Abstract
Background
Recent surveillance studies for equine respiratory viruses have shown that equine influenza virus (EIV) continues to be a prevalent respiratory virus of equids throughout the United States and Europe.
Objectives
To gain a better understanding of the prevalence and epidemiology of EIV shed by horses, mules and donkeys in the United States from March 2010 to November 2013.
Animals
2,605 equids.
Methods
Nasal secretions from index cases with acute onset of respiratory disease were tested by qPCR for EIV. Multilevel logistic regression was used to model the association between EIV status and prevalence factors. Furthermore, observations from EIV‐positive study horses were compared to previous data from March 2008 to February 2010.
Results
A total of 230 (9.7%) index cases tested qPCR positive for EIV. A higher‐than‐expected proportion of EIV qPCR‐positive horses occurred in the 1–5, 6–10, and 11–15 age groups when compared to the <1 year of age group. Fever, nasal discharge and coughing were positively associated with EIV‐positive horses. EIV qPCR‐positive study cases were significantly older and more often vaccinated against EIV compared to EIV qPCR‐positive animals from the 2008‐2010 study period.
Conclusions and Clinical Importance
This study provides valuable and contemporary information on the frequency of EIV detected by qPCR in the United States. The results also underscore that older and previously vaccinated horses were susceptible to EIV.
Keywords: EIV, Surveillance, United States
Abbreviations
- EIV
equine influenza virus
- eGAPDH
equine glyceraldehyde‐3‐phosphate dehydrogenase
- EHV‐4
equine herpesvirus type‐4
- qPCR
quantitative polymerase chain reaction
- OIE
Office International des Epizooties
Equine influenza virus (EIV) is considered one of the leading causes of infectious respiratory diseases in equids worldwide.1, 2, 3 Because of the highly contagious nature and rapid spread of EIV, this virus has severe financial implications for the horse industry.4 The global transportation of horses has been responsible for numerous outbreaks of EIV by introduction of the virus into previously unexposed horse populations.5, 6 Adherence to strict quarantine and vaccination protocols and vigilant monitoring are required to avoid the introduction and spread of EIV among all horse populations. Two distinct subtypes of influenza virus have been isolated from horses since 1956.7 These are represented by the following prototype strains: influenza A/equine/Prague/56 (H7N7) and influenza A/equine/Miami/63 (H3N8). EIV diverged into the Eurasian and American lineages in the late 1980s, with the American lineage diverging further into the Kentucky, South American, and Florida sublineages.8 Recent EIV surveillance data showed that Florida sublineage viruses from both clades 1 and 2 circulate in Europe, whereas only clade 1 viruses have been reported from North America.1, 9, 10, 11 Although the circulating EIV strains generally cause sporadic disease mainly in unvaccinated horses, recent surveillance data from the OIE (Office International des Epizooties) documented lack of vaccine efficacy against Florida sublineage viruses from both clades 1 and 2.9 A previous study by the authors on the prevalence and epidemiology of important viral and bacterial respiratory pathogens associated with upper respiratory tract diseases showed that EHV‐4 and EIV were the 2 most commonly detected respiratory viruses in horses in the United States.12 The same study documented that EIV‐positive cases were reported with increased frequency during the winter through spring months, in all ages with increased frequency in 1–5‐year‐old horses, and in all breeds and uses. Furthermore, among the 60 EIV qPCR‐positive cases reported in that study, nasal discharge, fever and coughing were the most commonly reported clinical signs. The objective of this study was to gain a better understanding of the prevalence and epidemiology of EIV shed by horses presented to veterinarians with upper respiratory tract infections from March 2010 to November 2013 and to compare categorical observations from EIV‐positive horses from March 2010 to November 2013 to previous data from March 2008 to February 2010.
