μg/kg, IV lipopolysaccharide (LPS, Escherichia coli 0127:B8)3 or an equal volume of placebo (0.9% NaCl)1 (T0) via a cephalic catheter.4 Thirty minutes later (T0.5), each dog received 40 mL/kg IV of the designated resuscitation fluid delivered over 30 minutes. The study was repeated with dogs receiving the opposite prefluid treatment (LPS or placebo) after a minimum 14‐day washout period during which no treatments were administered outside of normal husbandry and care. The first author was blinded to the type of fluid given and randomization was performed using an online number generating program.
Dogs were fasted 12 hours prior5 to the study and allowed free access to water. At the start of the experiment, butorphanol5 (0.2 mg/kg, IM) was given for analgesia and was repeated IV at 2 (T2) and 4 (T4) hours after baseline (T0). A 20‐gauge catheter4 was placed in the cephalic vein to enable IV administration of either placebo or LPS and butorphanol. At the completion of the experimental period (T6), the cephalic catheter was removed and the dogs were rehoused and offered food.
Sample Collection and Tests
Blood samples (5 mL) were collected atraumatically via jugular venipuncture at baseline (T0) and 1 (T1), 2 (T2), 4 (T4), and 24 (T24) hours after LPS or placebo administration. Blood samples were collected into a max‐ACT tube,6 EDTA tube,7 and two plastic vacutainer tubes with sodium citrate (3.2% trisodium citrate and blood in a 1 : 9 ratio).8 The max‐ACT tube was used immediately to determine the activated clotting time (ACT) by the axillary method as described previously.16 The EDTA blood was used for white blood cell and platelet count, which were performed at a commercial laboratory using an automated analyzer with laser light scatter and cytochemical staining methods.9 One of the two vacutainer tubes containing sodium citrate was stored at room temperature for 30 minutes and used for TEG analysis and the other was centrifuged immediately at 700g for 15 minutes. The plasma was then removed and stored at −70°C for batch analysis of PT, aPTT, FVIII activity, von Willebrand factor antigen activity (vWFAg) and collagen binding activity (vWFCBA), and D‐dimer concentration.
Coagulation Testing
PT and aPTT were performed at the coagulation laboratory at the Foster Hospital for Small Animals at Tufts University. PT and aPTT were both determined by means of an automated analyzer10 and commercially available reagents,11 , 12 as described previously.17 Reference intervals were based on pooled plasma obtained from 35 healthy dogs. FVIII activity, vWFAg and vWFCBA, and D‐dimer concentration were measured at the Comparative Coagulation Section of the Animal Health Diagnostic Center at Cornell University. FVIII activity was performed in a modified one‐stage aPTT technique using a semiautomated clot detection instrument13 and canine congenital FVIII deficient substrate plasma, as previously described.18, 19 vWFAg was measured by ELISA with monoclonal anticanine vWF antibodies.20 vWFCBA was measured by ELISA with bovine mixed, types I and III, collagen and the same monoclonal anticanine vWF antibodies.,14 21 The standard curves for all factor assays were derived from dilutions of a pooled canine plasma standard, prepared from 15 healthy dogs and stored in single‐use aliquots at −70°C. Plasma D‐dimer concentration was measured in a quantitative, immunoturbidometric method using a commercial kit.15 The assay standard was generated from dilutions of the manufacturer's human D‐dimer calibrator.
Thromboelastography
TEG was performed using a commercial analyzer16 running kaolin17 activated samples 30 minutes after sample collection. CaCl2 18 (20 μL) was added to 340 μL of the activator and citrated whole blood mixture, for a total volume of 360 μL per cup.19 The TEG analyses were run for a maximum of 90 minutes or until R, K, α, MA, global clot strength (G), clot lysis index at 30 minutes (CL30%), and clot lysis rate at 30 minutes (LY30%) values were obtained. G represents the global clot strength and was calculated from the MA (G = 5,000 × MA/[100‐MA]). When possible, clot lysis index at 60 minutes (CL60%), and clot lysis rate at 60 minutes (LY60%) values were also recorded. TEG results were compared with a reference interval established at the same institution from analysis of 40 healthy dogs.22
Statistical Methods
A Latin square design with repeated measures over time and a generalized linear mixed‐model was employed to analyze the data using proc mixed. The AKAIKE information criterion (AIC) was used to determine an error structure for the auto‐regression. The assumptions of the ANOVA were assessed by comprehensive residual analyses. Shapiro–Wilk test was conducted to analyze for normal distribution. Residuals were plotted against predicted values and explanatory variables (treatment, fluid, time, dogs) to look for outliers or unequal variance. If residual analyses suggested a need for data transformation, logarithmic transformation was done before data analysis. If comparisons of the factors “treatment” and “time” were significant, differences between dogs receiving 0.9% NaCl or tetrastarch were compared using paired t‐tests. The level of significance for the t‐tests was corrected using a Tukey's or Dunnett's test. Significance was set at P < .019 for all variables. Correlations between ACT, platelet count, PT, aPTT, FVIII activity, vWFAg, vWFCBA, and D‐dimer concentration, and individual TEG variables were performed via a Pearson correlation for normally distributed data and a Spearman correlation for nonnormally distributed data. The authors categorized correlation as follows: r ≥ 0.95 excellent, r ≥ 0.85 to 0.94 very good, r ≥ 0.75 to 0.84 good, r ≥ 0.50 to 0.74 fair, and r < 0.50 poor. Significance was set at P < .05 for all correlations. Commercially available software was used for the statistical analyses20 and graph generation.21
Results
All dogs were female (7 intact, 9 spayed) ranging in age from 16 to 44 months (median 18 months). The median body weight was 8.85 kg (range: 7.8–11 kg).
