Abstract
Background
Rapid determination of blood electrolyte concentrations can help determine electrolyte status and delivery of effective volume of electrolyte solutions in field conditions.
Objective
To evaluate reliability of the i‐STAT, a point‐of‐care (POC) device, in measuring blood K+, Na+, and CI − concentrations in cattle.
Animals
Ninety‐eight cattle with various diseases.
Methods
In this prospective study, blood samples collected from the jugular vein were processed for determination of K+, Na+, and CI − concentrations in blood and plasma using the i‐STAT and auto‐analyzer (Cobas C501), respectively. Blood and plasma electrolyte data were subjected to student t‐test for comparison, the concordance analysis for agreement, accuracy, and precision, the Passing‐Bablok regression and the Bland‐Altman plot for reliability, and receiver operating characteristics curves for sensitivity (Se) and specificity (Sp).
Results
Plasma concentrations of K+ (4.39 versus 4.2 mmol/L; P < .0001) and CI − (100.30 versus 99.4 mmol/L; P < .04) were greater than their concentrations in blood. Plasma and blood Na+ concentrations were similar (136.95 versus 136.8 mmol/L). The i‐STAT results were highly correlated with the Cobas C501 results (r = 0.970, 0.922, and 0.866 for K+, Na+, and CI −, respectively). Regression equations fitting blood (Y) and plasma (X) concentration did not deviate from the identity line for K+ (Y = −0.10 + 0.98 × X), Na+ (Y = X), and CI − (Y = 3.04 + 0.96 × X). The mean bias (blood concentration ‐ plasma concentration) was −0.20 for K+ (P = .03), −0.16 for Na+ (P = .12), and −0.87 for CI − (P = .93). The i‐STAT had 76–100% Se and 87.7–100% Sp for assessing electrolyte statuses.
Conclusions and Clinical Importance
The i‐STAT yielded results that were in agreement with the auto‐analyzer, with negligible biases in measurement of plasma K+, Na+, and CI − concentrations. The i‐STAT is a reliable POC device and can be used in field condition to assess electrolyte status in cattle.
Keywords: Accuracy, Bovine, Point‐of‐care device, Precision, Sensitivity, Specificity
Abbreviations
- CI−
chloride
- K+
potassium
- Na+
sodium
- POC
point‐of‐care
- ROC
receiver operating characteristics
- RSD
residual standard deviation
- Se
sensitivity
- Sp
specificity
Introduction
Evaluation of serum or plasma electrolytes is important part of the blood chemistry profile in cattle. Potassium (K+) and sodium (Na+) are the major intracellular and extracellular cations, respectively.1 Chloride (Cl−) is the major anion in extracellular fluid.1, 2 Changes in serum/plasma chloride concentration are generally parallel to those of sodium.2
Changes in blood electrolytes concentrations can occur in different diseases of cattle.3, 4, 5 Blood electrolytes are useful in the evaluation of body systems and implementing fluid and electrolyte treatment. Recognition of and monitoring disturbed electrolyte status is important for effective clinical interventions. Blood electrolyte concentration measurements have been performed by clinical chemistry laboratories at hospitals, which cannot be convenient to many field practitioners. In recent years, hand‐held analyzers, also known as point‐of‐care (POC) devices, have been produced to measure blood electrolyte concentrations quickly. The i‐STAT1 is extensively used in veterinary hospitals and ambulatory clinics. A study conducted on healthy cattle (n = 24) showed that i‐STAT yielded K+ and Na+ concentrations in agreement with the reference method.6 However, its reliability should be assessed in cattle a wide range of electrolyte concentrations. The purpose of this study was to evaluate the reliability the i‐STAT in assessment of blood K+, Na+, and CI− concentrations using plasma K+, Na+, and CI− measured by a conventional auto‐analyzer.2
Materials and Methods
Animals
Ninety‐eight cattle in different breed and ages [Simmental (n = 63), Swiss Brown (n = 26), Holstein (n = 9), ranging from 1 day to 9 years of age] referred to Firat University Teaching and Training Animal Hospital were used in this experiment. Animals were tentatively diagnosed to have various diseases [alimentary disorders (n = 42, traumatic reticuloperitonitis, simple indigestion, enteritis, abomasal ulcer, vagus indigestion), neonatal problems (n = 29, neonatal infection, neonatal diarrhea, cleft palate), respiratory disorders (n = 10, pneumonia, aspiration pneumonia), theileriosis (n = 6), mastitis (n = 4), ketosis (n = 2), uterine torsion (n = 2), pericarditis traumatica (n = 1), otitis media (n = 1), and tetanus (n = 1)]. No animal was excluded in order to obtain an extreme electrolyte range. In order to achieve reliable regression delivering acceptable R 2 and minimizing bias, animals were enrolled prospectively until meeting sample size and power calculations. Approval for all procedures was obtained from the Firat University Ethics Committee on Animal Experimentation.