Materials and Methods
Animals and Collection of Samples
Veterinarians in 239 equine veterinary practices located in 38 states and previously enrolled in a voluntary surveillance program for equine respiratory pathogens were asked to collect nasal secretions from horses with signs of acute upper respiratory tract infection (Fig. 1). The case definition of horses to be sampled included unexplained fever (T > 101.5°F) and ≥1 of the following signs: lethargy, nasal discharge and coughing. Case submission occurred over a 45‐month period (March 2010 to November 2013). A diagnosis of EIV infection was made based on the presence of clinical signs and laboratory detection of EIV by qPCR as previously described.12
Figure 1.
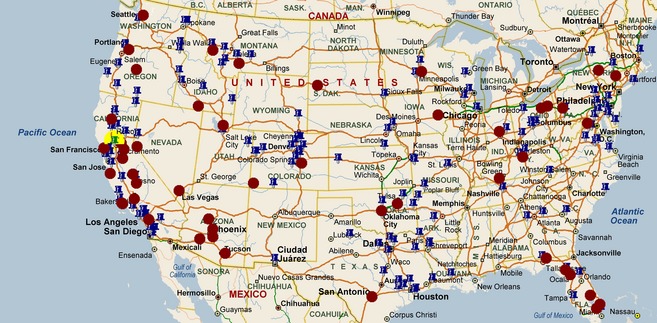
Geographic location of equine veterinary clinics enrolled in the voluntary surveillance program for equine respiratory pathogens. Blue pins represent veterinary clinics which submitted nasal secretions from horses negative for EIV by qPCR. Red dots represent veterinary clinics which submitted nasal secretions from horses positive for EIV by qPCR. The location of the laboratory which performed all the molecular analysis is marked with a yellow circle.
A questionnaire was used to collect information pertaining to the patient and its clinical signs. The questionnaire included signalment (age, breed, and sex), use (racing, show, pleasure, breeding, and others), vaccination history (date of vaccination and type of vaccine used), and presence of clinical signs at the time of sample collection (general attitude, appetite, rectal temperature, nasal discharge, ocular discharge, presence of cough, and distal limb edema). Information pertaining to vaccine brand and manufacturer was not requested.
The study veterinarians collected nasal secretions using two 6 inch rayon‐tipped swabs.1 Recommendations were given to collect nasal secretions from the nostrils with the most obvious discharge. After collection of nasal secretions, both swabs were placed in a 15 mL conical tube containing 2 mL of viral transport medium (minimal essential medium with 0.125% gentamicin and 0.1% amphotericin B). Samples were kept refrigerated and shipped on ice overnight to the laboratory at the University of California, School of Veterinary Medicine, Davis.
Nucleic acid extraction from nasal secretions was performed the day of sample arrival to the laboratory using an automated nucleic acid extraction system2 according to the manufacturer's recommendations. Total RNA was purified from nasal secretions and transcribed to complementary DNA as previously described.12 Nasal secretions were assayed for the presence of the HA1 gene of EIV using a previously reported qPCR assay.12 To determine the sample quality and efficiency of nucleic acid extraction, all samples were assessed for the presence of the housekeeping gene eGAPDH as previously described.13
Statistical Analysis
Multilevel logistic regression was used to model the association between EIV status and prevalence factors. State and clinic were used in the hierarchical model as nested random effects, whereas prevalence factors were treated as fixed effects. Factors were individually analyzed, and those with P ≤ .05 were included in a multivariable model. Results are reported as prevalence odds ratios and 95% confidence intervals. Furthermore, categorical observations from 230 EIV qPCR‐positive horses from March 2010 to November 2013 were compared to previous data from March 2008 to February 2010 (60 EIV qPCR positives).12 For all statistical analyses, values of P ≤ .05 were considered significant.
Results
A total of 230 (9.7%) index cases tested qPCR positive for EIV. The ages of the affected horses ranged from 3 weeks to 24 years (median, 6 years; Table 1). The breeds of affected animals included Quarter Horse (107), Thoroughbred (20), Warmblood (19), American Paint Horse (16), Pony breed (15), Arabian (12), Standardbred (4), Draft Horse breed (4), others (20) and not reported (13). There were 89 mares or fillies, 20 stallions or colts, 105 geldings, and 16 animals with no reported sex. The actual or intended use for the affected animals was pleasure riding (84), show (74), racing (21), breeding (7), others (22), and not reported (22). Lethargy, nasal discharge, fever and coughing were the most commonly reported clinical signs among affected animals. Vaccination status was unknown for 131 horses. Eighty‐four horses had been vaccinated against EIV, whereas 15 horses had never been vaccinated.