Healthy Dogs (Placebo Phase)
Compared to dogs given a fluid bolus of 0.9% NaCl, dogs given tetrastarch had a decreased platelet count (mean difference = −63.40, C.I. = −116.06 to −10.74, P = .019, Fig. 1A) and increased aPTT (mean difference = 3.39, C.I. = 1.50–5.29, P < .001, Fig. 1C) at T1, decreased vWFAg at T1 (mean difference = −0.86, C.I. = −1.23 to −0.50, P < .001) and T2 (mean difference = −0.67, C.I. = −1.03 to −0.30, P < .001) (Fig. 1E), and decreased vWFCBA at T1 (mean difference = −37.33, C.I. = −62.10 to −12.56, P = .003) and T2 (mean difference = −38.67, C.I. = −63.44 to −13.90, P = .003) (Fig. 1G). There was no significant difference in ACT, PT, FVIII activity, vWFAg/CBA ratio, or D‐dimer concentration between fluid groups.
Figure 1.
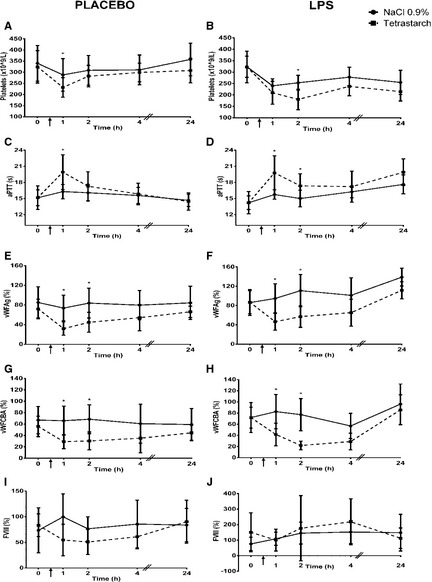
Comparison of platelet count (Reference interval: 117–418 × 109/L), aPTT (Reference interval: 10–20 seconds), vWFAg (Reference interval: 50–108%), vWFCBA (Reference interval: > 50%), and FVIII (Reference interval: 50–200%) in dogs given 0.9% NaCl or tetrastarch after placebo or LPS administration. Time of fluid bolus represented as ↑. Significant differences between fluids (P < .019) are indicated with *. All values are expressed as mean ± SD.
Compared to dogs given 0.9% NaCl, dogs receiving tetrastarch had a decreased R (mean difference = −1.07, C.I. = −1.94 to −0.19, P = .017, Fig. 2A) and increased K (mean difference = 0.60, C.I. = 0.29–0.90, P < .001, Fig. 2C) at T1, decreased α between T1 (mean difference = −7.00, C.I. = −10.17 to −3.83, P < .001) and T2 (mean difference = −5.32, C.I. = −8.52 to −2.12, P = .001) (Fig. 2E), decreased MA between T1 (mean difference = −13.38, C.I. = −17.39 to −9.36, P < .001) and T4 (mean difference = −4.94, C.I. = −8.95 to −0.92, P = .016) (Fig. 2G), decreased G between T1 (mean difference = −3.60, C.I. = −4.92 to −2.28, P < .001) and T4 (mean difference = −1.87, C.I. = −3.19 to −0.55, P = .006) (Fig. 2I), increased CL30% between T1 (mean difference = 2.52, C.I. = 1.13–3.92, P < .001) and T4 (mean difference = 1.66, C.I. = 0.31–3.01, P = .016) (Fig. 3A), increased LY30% at T1 (mean difference = 10.37, C.I. = 4.88–15.87, P < .001) (Fig. 3C), increased CL60% at T2 (mean difference = 1.42, C.I. = 0.56–2.29, P = .002) (Fig. 3E), and increased LY60% between T2 (mean difference = 1.45, C.I. = 0.47–2.43, P = .004) and T4 (mean difference = 1.41, C.I. = 0.43–2.39, P = .005) (Fig. 3G).
Figure 2.
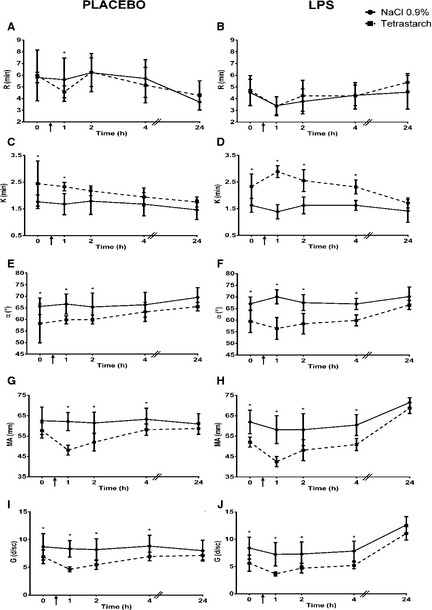
Comparison of kaolin‐activated R (Reference interval: 2–6 minutes), K (Reference interval: 1–3 minutes), α (Reference interval: 48–75°), MA (Reference interval: 46–64 mm), and G (Reference interval: 4.2–8.0K d/sc) in dogs given 0.9% NaCl or tetrastarch after placebo or LPS administration. Time of fluid bolus represented as ↑. Significant differences between fluids (P < .019) are indicated with *. All values are expressed as mean ± SD.