Blood Samples and Measurements
Blood samples were collected from the jugular vein of all cattle into blood collection tubes containing lithium heparin via vacutainer device.3 A portion of blood samples was used for immediate determination of blood K+, Na+, and CI− concentrations using the i‐STAT equipped with CHEM8+ cartridges.4 The remaining blood was immediately centrifuged5 at 3,000 × g for 15 minutes at 4°C to harvest plasma. Plasma samples were stored −20°C for plasma K+, Na+, and CI− concentration measurement within 3 days using an auto‐analyzer. The CHEM8+ cartridges were stored in a refrigerator (4–8°C) and the cartridges were used before the expiration date. Each cartridge was allowed to warm for 10 minutes in the ambient temperature before using. The i‐STAT control solutions6 were administered to 2 cartridges out of each cartridge batch. The i‐STAT and auto‐analyzer were calibrated and used in accordance with their manufacturers’ specifications.
Statistical Analysis
Sample size7 was calculated for K+ concentration, the main interest, using available free software.7 In order to reject the null hypothesis 64 observations were needed at Type I error (α) of 0.001 and Type II error (power, β) of 0.99. Entered standard deviation, standard deviation of the regression error, and slope of the regression line were computed to be 5.0, 6.0, 0.95, respectively in the sample size calculation.
After attaining normal distribution by the Kolmogorov‐Smirnov test8 data were subjected to paired Student t‐test and Box‐and‐Whisker Plot distribution for comparison of data obtained from both methods. The auto‐analyzer was considered the reference method, whereas the i‐STAT was considered the test method in the assessment of reliability. Accuracy and precision of the i‐STAT were attained using the concordance analysis. Pearson's correlation coefficients between 2 methods as described by Jensen and Kjelgaard‐Hansen.9 The Passing‐Bablok regression analysis was employed to determine the best linear fit for the i‐STAT.10 Systematic, proportional, and random difference (bias) between the i‐STAT and the auto‐analyzer were determined by the Bland‐Altman plot analysis.11 Finally, the receiver operating characteristics (ROC) curves were developed at the cut‐off plasma K+ concentrations of <3.9 mmol/L (hypokalemia) and >5.8 mmol/L (hyperkalemia); plasma Na+ concentrations of <132 mmol/L (hyponatremia), and >152 mmol/L (hypernatremia); plasma Cl− concentrations of <97 mmol/L (hypochloremia) and >111 mmol/L (hyperchloremia)12 to determine sensitivity (Se) and specificity (Sp) of the i‐STAT at the highest Youden Index.13 In data analyses, a commercial software8 was used and statistical significance was declared at P < .05.
Results
Descriptive statistical measurements and comparisons of plasma and blood electrolyte concentrations are shown in Fig 1. Plasma and blood K+ (4.39 versus 4.2 mmol/L; P < .0001) and Cl− (100.30 versus 99.4 mmol/L; P < .04) concentrations were different. However, plasma and blood Na+ concentrations (136.95 versus 136.8 mmol/L; P = .49) were similar.
Figure 1.
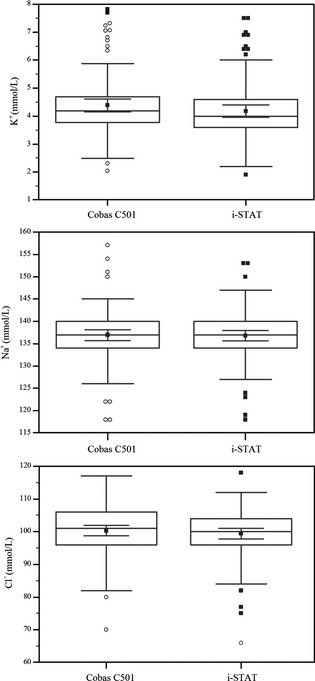
The box‐whisker plot of plasma and blood electrolyte concentrations determined using the auto‐analyzer (Cobas C501) and the POC meter (i‐STAT). Data show quartiles and group mean with 95% CI within the boxes (n = 98).