Table 1.
Signalment, use, vaccination history, clinical signs, and qPCR results for EIV in horses participating in a voluntary surveillance program for equine infectious respiratory pathogens from March 2010 to November 2013 and from March 2008 to February 2010
| Control study population 2010–2013 (2,375) | EIV qPCR positive (230) | Control study population 2008–2010 (761) | EIV qPCR positive (60) | |
|---|---|---|---|---|
| Age (years) | ||||
| <1 | 154 (6.5%) | 3 (1.3%) | 130 (17.1%) | 5 (8.3%) |
| 1–5 | 768 (32.3%) | 104 (45.2%) | 255 (33.5%) | 37 (61.7%) |
| 6–10 | 562 (23.7%) | 80 (34.8%) | 142 (18.7%) | 13 (21.6%) |
| 11–15 | 369 (15.5%) | 25 (10.9%) | 105 (13.8%) | 3 (5.0%) |
| 16–20 | 184 (7.7%) | 3 (1.3%) | 42 (5.5%) | 1 (1.7%) |
| >20 | 123 (5.2%) | 2 (0.9%) | 21 (2.7%) | 0 |
| Not reported | 215 (9.1%) | 13 (5.6%) | 66 (8.7%) | 1 (1.7%) |
| Breed | ||||
| Thoroughbred | 287 (12.1%) | 20 (8.7%) | 160 (21.0%) | 2 (3.3%) |
| Quarter Horse | 842 (35.4%) | 107 (46.5%) | 144 (18.9%) | 25 (41.7%) |
| Warmblood | 256 (10.8%) | 19 (8.3%) | 89 (11.7%) | 0 |
| American Paint | 151 (6.4%) | 16 (7.0%) | 46 (6.0%) | 9 (15.0%) |
| Pony | 102 (4.3%) | 15 (6.5%) | 38 (5.0%) | 2 (3.3%) |
| Arabian | 164 (6.9%) | 12 (5.2%) | 29 (3.8%) | 1 (1.7%) |
| Standardbred | 43 (1.8%) | 4 (1.7%) | 14 (2%) | 0 |
| Spanish | 0 | 0 | 9 (1.2%) | 0 |
| Draft | 29 (1.2%) | 4 (1.7%) | 9 (1.2%) | 0 |
| Mule/donkey | 5 (0.2%) | 0 | 4 (0.5%) | 1 (1.7%) |
| Others | 301 (12.7%) | 20 (8.7%) | 119 (15.6%) | 14 (23.3%) |
| Not reported | 195 (8.2%) | 13 (5.7%) | 100 (13.1%) | 6 (10.0%) |
| Gender | ||||
| Mare/filly | 846 (35.6%) | 89 (38.7%) | 257 (33.8%) | 20 (33.3%) |
| Gelding | 1035 (43.6%) | 105 (45.6%) | 300 (39.4%) | 23 (38.4%) |
| Stallion/colt | 338 (14.2%) | 20 (8.7%) | 140 (18.4%) | 12 (20.0%) |
| Not Reported | 156 (6.6%) | 16 (7.0%) | 64 (8.4%) | 5 (8.3%) |
| Use | ||||
| Show | 661 (27.8%) | 74 (32.2%) | 242 (31.8%) | 19 (31.6%) |
| Pleasure | 891 (37.5%) | 84 (36.5%) | 210 (27.6%) | 24 (40.0%) |
| Racing | 200 (8.4%) | 21 (9.1%) | 152 (20%) | 6 (10.0%) |
| Breeding | 110 (4.6%) | 7 (3.0%) | 23 (3.0%) | 1 (1.7%) |
| Others | 191 (8.1%) | 22 (9.6%) | 56 (7.4%) | 4 (6.7%) |
| Not reported | 322 (13.6%) | 22 (9.6%) | 78 (10.2%) | 6 (10.0%) |
| Vaccination | ||||
| EHV‐1/‐4/EIV yes | 855 (36.0%) | 84 (36.5%) | 300 (39.4%) | 9 (15.0%) |
| EHV‐1/‐4/EIV no | 232 (9.8%) | 15 (6.5%) | 103 (13.5%) | 10 (16.7%) |
| EHV‐1/‐4/EIV unknown | 1288 (54.2%) | 131 (57.0%) | 358 (47.1%) | 41 (68.3%) |
| Clinical signs | ||||
| Depression yes | 1363 (57.4%) | 143 (62.2%) | 390 (51.3%) | 34 (56.7%) |