Figure 3.
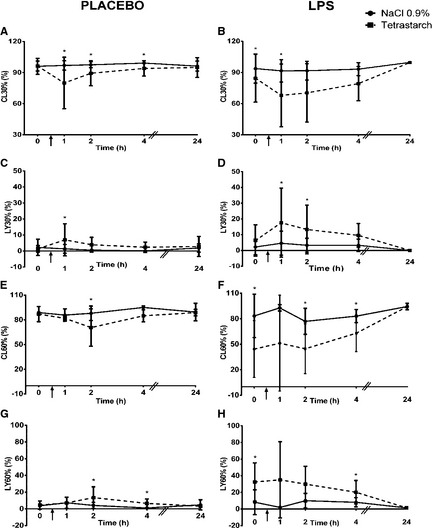
Comparison of kaolin‐activated CL30% (Reference interval: 92–100%), LY30% (Reference interval: 0–2%), CL60% (Reference interval: 85–100%), and LY60% (Reference interval: 0–15%) in dogs given 0.9% NaCl or tetrastarch after placebo or LPS administration. Time of fluid bolus represented as ↑. Significant differences between fluids (P < .019) are indicated with *. All values are expressed as mean ± SD.
When comparing TEG variables to traditional coagulation tests, vWFAg had fair correlation with MA (r = 0.521; P < .001) and G (r = 0.507; P < .001). Other TEG variables and traditional coagulation tests were either poorly or not correlated.
Dogs with Systemic Inflammation (LPS Phase)
Compared to dogs given 0.9% NaCl, dogs given tetrastarch had a decreased platelet count at T2 (mean difference = −62.22, C.I. = −114.01 to −10.43, P = .019, Fig. 1B), increased aPTT at T1 (mean difference = 3.38, C.I. = 2.05–5.84, P < .001) and T2 (mean difference = 2.30, C.I. = 0.43–4.16, P = .016) (Fig. 1D), decreased vWFAg at T1 (mean difference = −0.68, C.I. = −1.04 to −0.32, P < .001) and T2 (mean difference = −0.65, C.I. = −1.01 to −0.28, P < .001) (Fig. 1F), and decreased vWFCBA at T1 (mean difference = −39.40, C.I. = −63.72 to −15.08, P = .002) and T2 (mean difference = −40.03, C.I. = −64.35 to −15.70, P = .002) (Fig. 1H). There was no significant difference in ACT, PT, FVIII, vWFAg/CBA ratio, or D‐dimer concentration between dogs in either fluid group.
There was no significant difference in R between fluid groups (Fig. 2B). Compared to dogs given 0.9% NaCl, dogs given tetrastarch had increased K (T1 mean difference = 1.49, C.I. = 1.20–1.79; T2 mean difference = 0.89, C.I. = 0.59–1.18; T4 mean difference = 0.64, C.I. = 0.35–0.94; Fig. 2D) and decreased α (T1 mean difference = −13.30, C.I. = −16.40 to −10.20; T2 mean difference = −9.04, C.I. = −12.13 to −5.94; T4 mean difference = −6.05, C.I. = −9.15 to −2.96; Fig. 2F), MA (T1 mean difference = −16.27, C.I. = −20.23 to −12.32; T2 mean difference = −9.72, C.I. = −13.67 to −5.77; T4 mean difference = −8.93, C.I. = −12.89 to −4.98; Fig. 2H), and G (T1 mean difference = −3.56, C.I. = −4.86 to −2.26; T2 mean difference = −2.43, C.I. = −3.73 to −1.13; T4 mean difference = −2.37, C.I. = −3.67 to −1.06; Fig. 2J) between T1 (P < .001) and T4 hours (P < .001). Dogs given tetrastarch also had increased CL30% at T1 (mean difference = 1.67, C.I. = 0.34–2.99, P = .014) (Fig. 3B), increased LY30% at T1 (mean difference = 10.64, C.I. = 5.50–15.80, P < .001) and T2 (mean difference = 7.39, C.I. = 2.24–12.54, P = .005) (Fig. 3D), increased CL60% at T2 (mean difference = 1.21, C.I. = 0.36–2.05, P = .006) and T4 (mean difference = 1.24, C.I. = 0.38–2.10, P = .005) (Fig. 3F), and increased LY60% at T4 hours (mean difference = 1.22, C.I. = 0.25–2.19 P = .014) (Fig. 3H) compared to dogs administered 0.9% NaCl.
When comparing TEG variables to traditional coagulation tests, vWFAg had fair correlation with K (r = −0.560; P < .001), α (r = 0.578; P < .001), MA (r = 0.642; P < .001), and G (r = 0.642; P < .001). vWFCBA had fair correlation with MA (r = 0.506; P < .001). Other TEG variables and traditional coagulation tests were either poorly or not correlated.
All numeric values for the TEG variables in dogs given 0.9% NaCl or HES after placebo (Table S1) or LPS (Table S2) administration are included as supplemental information.