Concordance analysis revealed that correlation coefficients (r), precision (Pearson ρ), and accuracy (bias correction factor, C b) between the i‐STAT and the Cobas C501 were 0.970, 0.995, and 0.984 for K+; 0.922, 0.924, and 0.998 for Na+; and 0.866, 0.871, and 0.994 for CI−, respectively.
Figure 2 shows distribution of plasma and blood electrolyte concentrations. None of the blood electrolytes measured by the i‐STAT exhibited significant deviation from the identity line (Fig 2). Regression equations fitting blood (Y) and plasma (X) concentration [95% CI], were Y = −0.10 [−0.19/0.01] + 0.98 [0.96/1.00] × X, with a residual deviation (RSD) of 0.07 [−0.14/0.14] for K+; (P = 0.50); Y = 0 [0.00/17.06] + 1 [0.88/1.00] × X, with a RSD of 1.65 [−3.24/3.24] for Na+ (P = 0.27); and Y = 3.04 [−3.98/17.00] + 0.96 [0.82/1.03] × X, with a RSD of 2.91 [−5.71/5.71] for CI− (P = .15). Intercept, slope, and RSD in these equations represent systematic, proportional, and random errors, respectively.
Figure 2.
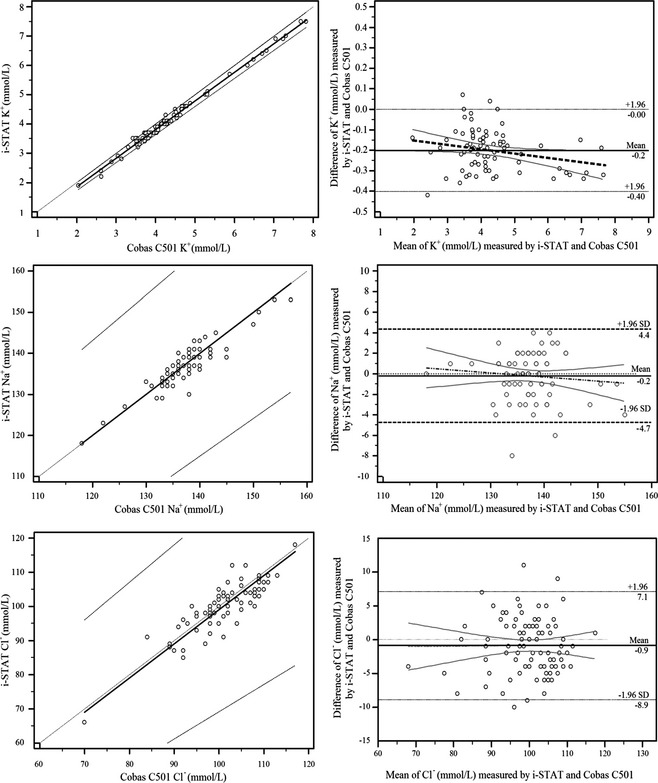
The Passing‐Bablok regression analysis (left column) of blood electrolyte concentrations determined using the POC meter (i‐STAT) versus plasma electrolyte concentrations determined using the auto‐analyzer (Cobas C501, X) (n = 98). The diagonal dashed line is the identity line; the solid line is best fit; and the dashed lines are 95% CI. The Bland‐Altman plot analysis (right column, Table 1) of differences between blood electrolyte concentrations determined using the POC meter and plasma electrolyte concentrations determined using the auto‐analyzer against their averages. The parallel dashed lines are upper and lower limits of the bias; the parallel solid straight line is the mean bias; the thick dashed line is the best fit; and the thin straight lines are 95% CI of the best fit.
Figure 2 and Table 1 summarize the detail of bias. The i‐STAT underestimated plasma K+ concentration [mean bias = −0.20 mmol/L, with a standard error of estimate (Sy.x) of 0.11], which was statistically significant (P = .03). However, underestimation of plasma Na+ (−0.16 mmol/L, with a Sy.x of 2.32; P = .12) and CI− (−0.87 mmol/L, with a Sy.x of 4.08; P < .93) concentrations by the i‐STAT was statistically insignificant.