| Depression no | 892 (37.6%) | 78 (33.9%) | 320 (42.0%) | 21 (35.0%) |
| Depression unknown | 120 (5.0%) | 9 (3.9%) | 51 (6.7%) | 5 (8.3%) |
| Anorexia yes | 1076 (45.3%) | 114 (49.6%) | 327 (43.0%) | 29 (48.4%) |
| Anorexia no | 1173 (49.4%) | 107 (46.5%) | 384 (50.5%) | 26 (43.3%) |
| Anorexia unknown | 126 (5.3%) | 9 (3.9%) | 50 (6.5%) | 5 (8.3%) |
| Fever yes | 1297 (54.6%) | 151 (65.6%) | 426 (56%) | 37 (61.6%) |
| Fever no | 720 (30.3%) | 43 (18.7%) | 228 (30%) | 19 (31.7%) |
| Fever unknown | 358 (15.1%) | 36 (15.7%) | 107 (14%) | 4 (6.7%) |
| Nasal discharge yes | 1586 (66.8%) | 214 (93.0%) | 580 (76.2%) | 53 (88.4%) |
| Nasal discharge no | 686 (28.9%) | 7 (3.1%) | 96 (12.6%) | 2 (3.3%) |
| Nasal discharge unknown | 103 (4.3%) | 9 (3.9%) | 85 (11.2%) | 5 (8.3%) |
| Ocular discharge yes | 509 (21.4%) | 66 (28.7%) | 64 (8.4%) | 12 (20.0%) |
| Ocular discharge no | 1738 (73.2%) | 155 (67.4%) | 640 (84.1%) | 43 (71.7%) |
| Ocular discharge unknown | 128 (5.4%) | 9 (3.9%) | 57 (7.5%) | 5 (8.3%) |
| Coughing yes | 1073 (45.2%) | 195 (84.8%) | 345 (45.3%) | 52 (86.7%) |
| Coughing no | 1147 (48.3%) | 22 (9.6%) | 51 (6.7%) | 3 (5.0%) |
| Coughing unknown | 155 (6.5%) | 13 (5.6%) | 365 (48%) | 5 (8.3%) |
| Limb edema yes | 178 (7.5%) | 5 (2.2%) | 65 (8.5%) | 1 (1.7%) |
| Limb edema no | 2062 (86.8%) | 213 (92.6%) | 642 (84.4%) | 54 (90.0%) |
| Limb edema unknown | 135 (5.7%) | 12 (5.2%) | 54 (7.1%) | 5 (8.3%) |
Multivariable analysis showed significant results for various factors, including age, breed, and specific clinical signs (Table 2). Significant clustering was noted at the clinic level (P < .001), but was negligible at the state level. A higher‐than‐expected proportion of the EIV qPCR‐positive horses occurred in the 1–5, 6–10, and 11–15 age groups when compared to the <1 year of age group. Fewer EIV qPCR‐positive horses were found in the age groups 16–20 and >20 years of age. No associations were found for the majority of the breeds when compared to the most prevalent breed (Quarter Horse breed). The prevalence odds of being EIV positive in the “other” breed category were half the odds in the Quarter Horse breed. Spanish breed and the category mule or donkey were omitted from the analysis because of the small number of animals. The presence of fever, coughing and nasal discharge was positively associated with EIV, whereas limb edema was negatively associated with EIV. No significant associations were found for use, sex, vaccination history and specific clinical signs such as ocular discharge, lethargy and anorexia.