Discussion
The results of this study revealed that administration of tetrastarch was associated with an immediate decrease in platelet count and prolongation in aPTT compared to 0.9% NaCl. Given that tetrastarch administration has no known direct effects on platelet count,3, 4, 10 this suggests a greater hemodilution effect with tetrastarch compared to 0.9% NaCl administration. The blood volume expansion that is achieved with isotonic crystalloids and synthetic colloids differs as administration of the latter produces fluid recruitment from the interstitial compartment.23 In a recent systematic review of randomized controlled trials performed in humans when tetrastarch was given as part of fluid resuscitation, the amount of crystalloids required for correction of the volume deficit was 1.8 times that used when administering colloids.12 Unfortunately, considering the paucity of literature regarding the volume expansive effects of tetrastarch in dogs, a dosage of tetrastarch that would produce equal volume expansion to that obtained with 0.9% NaCl could not be determined for this study. Therefore, the same dosage for 0.9% NaCl and tetrastarch was chosen because although volume expansion would ultimately be greater and more sustained with tetrastarch, the immediate volume expansion of the intravascular compartment would be equivalent for both. The dilutional effect of HES on platelets and clotting factors leading to prolongation of coagulation times has been demonstrated in other in vivo human studies.24, 25 However, to date there are no published veterinary studies investigating the effect of HES on traditional coagulation tests.
Although FVIII activity was not statistically different between the two fluid groups, it was decreased after tetrastarch administration and increased after 0.9% NaCl administration. Therefore, the prolongation in aPTT might be secondary to an effect of tetrastarch on FVIII activity, thereby affecting the intrinsic pathway of coagulation, while the extrinsic pathway measured by PT was unaffected.
Interestingly, both vWFAg and vWFCBA were also decreased for less than 2 hours after tetrastarch administration compared to 0.9% NaCl administration and then returned to baseline, suggesting transient acquired type 1 von Willebrand disease. This is consistent with findings from studies in humans documenting mild acquired von Willebrand disease after administration of rapidly degradable low MW HES such as tetrastarch, with activities returning to normal 5 hours after administration.10 Decreased FVIII activity occurs in dogs with von Willebrand disease because of the stabilizing role of vWF on circulating FVIII levels;26 therefore, one would expect decreased FVIII activity would be seen in conjunction with the decreased vWFAg and vWFCBA. Conversely, two human studies reveal that tetrastarch doses up to 50–70 mL/kg IV were administered without changes in FVIII concentration.27, 28 Although FVIII activity decreased after tetrastarch administration in the present study, it was not statistically different from 0.9% NaCl administration. However, it is possible that this study was underpowered to detect significant changes in FVIII activity after tetrastarch administration.
In this study, TEG variables were indicative of increased hypocoagulability after tetrastarch administration compared to 0.9% NaCl. This is consistent with findings from in vitro studies in healthy humans and humans undergoing liver transplant surgery that found hypocoagulability characterized by increased R and K, and decreased α and MA after tetrastarch administration.13, 15 Interestingly, in vivo studies in humans reveal that similar hypocoagulable effects are only seen immediately after HES administration, but do not persist hours after the HES bolus.29, 30 This finding is attributed to the more rapid elimination of tetrastarch because of its lower MW and shorter half‐life compared to other HES.29, 30 Similarly, all TEG variables that were altered in dogs in the present study 1–4 hours after administration of tetrastarch, but returned to baseline 24 hours after the fluid bolus.
There are some veterinary studies investigating the effects of HES on coagulation in dogs that also confirm the development of hypocoagulabilty after HES administration. In vitro and in vivo reports demonstrate that higher MW HES are associated with platelet dysfunction characterized by prolonged closure time using PFA‐100.31, 32 In vitro dilution with a lower MW product HES 130/0.42 also causes hypocoagulability manifested by prolonged closure time using PFA‐100.33 However, a recent study investigating the effect of tetrastarch on coagulation in a hemorrhagic shock model in dogs found no difference in platelet function as assessed with PFA‐100.34 Another study used TEG to investigate the effect of HES on coagulation and found that tetrastarch had less impact on coagulation than hetastarch at higher dilutions in vitro, but still resulted in hypocoagulability characterized by changes in K, α, and MA.22 Similarly, in vitro dilution with tetrastarch caused hypocoagulability secondary to a dose‐dependent alteration in fibrinogen concentration and decreased platelet function using thromboelastometry.35 Whereas none of these studies investigate the in vivo effects of tetrastarch on TEG in dogs, they consistently show that in vitro hemostatic testing confirms a hypocoagulable effect of HES in dogs.
The changes in TEG variables CL30%, LY30%, CL60%, and LY60% observed in this study in dogs receiving tetrastarch are suggestive of hyperfibrinolysis. This has been previously reported in vivo in humans given higher MW HES solutions and is hypothesized to be secondary to incorporation of HES molecules into the blood clot, or to increased plasminogen activator activity.36 Despite evidence of hyperfibrinolysis, there was no difference in D‐dimer concentration in dogs receiving tetrastarch compared to dogs given 0.9% NaCl in the present study. Likewise, a recent case series of dogs with disseminated intravascular coagulation revealed no association between D‐dimer concentration and fibrinolytic TEG variables.37 Other assays of fibrinolysis such as fibrin degradation products, fibrinogen, or plasminogen concentrations could be measured in future studies to further assess the effect of tetrastarch on fibrinolysis.