Table 1.
Evaluation of bias (blood concentration ‐ plasma concentration) of the i‐STAT for measuring electrolyte concentrations as compared to the auto‐analyzer (Cobas C501) (n = 98)
| Difference (mmol/L) | |||||
|---|---|---|---|---|---|
| Electrolyte | Mean (95% CI) | Sy.x | Lower limit | Upper limit | Regression equation |
| [K], mmol/L | −0.20 (−0.22/−0.18) | 0.11 | −0.40 (−0.44/−0.36) | 0.001 (−0.04/0.04) |
Y = −0.11 ± 0.04 ‐ 0.02 ± 0.01•X, P = .02 for intercept and P = .03 for slope |
| [Na], mmol/L | −0.16 (−0.63/0.30) | 2.32 | −4.71 (−5.50/−3.91) | 4.38 (3.58/5.18) |
Y = 8.58 ± 5.55 ‐ 0.06 ± 0.04•X, P = .13 for intercept and P = .12 for slope |
| [CI], mmol/L | −0.87 (−1.69/−0.05) | 4.08 | −8.87 (−10.27/−7.46) | 7.13 (5.73/8.53) |
Y = −1.32 ± 5.36 + 0.005 ± 0.05•X, P = .81 for intercept and P = .93 for slope |
Sy.x = standard error of estimate.
Figure 3 illustrates ROC curves for electrolyte status and Table 2 summarizes sensitivity and specificity of the i‐STAT in assessment of electrolyte status. The cut‐off values yielding the highest Youden index were slightly different from the projected cut‐off values stated previously. The i‐STAT had 100% Se for assessing all electrolyte statuses, except for hypochloremia (76.0%, 54.9–90.6%). Specificity of the i‐STAT for hypokalemia and hyperkalemia was 93.9% (85.2–98.3%) and 100% (95.8–100%), respectively. For hyponatremia and hypernatremia, the i‐STAT had Sp of 89.4 and 100%, respectively. Specificity of the i‐STAT was 87.7% (77.9–94.2%) and 95.7% (89.5–98.8%) in assessing hypo‐ and hyperchloremia, respectively.
Figure 3.
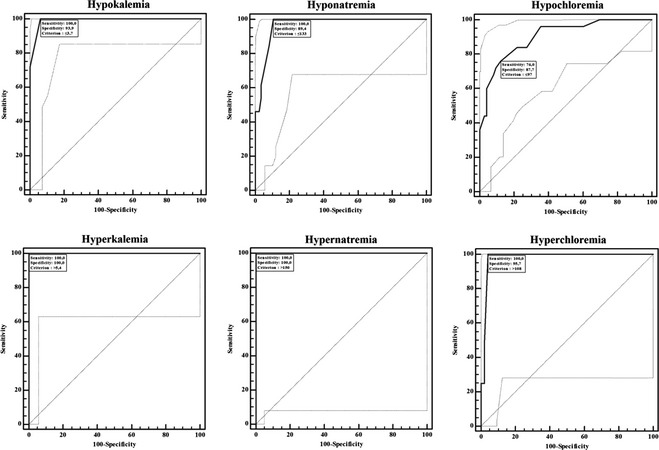
Sensitivity and specificity of the POC meter (i‐STAT) at a cut‐off plasma electrolyte concentrations measured by the auto‐analyzer (Cobas C501) for hypokalemic (≤3.70 mmol/L, n = 32) versus hyperkalemic (>5.40 mmol/L, n = 11); for hyponatremic (≤133 mmol/L, n = 13) versus hypernatremic (>150 mmol/L, n = 2); and hypochloremic (≤97 mmol/L, n = 25) versus hyperchloremic (>108 mmol/L, n = 4) statuses in cattle.
Table 2.