Table 2.
Univariable and multivariable analysis or selected demographic and clinical factors associated with EIV qPCR status. Results are reported as prevalence odds ratios and 95% confidence intervals
| Factors | Univariable analysis | Multivariable analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| Prevalence odds ratio | 95% confidence interval | P‐value | Prevalence odds ratio | 95% confidence interval | P‐value | |||
| Lower bound | Upper bound | Lower bound | Upper bound | |||||
| Age (years) | ||||||||
| <1 | 1.00 | 1.00 | ||||||
| 1–5 | 9.46 | 2.85 | 31.33 | <.001 | 7.37 | 2.16 | 25.11 | .001 |
| 6–10 | 10.24 | 3.04 | 34.45 | <.001 | 8.94 | 2.58 | 31.06 | .001 |
| 11–15 | 4.22 | 1.19 | 14.91 | .025 | 4.85 | 1.31 | 17.97 | .018 |
| 16–20 | 0.91 | 0.17 | 4.77 | .911 | 1.04 | 0.19 | 5.65 | .966 |
| >20 | 0.93 | 0.15 | 5.89 | .936 | 1.31 | 0.20 | 8.70 | .780 |
| Breed | ||||||||
| Quarter Horse | 1.00 | 1.00 | ||||||
| Thoroughbred | 0.54 | 0.29 | 0.99 | .045 | 0.49 | 0.24 | 1.03 | .060 |
| Warm blood | 0.42 | 0.22 | 0.81 | .009 | 1.00 | 0.48 | 2.10 | .994 |
| American Paint | 0.76 | 0.42 | 1.39 | .374 | 0.83 | 0.41 | 1.66 | .597 |
| Pony | 1.16 | 0.59 | 2.27 | .668 | 1.41 | 0.62 | 3.21 | .411 |
| Arabian | 0.50 | 0.26 | 0.97 | .040 | 0.67 | 0.31 | 1.44 | .302 |
| Standardbred | 0.45 | 0.11 | 1.80 | .263 | 0.69 | 0.12 | 3.90 | .673 |
| Draft | 1.06 | 0.33 | 3.40 | .916 | 1.98 | 0.41 | 9.63 | .397 |
| Others | 0.45 | 0.26 | 0.77 | .004 | 0.50 | 0.26 | 0.96 | .038 |
| Clinical signs | ||||||||
| Nasal discharge | 13.87 | 6.37 | 30.21 | <.001 | 7.19 | 2.99 | 17.28 | <.001 |
| Ocular discharge | 1.42 | 1.00 | 2.03 | .050 | 1.05 | 0.69 | 1.60 | .805 |
| Coughing | 10.59 | 6.64 | 16.89 | <.001 | 7.91 | 4.52 | 13.83 | <.001 |
| Fever | 2.02 | 1.39 | 2.93 | <.001 | 2.43 | 1.59 | 3.70 | <.001 |
| Limb edema | 0.26 | 0.10 | 0.66 | .005 | 0.21 | 0.06 | 0.71 | .013 |
Overall, 855 control horses had been vaccinated against EIV. Seven‐hundred‐and‐forty‐five horses had received a killed adjuvanted EIV vaccine and 76 horses had received a modified‐live EIV vaccine. For 34 horses, there was no record of which type of EIV vaccine had been administered. Time of vaccine administration to development of respiratory signs in the control horses was <6 months for 565 horses, 6–12 months for 134 horses, >12 months for 48 horses, and not reported for 108 horses. When evaluating the 230 EIV PCR‐postive horses, 84 had a vaccination history. Among these horses, 69 had received a killed adjuvanted EIV vaccine, 5 had received a modified‐live EIV vaccine and in 10 horses no EIV vaccine type was listed. For 39 EIV PCR‐positive horses, the EIV vaccine had been given <6 months before onset of respiratory signs, between 6 and 12 months for 26 horses, >12 months for 9 horses, and for 10 horses the time from vaccine administration was not reported. There was no statistical significance (P > .05) in the type of EIV vaccine used (killed adjuvanted vs modified‐live vaccine) between control and EIV PCR‐positive horses. Furthermore, significantly more horses in both groups (control and EIV PCR‐positive) had been vaccinated in the time period <6 months when compared to the time period 6–12 months and >12 months (P < .01).