In this study, traditional coagulation tests (platelet count, ACT, PT, aPTT, and D‐dimer concentration) had weak correlation with TEG variables. R is typically associated with soluble clotting factors, thus representing the intrinsic pathway of coagulation.38 Some TEG studies have reported good correlation between R and PT or aPTT.39, 40, 41 Similarly in a canine study using rotational thromboelastometry, PT was significantly correlated with coagulation time (similar to R), clot formation time (equivalent to K), α angle, and maximum clot firmness (similar to MA), but aPTT was not. Overall, rotational thromboelastometry results were not highly correlated with individual factor concentrations, while platelet concentration was correlated with clot formation time, α angle, and maximum clot firmness.42 In the present study, administration of tetrastarch was associated with prolongation of aPTT, but decreased R in healthy dogs and no change in R in dogs with LPS‐induced systemic inflammation. Similar findings were observed in a study comparing TEG variables in human volunteers administered either HES (200/0.5) or normal saline.25 The authors hypothesized that difference in methodology between TEG and PT or aPTT explained the differences observed, since TEG evaluates whole blood coagulation, whereas PT and aPTT are platelet‐poor plasma based assays.25 The slightly prolonged PT and aPTT after administration of HES (200/0.5) were attributed to dilution of coagulation factors in plasma, while decreases of R and K was explained by platelet activation or other cell‐mediated effects not assessed by cell‐depleted plasma assays.25 In the present study, platelet count was poorly correlated with MA, G, and α in healthy dogs. K and α are generally affected by platelet count and function, fibrinogen concentration and function, and clotting factor activity, whereas MA and G are influenced by platelet count and function, and fibrinogen concentration.43
This study has some limitations that warrant discussion. First, the small sample size rendered some of the analyses underpowered to detect significant differences. Likewise, some TEG variables had significant differences at baseline between dogs receiving 0.9% NaCl and those given tetrastarch. However, the subsequent significant changes at each time point in dogs given tetrastarch were in the opposite direction compared to dogs given 0.9% NaCl and at 24 hours after fluid administration, there were no longer significant differences between the two fluids. All dogs were deemed healthy based on physical examination, routine blood work, and PT and aPTT, but it is possible that subclinical diseases were missed that affected our results. More likely, type I error because of the small sample size created the baseline differences. Second, platelet function was not specifically assessed in this study. Therefore, the effect of tetrastarch on platelet function might have been underestimated. TEG is insensitive compared to flow cytometry and aggregometry at assessing platelet function in people.44 Other techniques used to evaluate platelet function including platelet aggregometry, PFA‐100, flow cytometry, and TEG platelet mapping45 should be considered in future studies investigating the coagulation effects of HES in dogs. Third, all dogs enrolled in this study were female and almost evenly distributed between intact and spayed. Influence of gender on viscoelastic evaluation of hemostasis has been demonstrated in people resulting in recommendations to report different reference intervals for each sex;46, 47 however, no such difference has been found in dogs.48 To date, the influence of reproductive hormones on TEG in intact dogs has not been investigated. Fourth, the washout period was selected based on the plasma terminal half‐life (t 1/2ß) of tetrastarch in humans, which is 12.1 hours after administration of a single dose of 500 mL.49 This information is not available for dogs and the presence of persistent circulating tetrastarch molecules for longer than the 14‐day washout period cannot be excluded. Fifth, it is also important to consider that while an LPS model was used in the present study to mimic the systemic changes encountered during naturally occurring sepsis,50, 51, 52, 53 it does not necessarily replicate all changes that occur in hospitalized dogs. Sixth, when interpreting TEG results, it is also important to consider the effects of fibrinogen and hematocrit. Fibrinogen was not measured. Packed cell volume was measured in these dogs for a separate study,54 which revealed that packed cell volume was decreased after fluid administration, but remained within the reference interval. Considering the reported hypercoagulable effect of a decreasing hematocrit on TEG results,23,24 the hypocoagulability noted after tetrastarch administration in the present study remains relevant. Finally, the additional dilution effect garnered by the increased volume expansion of the tetrastarch in comparison to the 0.9% NaCl cannot be ruled out as contributing to the hemostatic changes, irrespective of the direct effects on coagulation factors. Moreover, the rapid infusion of 40 mL/kg of tetrastarch IV over 30 minutes does not necessarily reflect clinical practice. As such, the results from the present study cannot necessarily be extrapolated to all hospitalized dogs with systemic inflammation or naturally occurring sepsis receiving synthetic colloids.
Conclusions
Healthy dogs and dogs with LPS‐induced systemic inflammation administered tetrastarch had prolonged aPTT, hypocoagulable and hyperfibrinolytic TEG variables, and acquired type 1 von Willebrand disease (decreased vWFAg and vWFCBA) compared to dogs given 0.9% NaCl, which resolved within 4 hours. Most coagulation variables were within the reference interval, which makes the hypocoagulable changes associated with tetrastarch administration of uncertain clinical significance and precludes any absolute contraindication to the use of tetrastarch in healthy dogs or dogs with systemic inflammation. Future studies investigating the effect of other doses or constant rate infusions of tetrastarch on coagulation, as well as in dogs with naturally occurring sepsis, are warranted and more sensitive methods for assessing platelet dysfunction should be included.
Supporting information
Table S1. Comparison of TEG variables in 16 dogs receiving NaCl 0.9% or tetrastarch after placebo administration. Significant differences (P < .019) indicated in bold. All values are expressed as mean ± SD.
Table S2. Comparison of TEG variables in 16 dogs receiving NaCl 0.9% or tetrastarch after LPS administration. Significant differences (P < .019) indicated in bold. All values are expressed as mean ± SD.
Acknowledgments
The authors thank Jessica Leader, Mandy Hathway, and Hiroshi Fujita for their assistance with data collection, as well as Gabrielle Monteith and William Sears for their help with statistical analyses.
This study was funded by the Ontario Veterinary College Pet Trust Fund.
Conflict of Interest: Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
This study was performed at the Ontario Veterinary College of the University of Guelph.
Presented in abstract form at the International Veterinary Emergency and Critical Care Society meeting in San Antonio, TX, September 2012.