The receiver operating characteristics curve parameters for the i‐STAT in assessing electrolyte status in cattle (n = 98)
| Area Under Curve | ||||||
|---|---|---|---|---|---|---|
| Category | Criteriona | Sensitivity (95% CI) | Specificity (95% CI) | Mean ± SE (95% CI) | z | P |
| Hypokalemia (n = 32) | ≤3.70 | 100 (89.1–100) | 93.9 (85.2–98.3) | 0.991 ± 0.006 (0.947–1) | 81.65 | <.0001 |
| Hyperkalemia (n = 11) | >5.40 | 100 (71.5–100) | 100 (95.8–100) | 1 ± 0 (0.963–1) | – | <.0001 |
| Hyponatremia (n = 13) | ≤133 | 100 (75.3–100) | 89.4 (80.8–95.0) | 0.967 ± 0.016 (0.910–1) | 28.855 | <.0001 |
| Hypernatremia (n = 2) | >150 | 100 (15.8–100) | 100 (96.2–100) | 1 ± 0 (0.963–1) | – | <.0001 |
| Hypochloremia (n = 25) | ≤97 | 76.0 (54.9–90.6) | 87.7 (77.9–94.2) | 0.902 ± 0.035 (0.825–0.953) | 11.51 | <.0001 |
| Hyperchloremia (n = 4) | >108 | 100 (39.8–100) | 95.7 (89.5–98.8) | 0.979 ± 0.014 (0.927–0.997) | 33.19 | <.0001 |
Corresponding with highest Youden index.
Discussion
Electrolyte minerals are vital and play in a number of metabolic activities and homeostasis. Laboratory‐based methods for measurement of plasma K+, Na+, and CI− concentrations are expensive and require specific equipments. Moreover, time to obtain laboratory results is one of the limitations to field practitioners. The i‐STAT produces potentially accurate and reliable results within minutes and can be operated easily in field settings. The i‐STAT has been in use in veterinary hospitals and ambulatory clinics in horses and dogs.14, 15
We evaluated the reliability of the i‐STAT in measuring blood K+, Na+, and CI− concentrations in cattle. Studies evaluating the POC device to assess electrolyte status in cattle are few. In a study involving blood samples collected from 24 clinically healthy cattle, K+ and Na+ measurements by the i‐STAT were shown to range within the reference values.6 Using data from 98 cattle with various diseases in this study allowed us to evaluate a wide range of K+, Na+, and CI− concentrations to determine reliability of the i‐STAT.
In measurement of blood K+ concentration, the i‐STAT was positively correlated with the reference method in clinically healthy cattle.6 The i‐STAT yielded reliable blood K+ measurement results with the reference methods in dogs and horses, as well.14 Blood K+ concentrations measured by the i‐STAT were typically 0.5–1.5 mmol/L less than concentrations obtained from reference method in healthy dogs, cats, and horses.16
Blood Na+ concentration in horses measured by the i‐STAT was shown to be highly correlated (r = 1.00) with plasma Na+ measured by the reference method.14 In clinically healthy cattle, the i‐STAT had excellent correlation with the reference method in measurement of blood Na+ concentration (r = 0.98).6 In the present report, mean blood and plasma Na+ concentrations were not different (Fig 1). Moreover, blood Na+ concentrations were highly correlated with plasma Na+ concentrations (r = 0.922, P < .0001), with a considerable accuracy (0.998) and precision (0.924).
To our knowledge, such evaluation of the i‐STAT for Cl− concentrations in cattle is lacking. The i‐STAT yielded bias and variability within clinically acceptable limits for blood CI− measurement in dogs, cats and horses.16 The i‐STAT is reported to be a reliable and useful device to measure blood CI− concentration in human.17 Our results showed that the i‐STAT could be safely used to determine plasma CI− concentration in cattle.
Although plasma K+ and Cl− concentrations were higher than blood K+ and Cl− concentrations (Fig 1), both variables were also correlated. Moreover, i‐STAT had considerably high precision and accuracy for determination of K+ concentration (0.995 and 0.984, respectively) and high accuracy for Na+ and Cl− concentration (0.998 and 0.994, respectively).
Despite significant mean difference for blood and plasma K+ and Cl− concentrations (Fig 1), the best fit describing relationship between blood and plasma electrolyte concentrations did not deviate from the identity line (Fig 2), suggesting that the i‐STAT produces accurate and precise blood electrolyte data on which to rely. Peiro et al.6 also reported a negative bias for the i‐STAT for Na+ concentration. The i‐STAT underestimated K+ (−0.20), Na+ (−0.16), and CI− (−0.87) concentrations in cow (Fig 2; Table 1). These corresponded to 4.6, 0.1, and 0.9% underestimation of plasma K+, Na+, CI− concentrations measured by the auto‐analyzer, respectively. Despite no deviation the best fit from the identity line, negative biases (an indication of underestimation) are too small considering the mean concentrations and physiological range of electrolyte. Thus, biases could be disregarded from a clinical stand‐point.