When both EIV qPCR‐positive groups were compared, several statistical differences were noticed. Horses qPCR‐positive for EIV in 2010–2013 tended to be older than horses in the EIV 2008–2010 group (P = .001). EIV‐positive stallions and colts were more commonly identified during the 2008–2010 period (P = .039). Horses qPCR‐positive for EIV in 2010–2013 tended to be vaccinated more often against EIV than the qPCR‐positive EIV horses from the 2008–2010 period (P = .001). There were no significant differences between the 2 EIV groups with regard to use and clinical presentation.
Discussion
Recently, the OIE expert surveillance panel on EIV reported on outbreaks of equine influenza in a variety of countries including the United States.9 Vaccination breakdowns were observed in Thoroughbred yearlings in Kentucky, sport horses in France, racehorses in Ireland and, recently, horses imported into South Arabia and Japan. Sequence analysis of the HA1 gene of various EIV isolates identified clade 1 and clade 2 viruses of the Florida sublineage with only clade 1 viruses found in the United States. Our study results are in agreement with the equine influenza activity reported by the OIE and also showed that contemporary EIV cases were reported with higher frequency in middle‐aged horses and horses previously vaccinated with EIV vaccines.
Although index cases represented a wide range of ages, 62% of them were ≤10 years of age, which reflects the higher susceptibility of young animals to infectious respiratory pathogens.14 Equine Influenza virus in the age group 1–5 years of age was overrepresented, similar to a previous study.12 Also, a higher‐than‐expected proportion of the EIV qPCR‐positive horses occurred in the 6–10 and 11–15 age groups. Although every age group appeared to be susceptible to EIV, the age‐dependent susceptibility may be a nonspecific marker for differences in management, exposure and immunity. Equine Influenza virus infections were mainly characterized by fever, nasal discharge and coughing, which is in agreement with previous studies.12, 14, 15
Despite a large number of submissions with unknown vaccination history, a similar percentage of control study horses and EIV qPCR‐positive horses were vaccinated against EHV‐1/‐4 and EIV. Surprisingly, qPCR‐positive EIV cases in 2010–2013 tended to be vaccinated more often than qPCR‐positive EIV horses from the 2008–2010 period. This observation is in agreement with the OIE expert surveillance panel on EIV and questions the efficacy of EIV vaccines available in the United States. The suboptimal protection of vaccines commercially available in the United States also is supported by the observation that significantly more horses in the EIV PCR‐positive group had been vaccinated in the time period <6 months when compared to the time period 6–12 months and >12 months. Information pertaining to vaccine brand and manufacturer was unavailable for the study horses, and thus no conclusions can be drawn regarding the efficacy of specific vaccines. Commercial killed and inactivated EIV vaccines should contain epidemiologically relevant viruses and should be updated in a timely manner to confer optimal protection. The OIE expert surveillance panel on EIV recommends that vaccines for the international market should contain both clade 1 (A/eq/Ohio/2003‐like) and clade 2 (A/eq/Richmond/1/2007‐like) viruses of the Florida sublineage.9
In conclusion, this follow‐up surveillance study focused on 2,605 horses with upper respiratory tract infection for which epidemiological information was collected over a 45‐month period. This study provides valuable and contemporary information on the frequency of EIV detected by qPCR. The results also point to the fact that older and previously vaccinated horses were susceptible to EIV. This study highlights the importance of conducting biosurveillance for respiratory pathogens to gather epidemiological information and determine the frequency of vaccine breakdowns.