Footnotes
0.9% NaCl, Baxter, Mississauga, ON, Canada
Voluven, Fresenius Kabi, Mississauga, ON, Canada
Escherichia coli serotype 0127:B8, Sigma‐Aldrich, St. Louis, MO
Torbugesic, Fort Dodge Animal Health, NY
BD Insyte Autoguard catheter, BD Medical, Sandy, UT
Max‐ACT test tubes, Helena Laboratories, Beaumont, TX
Capiject EDTA microcollection tubes, Terumo, Somerset, NJ
BD Vacutainer Plus plastic citrate, Becton, Dickinson, and Company, Franklin Lakes, NJ
Advia 2120, Siemens Medical Solutions Diagnostics, Tarrytown, NY
ACL Elite, Instrumentation Laboratory, Bedford, MA
RecombiPlasTin 2G, Instrumentation Laboratory, Bedford, MA
SynthASil, Instrumentation Laboratory, Bedford, MA
ST4, Diagnostica Stago Inc., Parsippany, NJ
Vitrogen, Angiotech BioMaterials Corporation, Palo Alto, CA
HemosIL D‐dimer, Instrumentation Laboratory, Bedford, MA
TEG, Haemoscope Corporation, Niles, IL
Kaolin, Haemoscope Corporation, Niles, IL
Calcium chloride, Haemoscope Corporation, Niles, IL
Plain cups and pins, Haemoscope Corporation, Niles, IL
SAS Online v.9.1.3, SAS Institute, Cary, NC
Prism 5, GraphPad Software, La Jolla, CA
Bacek LM, Martin LG, Spangler EA, Macintire DK. Determination of the in vitro effects of two forms of hydroxyethyl starch solutions on thromboelastography and coagulation parameters in healthy dogs [abstract]. J Vet Emerg Crit Care 2012; 21(s1):S2
Vilar P, Hansell J, Westendorf N, et al. Effects of hematocrit on thromboelastography tracings in dogs [abstract]. J Vet Int Med 2008; 22(3):774
Jacquith SD, Brown AJ, Scott MA. Effects of decreased hematocrit on canine thromboelastography [abstract]. J Vet Emerg Crit Care 2009; 19(s1):A4
References
- 1. Hugues D, Boag A. Fluid therapy with macromolecular plasma volume expanders In: DiBartola SP, ed. Fluid, electrolyte, and acid‐base disorders in small animal practice. St. Louis: Elsevier Saunders; 2012:655–657. [Google Scholar]
- 2. Dart AB, Mutter TC, Ruth CA, Taback SP. Hydroxyethyl starch (HES) versus other fluid therapies: Effects on kidney function (Review). Cochrane Database Syst Rev 2010;1:CD007594. [DOI] [PubMed] [Google Scholar]
- 3. Kozek‐Langenecker SA. Effects of hydroxyethyl starch solutions on hemostasis. Anesthesiology 2005;103:654–660. [DOI] [PubMed] [Google Scholar]
- 4. Westphal M, James MFM, Kozek‐Langenecker S, et al. Hydroxyethyl starches: Different products – different effects. Anesthesiology 2009;111:187–202. [DOI] [PubMed] [Google Scholar]
- 5. Bayer O, Reinhart K, Kohl M, et al. Effects of fluid resuscitation with synthetic colloids or crystalloids alone on shock reversal, fluid balance, and patient outcomes in patients with severe sepsis: A prospective sequential analysis. Crit Care Med 2012;40:2543–2551. [DOI] [PubMed] [Google Scholar]
- 6. Perner A, Haase N, Guttormsen AN, et al. Hydroxyethyl starch 130/0.42 versus Ringer's acetate in severe sepsis. N Engl J Med 2012;367:124–134. [DOI] [PubMed] [Google Scholar]
- 7. Myburgh JA, Finfer S, Bellomo R, et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med 2013;368:775. [DOI] [PubMed] [Google Scholar]
- 8. Goggs R, Humm K, Hughes D. Fluid therapy in small animals: 3 Colloid solutions. In Practice 2008;30:136–142. [Google Scholar]
- 9. Sossdorf M, Marx S, Schaarschmidt B, et al. HES 130/0.4 impairs haemostasis and stimulates pro‐inflammatory blood platelet function. Crit Care 2009;13:R208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kozek‐Langenecker SA, Scharbert G. Effects of hydroxyethyl starch solutions on hemostasis. Transfus Altern Transfus Med 2007;9:173–181. [Google Scholar]
- 11. Jamnicki M, Bombeli T, Seifert B, et al. Low‐ and medium‐molecular‐weight hydroxyethyl starches: Comparison of their effect on blood coagulation. Anesthesiology 2000;93:1231–1237. [DOI] [PubMed] [Google Scholar]
- 12. Hartog CS, Reuter D, Loesche W, et al. Influence of hydroxyethyl starch (HES) 130/0.4 on hemostasis as measured by viscoelastic device analysis: A systematic review. Intensive Care Med 2011;37:1725–1737. [DOI] [PubMed] [Google Scholar]
- 13. Jamnicki M, Zollinger A, Seifert B, et al. Compromised blood coagulation: An in vitro comparison of hydroxyethyl starch 130/0.4 and hydroxyethyl starch 200/0.5 using thrombelastography. Anesth Analg 1998;87:989–993. [DOI] [PubMed] [Google Scholar]
- 14. Schols SE, Feijge MA, Lance MD, et al. Effects of plasma dilution on tissue‐factor induced thrombin generation and thromboelastography: Partly compensating role of platelets. Transfusion 2008;48:2384–2394. [DOI] [PubMed] [Google Scholar]