It is very important to diagnose insufficient and excessive concentrations of electrolytes, which are associated with various diseases and trigger occurrence of various complications. Sensitivity refers to ability to detect suspected disease/status and Sp refers to ability to avoid misclassifying healthiness as suspected illness. Using physiological cut‐off values,12 actualized cut‐off values by the electrolyte slightly differed (Table 2). Based on the highest Youden index, cattle in this study were hypo‐ (32.7%, ≤3.7 mmol/L) and hyperkalemic (11.2%, >5.4 mmol/L), hypo‐ (13.3%, ≤133 mmol/L) and hypernatremic (2.0%, >150 mmol/L), and hypo‐ (25.5%, ≤97 mmol/L) and hyperchloremic (4.1%, >108 mmol/L). Among these electrolyte disturbances, the diagnosis of hypo‐ and hyperkalemia may be very important because K+ plays a key role in cell function and neuromuscular transmission.18, 19 For instance, because of involvement of a number of factors, in addition to hypokalemia, in muscular weakness and recumbence,20, 21 i‐STAT can be used for more definitive diagnosis and effective treatment protocol. Hyperkalemia may require urgent clinical intervention because of its cardiac effects.22 Hypernatremia, another electrolyte disturbance, may also be life threatening and an immediate intensive medical treatment may be needed in cattle.23 The i‐STAT yielded high Se (ability to detect disease status) and Sp (ability to avoid misclassifying healthy individual as ill individual) for hypo‐ and hyperkalemic status as well as hypo‐ and hypernatremic status. The i‐STAT can be a very valuable tool in bovine medicine in both clinical and field settings for evaluating diagnosis and the efficacy of applied treatments. Except for hypchloremia (76.0%), the i‐STAT had 100% Se in defining tested electrolyte status (Fig 3; Table 2). Further studies evaluating data from a large set of cattle are needed in order to confirm Se and Sp for low and high levels of electrolyte statuses.
In summary, the i‐STAT yielded blood electrolyte results that were in agreement with plasma electrolyte results measured by the auto‐analyzer, with negligible biases and high accuracy and precision. Moreover, results for the i‐STAT predicted electrolyte statuses satisfactorily as evidenced by especially high Se and Sp values for K+ status. It can be concluded that the i‐STAT is a reliable POC device, which can be used in the field setting to assess plasma Na+, K+, and CI− concentrations in cattle.
Acknowledgment
This research was supported by Firat University Scientific Research Unity (FUBAP) project #: VF.13.02.
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Veterinary Teaching Hospital, Faculty of Veterinary Medicine, Firat University, 23119 Elazig, Turkey.
Footnotes
i‐STAT, Abbott Point of Care, Abbott Laboratories, Chicago, IL
Cobas C501 auto‐analyzer, Roche, Mannheim, Germany
BD Vacutainer, Becton, Dickinson and Company, Plymouth, UK
Disposable cartridge, Abbott Point of Care, Abbott Laboratories
SIGMA Laboratory Centrifuges 6K15, Ostrede am Harz, Germany
i‐STAT CHEM8 + control level 1 and 3, Abbott Point of Care, Abbott Laboratories
PS Power and Sample size Calculations, Version 3.0, 2009, Vanderbilt University, Nashville, TN
MedCalc Statistical Software version 13.1.2, Ostend, Belgium
References
- 1. Carlson GP, Bruss M. Fluid, electrolyte, and acid‐base balance In: Kaneko JJ, Harvey JW, Bruss ML, eds. Clinical Biochemistry of Domestic Animals, 6th ed San Diego: Elsevier; 2008:529–559. [Google Scholar]
- 2. Russel K, Roussel AJ. Evaluation of the ruminant serum chemistry profile. Vet Clin North Am Food Anim Pract 2007;23:403–426. [DOI] [PubMed] [Google Scholar]