Acknowledgments
The authors thank all the veterinarians enrolled in the biosurveillance program for assistance with sample collection. The study was supported by Merck Animal Health.
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Footnotes
Puritan Products Company LLC, Guilford, ME
CAS‐1820 X‐tractor Gene, Corbett Life Science, Australia
References
- 1. Landolt GA, Townsend GG, Lunn DP. Equine influenza infection In: Sellon DC, Long MT, eds. Equine Infectious Diseases, 2nd ed St. Louis, MO: Saunders Elsevier; 2014:141–151. [Google Scholar]
- 2. Legrand LJ, Pitel PH, Marcillaud‐Pitel CJ, et al. Surveillance of equine influenza viruses through the RESPE network in France from November 2005 to October 2010. Equine Vet J 2013;45:776–783. [DOI] [PubMed] [Google Scholar]
- 3. Gildea S, Fitzpatrick DA, Cullinane A. Epidemiological and virological investigations of equine influenza outbreaks in Ireland (2010–2012). Influenza Other Respir Viruses 2013;7:61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Smyth GB, Dagley K, Tainsh J. Insights into the economic consequences of the 2007 equine influenza outbreak in Australia. Aust Vet J 2011;89:151–158. [DOI] [PubMed] [Google Scholar]
- 5. Guthrie AJ, Stevens KB, Bosman PP. The circumstances surrounding the outbreak and spread of equine influenza in South Africa. Rev Sci Tech 1999;18:179–185. [DOI] [PubMed] [Google Scholar]
- 6. Watson J, Daniels P, Kirkland P, et al. The 2007 outbreak of equine influenza in Australia: Lessons learned for international trade in horses. Rev Sci Tech 2011;30:87–93. [DOI] [PubMed] [Google Scholar]
- 7. Kawaoka Y, Bean WJ, Webster RG. Evolution of the hemagglutinin of equine H3 influenza viruses. Virology 1989;169:283–292. [DOI] [PubMed] [Google Scholar]
- 8. Lewis NS, Daly JM, Russell CA, et al. Antigenic and genetic evolution of equine influenza A (H3N8) virus from 1968 to 2007. J Virol 2011;85:12742–12749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. OIE (2013) Conclusions and recommendations from the Expert Surveillance Panel on Equine Influenza Vaccines. Office International des Epizooties, http://www.oie.int/our-scientific-expertise/specific-information-and-recommendations/equine-influenza.
- 10. Bryant NA, Rash AS, Woodward AL, et al. Isolation and characterization of equine influenza virus (H3N8) from Europe and North America from 2008 to 2009. Vet Micro 2011;147:19–27. [DOI] [PubMed] [Google Scholar]
- 11. Gildea S, Quinlivan M, Arkins S, et al. The molecular epidemiology of equine influenza in Ireland from 2007–2010 and its international significance. Equine Vet J 2011;44:387–392. [DOI] [PubMed] [Google Scholar]
- 12. Pusterla N, Kass PH, Mapes S, et al. Surveillance programme for important equine infectious respiratory pathogens in the USA. Vet Rec 2011;169:12. [DOI] [PubMed] [Google Scholar]
- 13. Mapes S, Leutenegger CM, Pusterla N. Nucleic acid extraction methods for detection of EHV‐1 from blood and nasopharyngeal secretions. Vet Rec 2008;28:857–859. [DOI] [PubMed] [Google Scholar]
- 14. Mumford EL, Traub‐Dargatz JL, Carman J, et al. Occurrence of infectious upper respiratory tract disease and response to vaccination in horses on six sentinel premises in northern Colorado. Equine Vet J 2003;35:72–77. [DOI] [PubMed] [Google Scholar]
- 15. Morley PS, Townsend HG, Bodgan JR, et al. Descriptive epidemiologic study of disease associated with influenza virus infections during three epidemics in horses. J Am Vet Med Assoc 2000;216:535–544. [DOI] [PubMed] [Google Scholar]