- 15. Bang SR, Kim YH, Kim GS. The effects of in vitro hemodilution with 6% hydroxyethyl starch (HES) (130/0.4) solution on thrombelastograph analysis in patients undergoing liver transplantation. Clin Transplant 2010;25:450–456. [DOI] [PubMed] [Google Scholar]
- 16. Bateman SW, Mathews KA. Comparison of axillary and heating block methods of activated clotting time (ACT) in dogs. J Vet Emerg Crit Care 1999;9:79–82. [Google Scholar]
- 17. Iazbik C, Couto CG, Gray TL, Kociba G. Effect of storage conditions on hemostatic parameters of canine plasma obtained for transfusion. Am J Vet Res 2001;62:734–735. [DOI] [PubMed] [Google Scholar]
- 18. Stokol T, Brooks MB, Erb HN. Effect of citrate concentration on coagulation test results in dogs. J Am Vet Med Assoc 2000;217:1672–1677. [DOI] [PubMed] [Google Scholar]
- 19. Wardrop KJ, Brooks MB. Stability of hemostatic proteins in canine fresh frozen plasma units. Vet Clin Pathol 2001;30:91–95. [DOI] [PubMed] [Google Scholar]
- 20. Benson RE, Catalfamo JL, Brooks MB, Dodds WJ. A sensitive immunoassay for von Willebrand factor. J Immunoassay 1991;12:371–390. [DOI] [PubMed] [Google Scholar]
- 21. Sabino EP, Erb H, Catalfamo JL. Development of a collagen binding activity assay as a screening test for type II VWD in dogs. Am J Vet Res 2006;67:242–249. [DOI] [PubMed] [Google Scholar]
- 22. Flint SK, Wood RD, Abrams‐Ogg AC, et al. Comparison of citrated native and kaolin‐activated samples for thromboelastographic analysis in healthy dogs. Vet Clin Path 2012;41:249–255. [DOI] [PubMed] [Google Scholar]
- 23. Silverstein DC, Aldrich J, Haskins SC, et al. Assessment of changes in blood volume in response to resuscitative fluid administration in dogs. J Vet Emerg Crit Care 2005;15:185–192. [Google Scholar]
- 24. Eriksen C, Tønnesen E, Ingerslev J, Sørensen B. Mechanisms of hydroxyethyl starch‐induced dilutional coagulopathy. J Thromb Haemost 2009;7:1099–1105. [DOI] [PubMed] [Google Scholar]
- 25. Ruttmann TG, James MFM, Aronson I. In vivo investigation into the effects of haemodilution with hydroxyethyl starch (200‐0.5) and normal saline on coagulation. Br J Anesth 1998;80:612–616. [DOI] [PubMed] [Google Scholar]
- 26. Brinkhous KM, Sandberg H, Garris JB, et al. Purified human factor VIII procoagulant protein: Comparative hemostatic response after infusions into hemophilic and von Willebrand disease dogs. Proc Natl Acad Sci USA 1985;82:8752–8756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kasper SM, Meinert P, Kampe S, et al. Large‐dose hydroxyethyl starch 130/0.4 does not increase blood loss and transfusion requirements in coronary artery bypass surgery compared with hydroxyethyl starch 200/0.5 at recommended doses. Anesthesiology 2003;99:42–47. [DOI] [PubMed] [Google Scholar]
- 28. Neff TA, Doelberg M, Jungheinrich C, et al. Repetitive large‐dose infusion of the novel hydroxyethyl starch 130/0.4 in patients with severe head injury. Anesth Analg 2003;96:1453–1459. [DOI] [PubMed] [Google Scholar]
- 29. Felfernig M, Franz A, Bräunlich P, et al. The effects of hydroxyethyl starch solutions on thromboelastography in preoperative male patients. Acta Anaesthesiol Scand 2003;47:70–73. [DOI] [PubMed] [Google Scholar]
- 30. Jin SL, Yu BW. Effects of acute hypervolemic fluid infusion of hydroxyethyl starch and gelatin on hemostasis and possible mechanisms. Clin Appl Thromb Hemost 2010;16:91–98. [DOI] [PubMed] [Google Scholar]
- 31. Wieranga JR, Jandrey KE, Haskins SC, Tablin F. In vitro comparison of the effects of two forms of hydroxyethyl starch solutions on platelet function in dogs. Am J Vet Res 2007;68:605–609. [DOI] [PubMed] [Google Scholar]
- 32. Smart L, Jandrey KE, Kass PH, et al. The effect of Hetastarch (670/0.75) in vivo on platelet closure time in the dog. J Vet Emerg Crit Care 2009;19:444–449. [DOI] [PubMed] [Google Scholar]
- 33. Classen J, Adamik KN, Weber K, et al. In vitro effect of hydroxyethyl starch 130/0.42 on canine platelet function. Am J Vet Res 2012;73:1908–1912. [DOI] [PubMed] [Google Scholar]
- 34. McBride D, Hosgood GL, Mansfield CS, Smart L. Effect of hydroxyethyl starch 130/0.4 and 200/0.5 solutions on canine platelet function in vitro. Am J Vet Res 2013;74:1133–1137. [DOI] [PubMed] [Google Scholar]
- 35. Falco S, Bruno B, Maurella C, et al. In vitro evaluation of canine hemostasis following dilution with hydroxyethyl starch (130/0.4) via thromboelastometry. J Vet Emerg Crit Care 2012;22:640–645. [DOI] [PubMed] [Google Scholar]
- 36. Strauss RG, Pennell BJ, Stump DC. A randomized, blinded trial comparing the hemostatic effects of pentastarch versus hetastarch. Transfusion 2002;42:27–36. [DOI] [PubMed] [Google Scholar]