- 3. Constable P, Grünberg W, Staufenbiel R, Stämpfli HR. Clinicopathologic variables associated with hypokalemia in lactating dairy cows with abomasal displacement or volvulus. J Am Vet Med Assoc 2013;242:826–835. [DOI] [PubMed] [Google Scholar]
- 4. Vogel SR, Nichols S, Buczinski S, et al. Duodenal obstruction caused by duodenal sigmoid flexure volvulus in dairy cattle: 29 cases (2006–2010). J Am Vet Med Assoc 2012;241:621–625. [DOI] [PubMed] [Google Scholar]
- 5. Braun U, Beckmann C, Gerspach C, et al. Clinical findings and treatment in cattle with caecal dilatation. BMC Vet Res 2012;8:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peiro JR, Borges AS, Gonçalves RC, Mendes LC. Evaluation of a portable clinical analyzer for the determination of blood gas partial pressures, electrolyte concentrations, and hematocrit in venous blood samples collected from cattle, horses, and sheep. Am J Vet Res 2010;71:515–521. [DOI] [PubMed] [Google Scholar]
- 7. Dupont WD, Plummer WD. PS power and sample size program available for free on the Internet. Controlled Clin Trials 1997;18:274. [Google Scholar]
- 8. Stephens MA. EDF statistics for goodness of fit and some comparisons. Am J Statist Assoc 1974;69:730–737. [Google Scholar]
- 9. Jensen AL, Kjelgaard‐Hansen M. Method comparison in the clinical laboratory. Vet Clin Pathol 2006;35:276–286. [DOI] [PubMed] [Google Scholar]
- 10. Passing H, Bablok W. A new biometrical procedure for testing the equality of measurements from two different analytical methods. Application of linear regression procedures for method comparison studies in Clinical Chemistry, Part I. J Clin Chem Clin Biochem 1983;21:709–720. [DOI] [PubMed] [Google Scholar]
- 11. Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res 1999;8:135–160. [DOI] [PubMed] [Google Scholar]
- 12. Carlson GP. Clinical chemistry tests In: Smith BP, ed. Large Animal Internal Medicine, 3rd ed St. Louis, USA: Mosby Co; 2002:375–397. [Google Scholar]
- 13. Fawcett T. An introduction to ROC analysis. Pattern Recogn Lett 2006;27:861–874. [Google Scholar]
- 14. Looney AL, Ludders J, Erb HN, et al. Use of a handheld device for analysis of blood electrolyte concentrations and blood gas partial pressures in dogs and horses. J Am Vet Med Assoc 1998;213:526–530. [PubMed] [Google Scholar]
- 15. Silverman SC, Birks EK. Evaluation of the i‐STAT hand‐held chemical analyser during treadmill and endurance exercise. Equine Vet J Suppl 2002;34:551–554. [DOI] [PubMed] [Google Scholar]
- 16. Grosenbaugh DA, Gadawski JE, Muir WW. Evaluation of a portable clinical analyzer in a veterinary hospital setting. J Am Vet Med Assoc 1998;213:691–694. [PubMed] [Google Scholar]
- 17. Mock T, Morrison D, Yatscoff R. Evaluation of the i‐STAT system: A portable chemistry analyzer for the measurement of sodium, potassium, chloride, urea, glucose, and hematocrit. Clin Biochem 1995;28:187–192. [DOI] [PubMed] [Google Scholar]
- 18. Brobst D. Review of the pathophysiology of alterations in potassium homeostasis. J Am Vet Med Assoc 1986;188:1019–1025. [PubMed] [Google Scholar]
- 19. Cunningham CG, Klein BG. Textbook of Veterinary Physiology, 4th ed St Louis: Saunders Elsevier; 2007. [Google Scholar]
- 20. Peek SF, Divers TJ, Guard C, et al. Hypokalemia, muscle weakness, and recumbency in dairy cattle. Vet Ther 2000;4:235–244. [PubMed] [Google Scholar]
- 21. Johns IC, Whitlock RH, Sweeney RW. Hypokalaemia as a cause of recumbency in an adult dairy cow. Aust Vet J 2004;82:413–416. [DOI] [PubMed] [Google Scholar]
- 22. Radostits OM, Gay CC, Hinchcliff KW, Constable PD. Disturbances of Free Water, Electrolytes and Acid‐Base Balance. Veterinary Medicine, 10th ed St Louis: Saunders Elsevier; 2007: 73–102. [Google Scholar]
- 23. Cebra CK, Cebra ML. Altered mentation caused by polioencephalomalacia, hypernatremia, and lead poisoning. Vet Clin North Am Food Anim Pract 2004;20:287–302. [DOI] [PubMed] [Google Scholar]