- 37. Wiinberg B, Jensen AL, Johansson PI, et al. Thromboelastographic evaluation of hemostatic function in dogs with disseminated intravascular coagulation. J Vet Intern Med 2008;22:357–365. [DOI] [PubMed] [Google Scholar]
- 38. Zuckerman L, Cohen E, Vagher JP, et al. Comparison of thrombelastography with common coagulation tests. Thromb Haemost 1981;46:752–756. [PubMed] [Google Scholar]
- 39. Alexander DC, Butt WW, Best JD, et al. Correlation of thromboelastography with standard tests of anticoagulation in paediatric patients receiving extracorporeal life support. Thromb Res 2010;125:387–392. [DOI] [PubMed] [Google Scholar]
- 40. Martini WZ, Cortez DS, Dubick MA, et al. Thromboelastography is better than PT, aPTT, and activated clotting time in detecting relevant clotting abnormalities after hypothermia, hemorrhagic shock and resuscitation in pigs. J Trauma 2008;65:535–543. [DOI] [PubMed] [Google Scholar]
- 41. Wagg CR, Boysen SR, Bédard C. Thromboelastography in dogs admitted to an intensive care unit. Vet Clin Pathol 2009;38:453–461. [DOI] [PubMed] [Google Scholar]
- 42. Smith SA, McMichael MA, Gilor S, et al. Correlation of hematocrit, platelet concentration, and plasma coagulation factors with results of thromboelastometry in canine whole blood samples. Am J Vet Res 2012;73:789–798. [DOI] [PubMed] [Google Scholar]
- 43. Katori N, Tanaka KA, Szlam F, Levy JH. The effects of platelet count on clot retraction and tissue plasminogen activator‐induced fibrinolysis on thrombelastography. Anesth Analg 2005;100:1781–1785. [DOI] [PubMed] [Google Scholar]
- 44. Bowbrick VA, Mikhailidis DP, Stansby G. Value of thromboelastography in the assessment of platelet function. Clin Appl Thromb Hemost 2003;9:137–142. [DOI] [PubMed] [Google Scholar]
- 45. Jandrey KE. Assessment of platelet function. J Vet Emerg Crit Care 2012;22:81–98. [DOI] [PubMed] [Google Scholar]
- 46. Sucker C, Tharra K, Litmathe J, et al. Rotation thromboelastography (ROTEM) parameters are influenced by age, gender, and oral contraception. Perfusion 2011;26:336–340. [DOI] [PubMed] [Google Scholar]
- 47. Armstrong S, Fernando R, Ashpole K, et al. Assessment of coagulation in the obstetric population using ROTEM thromboelastometry. Int J Obstet Anesth 2011;20:293–298. [DOI] [PubMed] [Google Scholar]
- 48. Bauer N, Eralp O, Moritz A. Establishment of reference intervals for kaolin‐activated thromboelastography in dogs including an assessment of the effects of sex and anticoagulant use. J Vet Diagn Invest 2009;21:641–648. [DOI] [PubMed] [Google Scholar]
- 49. Waitzinger J, Bepperling F, Pabst G, et al. Pharmacokinetics and tolerability of a new hydroxyethyl starch (HES) specification [HES (130/0.4)] after single‐dose infusion of 6% or 10% solutions in healthy volunteers. Clin Drug Invest 1998;16:151–160. [DOI] [PubMed] [Google Scholar]
- 50. Aksu U, Bezemer R, Demirci C, Ince C. Acute effects of balanced versus unbalanced colloid resuscitation on renal macrocirculatory and microcirculatory perfusion during endotoxemic shock. Shock 2012;37:205–209. [DOI] [PubMed] [Google Scholar]
- 51. Baum TD, Wang H, Rothschild HR, et al. Mesenteric oxygen metabolism, ileal mucosal hydrogen ion concentration, and tissue edema after crystalloid or colloid resuscitation in porcine endotoxin shock: Comparison of Ringer's lactate and 6% hetastarch. Circ Shock 1990;30:385–397. [PubMed] [Google Scholar]
- 52. Hoffmann JN, Vollmar B, Laschke MW, et al. Hydroxyethyl starch (130 kD), but not crystalloid volume support, improves microcirculation during normotensive endotoxemia. Anesthesiology 2002;97:460–470. [DOI] [PubMed] [Google Scholar]
- 53. Schäper J, Ahmed R, Schäfer T, et al. Volume therapy with colloid solutions preserves intestinal microvascular perfusion in endotoxaemia. Resuscitation 2008;76:120–128. [DOI] [PubMed] [Google Scholar]
- 54. Gauthier V, Holowaychuk MK, Kerr CL, et al. Effect of synthetic colloid administration on hemodynamic and laboratory variables in healthy dogs and dogs with systemic inflammation. J Vet Emerg Crit Care 2014;24:251–258. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Comparison of TEG variables in 16 dogs receiving NaCl 0.9% or tetrastarch after placebo administration. Significant differences (P < .019) indicated in bold. All values are expressed as mean ± SD.
Table S2. Comparison of TEG variables in 16 dogs receiving NaCl 0.9% or tetrastarch after LPS administration. Significant differences (P < .019) indicated in bold. All values are expressed as mean ± SD.


